Good governance of resources and global warming has attracted interest on minimal flow (0.3–0.5l/min) inhalation anesthesia.
ObjectivesTo evaluate the predictive and clinical performance of a TCI (Target-controlled infusion) device, and its pharmacokinetic correlation for sevoflurane.
MethodsProspective, longitudinal, and analytical study on 25 adult patients. Fresh gas flow used 0.5l/min. Target concentration of 1.2% (v/v). Continuous and variable infusion into the circuit of the anesthesia workstation. Controller developed with LABVIEW 6.1. Hemodynamic, respiratory and anesthetic depth data collected every 5s using the anesthesia workstation software. Bias (Median of Predictive Error, MDPE%), inaccuracy (Absolute Mean of Predictive Error, MDAPE%), wobble, and divergence of the TCI device were determined in the first hour. STATA-12 pk collapse was used to analyze the area under the curve of the target and expired concentrations. The results are expressed as mean (95% CI) and median [IQR]*.
ResultsTarget concentration used 1.22 [1.14–1.37]%*, reached in 04:07 [03:15–06:15]* min:s (expiratory branch). Anesthetic duration 1:10:50 (00:56:57–1:24: 43) h:min:s. Sevoflurane consumption 6.9 (5.7–8.0) ml. MDPE% −12.8 (−17.6 to −8.1) %; MDAPE% 15.9 (11.9–19.8) %; wobble 6.9 (5.0–8.7)% and divergence 0.89% (−5.96 to 7.7)%h-1. Interactions per hour on the TCI of 3 (2–4). Correlation of the area under the curve, Spearman's rho=0.8577, p<0.00001. ≥15% inaccuracy was associated with age >65 years and obesity.
ConclusionsThe TCI sevoflurane© showed good performance, and the target concentration was rapidly reached and remained stable, with few interactions with the device needed during the first hour. There were neither overdosing nor clinically significant alterations.
La racionalización de recursos y el calentamiento global han despertado el interés en la anestesia inhalatoria con flujos mínimos (0,3-0,5 l/min).
ObjetivosConocer el desempeño predictivo, clínico y la correlación farmacocinética de un dispositivo target-controlled infusion (TCI) para sevofluorano.
MetodologíaEstudio prospectivo, longitudinal y analítico en 25 pacientes adultos. Flujo de gas fresco empleado 0,5 l/min. Concentración objetivo de sevofluorano 1,2% v/v. Infusión continua y variable en el circuito de la estación de anestesia. Controlador desarrollado con LABVIEW 6.1. Datos hemodinámicos, respiratorios y profundidad anestésica capturados cada 5 s con software de la estación de anestesia. Se determinó en la primera hora: sesgo (MDPE%), inexactitud (MDAPE%), oscilación y divergencia del TCI; análisis del área bajo la curva de las concentraciones objetivo y espirada con pkcollapse STATA-12. Resultados expresados en media (IC 95%) y mediana [IQR]*
ResultadosConcentración objetivo empleada 1,22 [1,14-1,37]%*, alcanzada en 04:07 [03:15-06:15]* min:s (en rama espiratoria). Duración anestésica 1:10:50 (0:56:57-1:24:43) h:min:s. Consumo de sevofluorano 6,9 (5,7- 8,0) ml. MDPE% -12,8 (−17,6 a −8,1)%; MDAPE% 15,9 (11,9-19,8) %; oscilación 6,9 (5,0-8,7) % y divergencia 0,89 (−5,96-7,7)%h-1. Interacciones por hora sobre el TCI de 3 (2-4). Correlación del área bajo la curva, Spermann rho = 0,8577; p < 0,00001.La inexactitud ≥ 15% se asoció con edad > 65 años y obesidad.
ConclusionesEl TCI sevoflurane© mostró buen desempeño, la concentración objetivo se alcanzó rápidamente y se mantuvo estable, siendo necesarias pocas interacciones sobre el dispositivo durante la primera hora. No hubo sobredosificación ni alteraciones clínicas significativas.
