Cerebral oximetry based on near-infrared spectroscopy can non-invasively measure hemoglobin oxygen saturation in mixed arterial, venous and capillary blood in the brain. In order to determine if this is a clinically desirable monitor, we need to answer three questions in order. The first question is if cerebral oximetry monitors an important aspect of physiology. The second question is if the physiology can be optimized based on this monitor. The third question is if the outcome can be improved based on cerebral oximetry-guided clinical care. In this review, we share our answers to these three questions.
La oximetría cerebral basada en la espectroscopia cercana al infrarrojo puede medir demanera no invasiva la saturación de oxígeno de la hemoglobina en la sangre mixta arterial, venosa y capilar en el cerebro. A fin de determinar si este monitor es deseable en la clínica, es preciso responder 3 preguntas, en su orden. La primera es si la oximetría cerebral monitorea un aspecto importante de la fisiología. La segunda es si se puede optimizar la fisiología con base en este monitor. La tercera es si se puede mejorar el desenlace mediante una intervención clínica basada en la oximetría cerebral. En esta revisión presentamos nuestras respuestas a estas 3 preguntas.
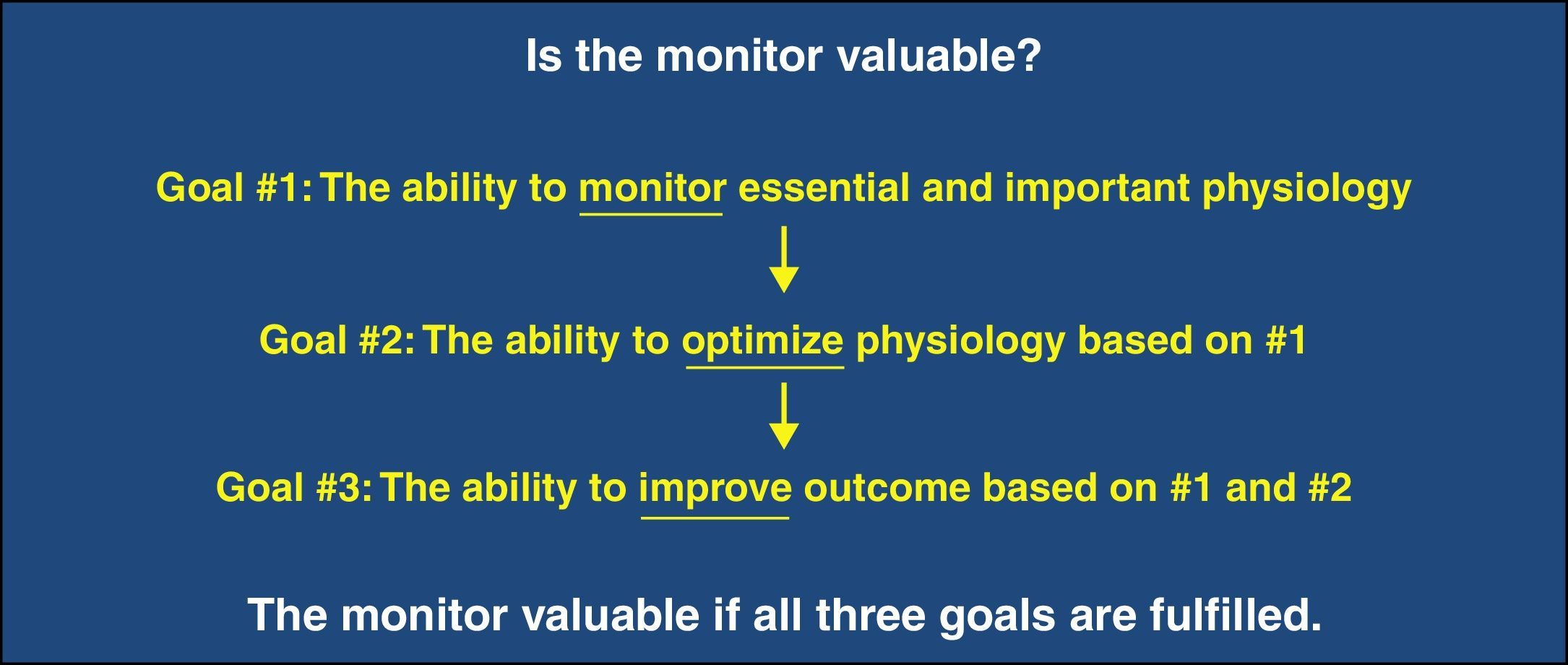
Cerebral oximetry based on near-infrared spectroscopy (NIRS) is a non-invasive, easy-to-use and still evolving monitoring modality. The technology itself and the status of its clinical application have been recently reviewed.1 Philosophically, the following three sequential qualifications characterize any monitor as being desirable (Fig. 1). The first is that it monitors an essential or important aspect of physiology. The second is that it facilitates the optimization of that essential physiology. The third is that it improves outcome based on monitoring and optimization of the physiology. In this review, we examine if cerebral oximetry meets these qualifications.