Low Flow Anesthesia (LFA), 0,5–1.0l/min, has shown lower consumption of anesthesia gas and reduced health costs,1 it has minimized labor exposure and environmental pollution,2 in addition it has enabled the warming and humidifying of inhaled gases;3 characteristics that have been optimized by the use of Minimal-Flow Anesthesia (MFA), 0.3–0.5l/min.
Despite the association of minimal-flow sevoflurane anesthesia with the accumulation of toxic substances and nephrotoxicity, this fact has not yet been demonstrated in humans4 during extended surgical procedures,5 or using soda lime.6 The use of nitrous oxide (N2O) is not recommended in order to avoid the administration of hypoxic mixtures; such as forgoing the use of MFA in patients with large hematomas, severe hemolysis and massive transfusion to prevent the endogenous accumulation of carbon monoxide from degradation of the heme group.7
Using a FGF of 2l/min (O2+Air) FGF and a minimum alveolar concentration for 1h (CAM-h) Ryan and Nielsen8 calculated the “20-year carbon dioxide emissions equivalent” (CDE20) for sevoflurane, and found that it was similar to the emission of a car in a distance of 28km (6980g of CO2/h); when associated to 60% N2O the effect was increased 5.9 fold.
Different applications have been used to implement LFA and MFA with vaporizer: the Gasman®9 simulator, the real-time halogenated agent concentration predictive software by Kennedy et al.10 and a software that alerts when the FGF exceeded 1l or 2 MAC-h for sevoflurane.11 These two latter software programs were able to lower the FGF down to 35%12 and 24% respectively.
The first workstation with closed circuit control and halogenated compound injection was PHISIOFLEX®,13 was removed from the market because of its high selling price. Later on, the DRAGER-ZEUS® station achieved constant concentrations of inspired oxygen and expired anesthetic agent, resulting in decreased halogenated compound consumption and greater hemodynamic stability.14 A 27% drop in consumption and a reduction of 44% greenhouse gas emissions were recently reported using the AISYS-GE™ workstation ET CONTROL® module.15
Initially, the syringe injection of liquid sevoflurane was administered to determine the closed circuit uptake model.16,17 The AnaConDa™ device is currently available for injecting the halogenated compound in a non-reinhalation circuit, with anesthetic savings similar to a LFA technique.18
In the mid-nineties, Candia and Acosta19 developed the first TCI model for halogenated compounds, based on the equation suggested by Lowe and Ernest.20 This IT application enabled the continuous injection of the halogenated agent, avoiding the administration of boluses and the use of the stopwatch. In a second version, other uptake models were simulated and the infusion was adjusted to the body surface. Later on, Candia and Roca developed the third TCI sevoflurane© version for the current workstations, using MFA.21
The primary objectives of the study were to assess the performance of TCI sevoflurane© in the general population of our hospital, using a TCI accepted methodology and to estimate the pharmacokinetic correlation of the model, establishing the area under the curve of the target and expired concentrations. The secondary objectives were: to learn about the behavior of the hemodynamic and respiratory variables, the depth of anesthesia during the first hour and to analyze the relationship between the controller's performance and the patients’ characteristics.
MethodsProspective, longitudinal, analytical study at the Cartagena University Hospital Complex. The protocol was approved by the hospital's Ethics and Clinical Research Committee and the informed consents were obtained. 30 adult patients meeting the following criteria were recruited: 16–90 years old, ASA I–III and scheduled for elective surgery lasting over 30min. The exclusion criteria were: obstetric patients, heavy smokers and patients with a history of drug addiction. 20min before the procedure the patients were pre-medicated with midazolam 1–2mg or fentanyl 50–100μg. Denitrogenation for 3min with O2 at 8l/min. TCI propofol and remifentanil induction at effect site concentrations of 3–4.5μg/ml and 3–4.7ng/ml respectively and rocuronium 2DE95. Following intubation, the FDF was reduced to 0.5l/min (O2 0.35l/min+air 0.15l/min), propofol was suspended and TCI sevoflurane© was initiated with a target concentration of 1.2% (v/v); mean value obtained from the analysis of the trials completed to determine the necessary concentration for a BIS ≤50 in patients ≤40 years old (MAC-BIS50).22–24 Remifentanil was used for maintenance based on its synergistic action with sevoflurane.25
The TCI was developed with LABVIEW® 6.1 (National Instruments, Austin, USA) software that enabled continuous and variable infusion of liquid sevoflurane at the AESPIRE-VIEW® (GE-Healthcare, Madison, USA) station, using the Harvard Apparatus 22 (South Natick, USA) infusion syringe. Using the parameters of age, weight, size and gender, the following estimates were made: age-adjusted target concentration26; oxygen consumption and minute volume27 and effective pulmonary volume.28 The system's purge dose (circuit-patient) was administered in the first minute. A negative bi-exponential model derived from a closed circuit uptake study was used.17 If needed, TCI interactions were preformed, changing the target concentration with the guidance of the gas analyzer (Fig. 1).
TCI sevoflurane© Main Screen. In the upper part of the screen enter the patient's characteristics. In the left lower quadrant enter the sevoflurane target concentration used, corrected for age. In the middle of the graph; in blue, the infusion rate and in red the volume of liquid sevoflurane administered.
The system's volume included an adult-type circuit (M1019499; GE, Helsinki, Finland), the humidifier, the analyzer connection and the oro-tracheal tube. The gas analyzer leaks were added to the model. The soda-lime absorption and degradation of sevoflurane were excluded from the calculations because they were considered of little clinical importance.29 The soda lime used was partially used and humidified. The sevoflurane was placed in a syringe with an adapter (26042; Sedana Medical AB, Sundbyberg, Sweden) and by means of an extension (MFX1954-ALARIS®, Höchberg, Germany) coupled to a modified metallic t-connector in the inspiratory branch, as described by Parra.30 The syringe and the plastic extension maintained their physical stability.31
The principal variables were: target concentrations, inspired and expired sevoflurane concentrations, captured every 5s using the S/5™Collect4 software (GE Healthcare, Helsinki, Finland); the measurement precision was 0.15% (v/v), as per the manufacturer's brochure. Secondary variables: systolic blood pressure (SBP), heart rate (HR), end-tidal CO2 (ETCO2), inspired oxygen fraction (FiO2), state entropies (SE) and response entropies (RE).
TCI performance32 was based on the Predictive Error calculation (PE%).
The bias (MDPE%), inaccuracy (MDAPE%) and the controller oscillation were inferred every 5min. The MDPE% is the PE% median. It is the error sign indicates: overdose (+) or underdose (−). MDAPE% is the PE% mean absolute value. It is the magnitude of error. Wobble is the mean absolute value of the difference between each PE% and the subject's MDPE. It measures each patient's predictive instability or variability. Divergence is the slope of the lineal regression curve of PE% absolute values vs. time, expressed as a divergence percentage per hour; its positive value indicates the trend of the target concentration to move away from the measured or expired concentration in time and its negative value indicates convergence.
The normal distribution of the data was determined using Shapiro–Wilks statistics. The comparison of target, inspired and expired means was done using the signs range test. Chi square or Fischer's Exact Test and the binary logistics regression analysis were used to establish an association between the variables of patients grouped by: age ≥65 years old; gender, BMI ≥30, ASA classification and number of user's TCI interactions <3per hour; compared against the visually grouped parameters of TCI performance: MDPE% <−13%, MDAPE% <15%, wobble <7% and negative divergence.