Question 1: Does cerebral oximetry monitor essential and important physiology?The first question asks if what cerebral oximetry monitors qualifies as essential physiology. Different to pulse oximetry which monitors arterial blood hemoglobin saturation (SpO2), cerebral oximetry monitors hemoglobin saturation in mixed arterial, venous, and capillary blood in cerebral tissue (SctO2). As a result, SctO2 is determined by two physiological considerations. The first is the proportional volumes of the arterial, venous and capillary blood in the brain region illuminated by cerebral oximetry. SctO2 is higher if the saturated arterial blood is more and/or the desaturated venous blood is less, and vice versa. The volume percentage of different cerebral blood compartment is not fixed. It varies inter-individually and possibly between different brain regions in the same individual.2 Moreover, it was suggested that it may change during hypoxia,2 hypercapnia/hypocapnia,3 neural excitation,4 and phenylephrine administration.5 The second consideration is the balance between cerebral oxygen supply and demand. Cerebral oxygen supply is determined by cerebral blood flow (CBF) and arterial blood oxygen content. If arterial blood oxygen content is stable, an increase in CBF will expand arterial blood volume and shift the volume ratio toward more arterial blood. Cerebral oxygen demand is determined by cerebral metabolic rate of oxygen. If cerebral oxygen supply is stable, an increase in cerebral metabolic rate of oxygen will expand venous blood volume and shift the volume ratio toward more venous blood. These physiological processes alter SctO2 reading. Therefore, it can be challenging to decipher the exact cause of a change in SctO2 when the needed supplementary information is not available. When cerebral metabolic rate of oxygen, arterial blood oxygen content, and the volume percentage of different blood compartments are all relatively stable, SctO2 can be regarded as a surrogate of cerebral perfusion.
Tissue perfusion and oxygenation are essential physiological components because ischemia and hypoxia are (rapidly) harmful. Tissue perfusion and oxygenation are arguably the end point of all physiological management in the operating room and intensive care unit. However, they are not routinely monitored. Therefore, any device that can monitor indices of tissue perfusion and oxygenation may be useful. Cerebral oximetry is viewed as such a technology because (1) if the same brain region is monitored continuously in the same patient, the trend of change in SctO2 reflects the balance between cerebral oxygen supply and consumption, and (2) if cerebral metabolic rate is relatively constant, SctO2 is determined by oxygen delivery to the brain. Therefore, it can be stated that NIRS-based cerebral oximetry monitors essential and important physiology – the perfusion and oxygenation of cerebral tissue as long as all components of SctO2-determing physiological processes are known or at a minimum considered.
Question-2: Can cerebral oximetry optimize the essential physiology?Monitoring is clinically meaningless if nothing can be done about the physiology it monitors. The universal feature among the standard monitors such as blood pressure and pulse oximetry used in anesthetized patients is that something can be done based on the monitoring. For NIRS-measured SctO2, intervention protocols aiming to increase SctO2 when it is decreased (also known as cerebral desaturation) have been adopted in cardiac6,7 and non-cardiac surgeries.8 The protocols adopted by various studies are similar but with some variations.6–8 An algorithm was proposed to treat intraoperative cerebral desaturation in a more systematic approach.9 However, its validation is still awaiting multicenter study.
The guiding principle in the treatment of cerebral desaturation is to increase oxygen delivery to the brain, and/or decrease cerebral metabolic rate of oxygen. Increasing CBF can augment cerebral oxygen supply. Interventions that can be considered for CBF augmentation include: (1) increasing cerebral perfusion pressure if it is below the lower limit of cerebral autoregulation and autoregulation is intact,10 (2) increasing cerebral perfusion pressure irrespective of the lower limit if the autoregulation is impaired,10 (3) augmenting cardiac output,11 (4) avoiding hyperventilation and hypocapnia,12 (5) administering cerebral vasodilator,6 and (6) using inhalational anesthetic agents based on their intrinsic cerebral vasodilatory property.13 In addition to CBF augmentation, interventions capable of improving arterial blood oxygen content such as increased inspired oxygen fraction and red blood cell transfusion should also be considered in order to boost oxygen deliver to the brain – the supply side. On the consumption side, deepening anesthesia causes a progressive decrease in cerebral metabolic rate of oxygen until electroencephalography becomes isoelectric.14 However, we caution against decreasing cerebral metabolic rate of oxygen via deepening anesthesia because there is emerging evidence that unnecessarily deep anesthesia may associate with poorer outcomes.15,16 Moreover, a deeper anesthesia plane may cause more hypotension and even impair cerebral autoregulation if >1 MAC inhalational agent is used. A study has reported that cerebral desaturation can be successfully managed in 80% of the patients in whom one or more episodes of cerebral desaturation had occurred.7