The data analysis was done with SPSS 21 (IBM®, Armonk, USA) and EXCEL 2007 (Microsoft®, Redmond, USA). The pharmacokinetic analysis of the area under the curve for the target concentration and expired sevoflurane concentration was performed with the pharmacokinetic data generator (pkcollapse) STATA 12 (College Station, USA). The results were expressed as Mean (95% CI) for normal distribution values and Median with interquartile range [IQR 25–75%]* for the non-parametric; the acceptable level of significance was p<0.05. The estimator used was odds ratio (95% CI). The sample size with an alpha error=0.05 and a beta power=0.8 was 25 patients, estimated according to our pilot study,22 using the variance of MDAPE%=9.86.
Results25 of 30 patients completed the study; three were excluded for missing data and two for circuit leaks. 80% of the patients had a BMI ≥25. 48% of the surgical procedures were laparoscopic (Table 1).
Patient characteristics, n=25.
| Age (years) | 44.8 | (37.0–52.5) |
| Weight (kg) | 83.8 | (76.0–91.7) |
| Height (cm) | 176.0 | 163.0–171.0) |
| Body surface (m2) | 2.0 | (1.9–2.1) |
| BMI (kg/m2) | 30.0 | (27.3–32.9) |
| Sex | ||
| Male | 13 | (52%) |
| Female | 12 | (48%) |
| Obesity (BMI>30) | ||
| Yes | 12 | (48%) |
| No | 13 | (52%) |
| ASA classification | ||
| ASA I | 8 | (32.%) |
| ASA II | 12 | (48%) |
| ASA III | 5 | (20%) |
| Surgical specialty | ||
| General surgery | 19 | (76%) |
| Maxillofacial surgery | 4 | (16%) |
| ENT | 2 | (8%) |
| Intravenous anesthesia | ||
| Propofol (mg) | 203.4 | (182–225) |
| Remifentanil (μg) | 833.4 | (592–1075) |
| Rocuronium (mg) | 63.5 | [50–65]* |
Qualitative variables in absolute numbers (percentage).
Quantitative variables expressed as mean (CI 95%) and median [IQR]*.
The target, inspired and expired concentrations showed non-parametric distributions; the sevoflurane expired mean was 18.4% below the target concentration mean used. 84.5% of the values of Predictive Error (PE%) showed normal distribution, so that MDPE%, MDAPE%, wobble and divergence of TCI, was expressed with mean (CI 95%) (Table 2).
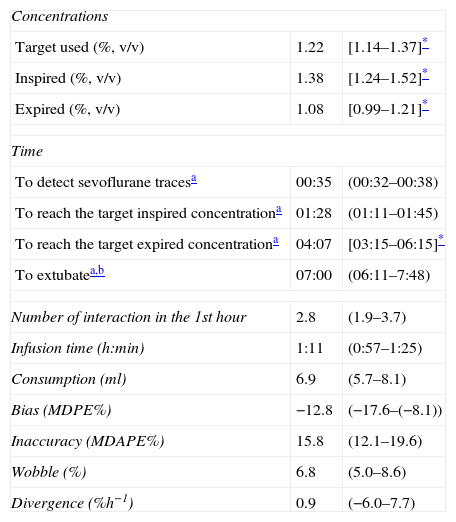
TCI sevoflurane© performance.
| Concentrations | ||
| Target used (%, v/v) | 1.22 | [1.14–1.37]* |
| Inspired (%, v/v) | 1.38 | [1.24–1.52]* |
| Expired (%, v/v) | 1.08 | [0.99–1.21]* |
| Time | ||
| To detect sevoflurane tracesa | 00:35 | (00:32–00:38) |
| To reach the target inspired concentrationa | 01:28 | (01:11–01:45) |
| To reach the target expired concentrationa | 04:07 | [03:15–06:15]* |
| To extubatea,b | 07:00 | (06:11–7:48) |
| Number of interaction in the 1st hour | 2.8 | (1.9–3.7) |
| Infusion time (h:min) | 1:11 | (0:57–1:25) |
| Consumption (ml) | 6.9 | (5.7–8.1) |
| Bias (MDPE%) | −12.8 | (−17.6–(−8.1)) |
| Inaccuracy (MDAPE%) | 15.8 | (12.1–19.6) |
| Wobble (%) | 6.8 | (5.0–8.6) |
| Divergence (%h−1) | 0.9 | (−6.0–7.7) |
In absolute values, MDPE% was consistent with an under-dosing of <0.16%(v/v) (Fig. 2B). The average MDAPE% was 16% until minute 40 (Fig. 2C). Oscillation in average was <10% (0.12%, v/v) between minutes 10 and 55 (Fig. 2D).
TCI sevoflurane performance in the first hour. (A) The predictive errors (PE) % of the 25 patients every 5s in the first hour. (B–D) The bias (MDPE%) MDAPE% and wobble from minute 3 through 60; n=14,761 observations. The solid line represents the mean (95% CI) and the dashed line represents the median.
TCI's clinical performance was represented with box-plots (Fig. 3). The SBP dropped by 13% in 50% of the patients at minute 0. HR, ETCO2, SE and RE showed no significant changes. FiO2 showed a rapid decrease following the nitrogen wash in the first 30min and was maintained between 70% and 60% from minutes 30 through 60. The concentration–time area under the curve correlation for target and expired concentrations in each patient was high (r2=0.766) (Fig. 4). The performance every 5s was represented in a PE% absolute value histogram (Fig. 5).
Hemodynamic, respiratory and anesthetic depth parameters. The upper part shows the systolic blood pressure (SBP) and the heart rate (HR) with basal pre-induction values and every 10min for the first TIC infusion hour. In the middle, the tele-expiratory CO2 (ETCO2) and the oxygen inspired fraction (FiO2); the dividing lines represent the median. The lower section shows the state entropy (SE) and response entropy (RE); the dividing lines represent the accepted anesthetic depth range. The data are expressed as median [IQR]*.
Sevoflurane TCI performance expressed in absolute value ranges for PE% (APE%). Ordered histogram of the APE% from minute 3 and every 5s. n=14.761 observations. Clinical performance was defined as “optimal”, “Good”, “acceptable”, “inadequate” and “poor” when APE% was ≤10%, 11–20%, 21–30%, 31–40%, or >40%, respectively.
Among the variables collected, an association was found between the number of user's interactions per hour over the TCI <3 and MDAPE <15%, with p<0.033 and an Odds Ratio (95% CI) of 7.77 (1.3–46.1). Age <65 years old was related to MDAPE <15% with a p<0.039 and an Odds Ratio of 0.381 (0.22–0.66). Obesity was related with MDAPE ≥15% with a significance of p<0.036 unilateral and an Odds Ratio (95% CI) of 4.5 (0.87–23.26). No association was found among the remaining parameters of the controller and grouped characteristics.
DiscussionThis study evaluated the predictive and clinical performance and the pharmacokinetic correlation of a sevoflurane TCI device, using MFA from the beginning of anesthesia. A target concentration of 1.2% (v/v) enabled a stable sevoflurane expired concentration. A 0.6 MAC maintained the stability of the hemodynamic, respiratory and depth of anesthesia parameters evaluated. The oxygen–air gas mixture used maintained the FiO2 at safe levels during the 1-h evaluation (Fig. 3).
Traditionally, MFA has used modified FGF and changes in the vaporizer dial.33 Its validation using sevoflurane showed an overdose of less than 20%34,35; on the contrary, TCI sevoflurane© showed less than 16% sub-dosing. TCI sevoflurane© detected halogenated compound traces and reached the target concentration in the expired branch in 35s and 04:07min respectively (Table 2). The AnaConDa™36 device achieved the same parameters in 3:50min and 13:09min. TCI sevoflurane© reached the target concentration in less time due to the administration of a bolus in the first minute; in contrast, AnaConDa™ requires a manual and constant infusion that delays the induction in the OR.