Question 3: Can cerebral oximetry-guided intervention improve outcome?It is common practice to believe that treating an inappropriate number on a monitor is the correct action. However, this is just a hypothesis. Physiology is complicated and multifactorial. In contrast, monitors often present a simplified and localized view of the big picture. If an intervention guided by a specific monitor is beneficial needs to be appraised by outcome studies. Clinical intervention that improves the readout of one parameter may hurt others. For example, when anesthesia-induced hypotension is corrected by phenylephrine bolus administration, both NIRS-measured SctO2 and esophageal Doppler-derived cardiac output are decreased.17 In contrast, SctO2 and cardiac output remain stable even though the arterial blood pressure is significantly decreased by anesthesia induction and the associated hypotension is not treated.18
Cerebral oximetry has been studied in patients undergoing cardiac surgery since its introduction to the operating room.6,7,19,20 In a retrospective study, Goldman et al. studied 1034 cardiac patients in whom cerebral oximetry was used to guide the maintenance of SctO2 at or near the preinduction baseline and compared the incidence of stroke in this group with the control group (n=1245) in which cerebral oximetry was not incorporated. They found that permanent stroke in the treatment group (n=10, 0.97%) was significantly less than the control group (n=25, 2.5%) (P<0.044).6 In a prospective study, Murkin et al. studied 200 patients undergoing coronary artery bypass surgery and randomized them into intervention group (n=100) and control group (n=100). Intraoperative cerebral desaturation was treated as per standardized protocol. The outcome measured was major organ morbidity and mortality that include death, ventilation more than 48hours, stroke, myocardial infarction, and return for re-exploration. They found that major organ morbidity and mortality was significantly less in the intervention group (n=3) than the control group (n=11) (P=0.048). Stroke occurred in one patient in the intervention group and 4 patients in the control group; however, the study was not powered to detect a difference in stroke rate.7 Zheng et al. conducted a systematic review to determine if NIRS-measured cerebral desaturation associated with stroke, postoperative cognitive dysfunction, or delirium in adult cardiac patients. They concluded that evidence linking cerebral desaturation to postoperative neurologic complications was low.20
In elderly patients (>65 years old) undergoing major abdominal surgery, Casati et al. prospectively studied 122 patients and randomized them into an intervention group (n=56) or a control group (n=66). The goal in the intervention group was to maintain SctO2≥75% of preinduction values. It was found that the mean value of SctO2 in the intervention group was significantly higher than the control group (66% vs. 61%, P=0.002), and the area under the curve below 75% of baseline in the intervention group was significantly less than the control group (0.4min% vs. 80min%, P=0.017), suggesting that cerebral oximetry helps to reduce the potential exposure of the brain to hypoxia. Further, they attributed this potential benefit to the observed higher Mini Mental State Examination score assessed at the 7th postoperative day (P=0.02), shorter post-anesthesia care unit stay time (P=0.01) and less hospitalization days (P=0.007) in the intervention group than the control group when considering only patients developing intraoperative cerebral desaturation.21 In another prospective cohort study with a nested randomized controlled intervention trial, Ballard et al. studied the effect of BiSpectral index and SctO2 monitoring on postoperative cognitive decline in older adults (>60 years old) undergoing orthopedic or abdominal surgery. Even though only 34 patients in the intervention group and 39 patients in the control group were included in the nested randomized controlled trial, the preliminary results suggest that cognitive impairment can be significantly reduced at one, 12 and 52 weeks postoperatively when a pragmatic intervention protocol focusing on BIS and SctO2 is instituted.8 However, this study does not differentiate the effect of BIS- and SctO2-guided management on postoperative cognitive decline. Cerebral oximetry has also been investigated in patients undergoing carotid endarterectomy to see if it can reliably predict cerebral ischemia during carotid cross-clamping. To date, it is still inconclusive due to the inconsistency in results.22–27
SummaryNIRS-based cerebral oximetry is different to pulse oximetry. The changes in SctO2 are essentially determined by the balance between oxygen delivery to the brain and cerebral metabolic rate of oxygen when the same brain region is continuously monitored in the same patient. Therefore, it reflects integrated information about cerebral oxygen supply and consumption. The match between metabolic substrate supply and consumption is intuitively essential for tissue well-being. In contrast, pulse oximetry only reflects information about the supply because it monitors only arterial blood. In this regard, cerebral oximetry is a promising technology because it monitors the essential and important physiology. It is important for clinicians to clearly understand how various physiological processes affect cerebral NIRS measurement. Intervention protocols have been proposed to optimize SctO2 or correct cerebral desaturation based on principles of CBF and arterial blood oxygen content augmentation and anesthetic depth increment. The success rate of various protocols is poorly reported. A very limited number of randomized controlled trials have been conducted to test if cerebral oximetry-guided intraoperative intervention improves neurologic or composite outcome. Even though the preliminary results seem promising, well-executed large scale randomized controlled trials are needed to definitively assess the beneficial effect of cerebral oximetry on short- and long-term outcome. The answers to the three questions we asked are still a work in progress.
FundingNone.
Conflicts of interestNone.
Please cite this article as: Meng L, Gelb AW. Oximetría cerebral: tres preguntas esenciales. Rev Colomb Anestesiol. 2015;43:52–56.