Time-to-extubation was 7min in average, which is consistent with the three time constants (τ=2.5min) needed to washout 95% of the sevoflurane in the brain and with the three half-lives of the proportional first order equilibrium constant between the alveolus and the effect site (T1/2 Keo=2.4min).37TCI sevoflurane© enabled normocapnia using a tidal volume between 7 and 8ml/kg; as opposed to the AnaConDa™ where the dead space requires an increased tidal volume of 10ml/kg for preventing hypercapnia and water vapor condensation.36 AnaConDa™ also absorbs the exhaled CO2 that is re-inhaled in the following breath, creating a dead space equivalent to 180ml additional to the 100ml of internal volume. In the opinion of the authors, this effect should be studied and changed prior to the adoption of this device in the practice of anesthesia.38
Initially, the performance of TCI devices was considered acceptable with MDPE% <±20% and MDAPE% 20–40%.39 Recently40 a good performance has been accepted as MDPE% <±15% and MDPE% <25%, a condition that is achieved with TCI sevoflurane© (Table 3).39–43
Intravenous TCI performance.
| Model | n | Bias MPDE % | Inaccuracy MDAPE % | Wobble % | Divergence % h−1 |
| Propofol | |||||
| 1. Marsh. Morbidly obese patients41 | 20 | −32.6 | 33.1 | 5.9 | −1.5 |
| 2. Marsh. Healthy adults39 | 46 | 16.3 | 24.1 | 21.9 | 7.6 |
| 3. Marsh. Orientals42 | 27 | 14.9 | 23.3 | 18.9 | −1.9 |
| 4. Schnider. Healthy adults40 | 9 | −15.6 | 23.6 | 18.8 | 1.4 |
| Remifentanil | |||||
| 5. Minto. Healthy adults43 | 30 | −15.0 | 20.0 | ||
The population age range in this study was wide (16–88); the association identified between MDAPE% ≥15% in patients over 65 years old may be explained by the presence of comorbidities as evidenced by the absence of ASA I patients in this group. In AnaConDa™ using the Enlund et al.44 model, the MDAPE% was higher because of several reasons: use of a re-breathing circuit, (0.5–8%), inclusion of pediatric patients and 30-s induction. In contrast to Belda et al.45 and Soro et al.46 that found lower MDAPE%, using non-rebreathing circuits, fixed concentrations per groups of patients, narrow age ranges and a 10-min induction. Soro et al.46 explained their differences to the Belda et al.45 study because the evaluation time was less than an hour and their measurements were affected by the early fatty tissue uptake. In terms of the association between MDAPE >15% and BMI >30, TCI sevoflurane© showed differences; the first evaluation21 did not include morbid obese patients, showing: positive MDPE%, low MDAPE% and wobble, with negative divergence. In the current evaluation, MDPE% was negative and the MDAPE% doubled, probably as a result of two factors: smaller calculated circuit volume and higher percentage of overweight and obesity in the population evaluated (Table 4).
Inhalation devices performance.
| Device | n | Time (min) | BMI | Bias MDPE% | Inaccuracy MDAPE% | Wobble % | Divergence % h−1 | |
| 1. Kennedy28 | 16 | 93 | Not available | 3.6 | 10.9 | 3.8 | 0.9 | |
| AnaConDa™ | ||||||||
| 2. Enlund44 | 38 | 248 | 26.0 | [23.0–28.0]* | 11.0 | 27.0 | ||
| 3. Belda45 | 15 | 360 | 23.8 | (23.7–27.8) | −5.0 | 5.0 | 6.8 | 0.9 |
| 4. Soro46 | 10 | 58 | 25.9 | [23.8–27.0]* | −11.0 | 13.5 | 6.6 | |
| TCI Sevoflurane© | ||||||||
| 5. Piloto21 | 10 | 60 | 27.1 | (23.9–30.3) | 2.0 | 8.3 | 6.9 | −15.7 |
| 6. Actual | 25 | 60 | 30.0 | (27.3–32.9) | −12.6 | 15.8 | 6.8 | 0.9 |
3 and 4: sevoflurane 1% target concentration.
TCI sevoflurane© showed an average sevoflurane consumption of 6.9ml in 71min. The closed loop ET Control®47 device showed a higher consumption of sevoflurane of 11ml/h. Our reduced consumption may be explained by the use of MFA from the start of the infusion and by the low concentration used. The ET Control® introduces automatic variations both in the FGF and in the electronic vaporizer dial.
TCI sevoflurane© environmental impact may be theoretically lower, because it uses half the MAC and one fourth of the FGF used in the Rian & Nielsen trial.8
The devices suggesting changes in the vaporizer and the FGF are able to temporarily reduce consumption; however, upon removal, FGF rises again and hence requires consistent supervision to maintain the reduction achieved.11,12,48 As TCI sevoflurane© is constantly operating with MFA from the start, savings may be maintained upon its implementation.
Because TCI sevoflurane© is an open loop controller, it was unable to correct the biological variability on its own. The calculations neglected the absorption and degradation of sevoflurane due to the soda lime,29 and the metabolism of sevoflurane (3–5%) was not corrected either. Consequently, the model may be optimized though it will never be perfect for every patient, as highlighted by Hendricks.49 A gas analyzer will be then required to make all the adjustments. It is therefore recommended to do control group validations with vaporizer, include validated longer and safe procedures MFA5,6 so that in the future TCI syringes may be available that include the sevoflurane option to simplify the MFA technique. Another potential development with this device is using the every 5-s gas analyzer measurements to automatically correct predictive errors with a fuzzy logic controller or PID (proportional-integral-derivative) controller. This would compensate the drift the area under the curve and the error trend, additionally using anesthetic depth measurements to adjust the halogenated agent expired concentration at the desired level of hypnosis, as has been published for isofluorane50 and more recently for propofol51 in our environment.
ConclusionsTCI sevoflurane© showed a good performance, the target concentration was rapidly achieved and was maintained stable with few interactions needed with the device in the first hour. There were no overdoses or clinically significant disruptions, maintaining an adequate depth of anesthesia. A TCI for sevoflurane was available at a conventional workstation; the vaporizer was not used in this study, while the MFA was used from the start of the anesthesia, guided by the halogenated agent concentrations. Performance was similar to other devices used for the administration of inhaled anesthesia such as AnaConDa and intravenous TCIs. Keeping in mind the low consumption of sevoflurane observed in our study, we may then conclude that there was a reduction in labor exposure, environmental pollution and monetary costs.
FundingThe authors’ own resources.
Conflicts of interestThe authors have no conflicts of interest to declare. The TCI sevoflurane© algorithm was written by César A. Candia Arana and programmed by Joaquín Roca González.
Acknowledgement to José Antonio Álvarez Gómez, Head of Service, for their peerless contribution on this project for over 10 years and Ana Isabel Pérez Torres, PhD, for critical reading of the manuscript.
Please cite this article as: Candia Arana CA, Castillo Monzón CG, Álvarez Gómez JA, González JR, Eslava Schmalbach JH. Desempeño predictivo y clínico de un dispositivo TCI para sevofluorano en una estación de trabajo convencional: Correlación farmacocinética del modelo empleado. Rev Colomb Anestesiol. 2014;42:255–264.















![Hemodynamic, respiratory and anesthetic depth parameters. The upper part shows the systolic blood pressure (SBP) and the heart rate (HR) with basal pre-induction values and every 10min for the first TIC infusion hour. In the middle, the tele-expiratory CO2 (ETCO2) and the oxygen inspired fraction (FiO2); the dividing lines represent the median. The lower section shows the state entropy (SE) and response entropy (RE); the dividing lines represent the accepted anesthetic depth range. The data are expressed as median [IQR]*. Hemodynamic, respiratory and anesthetic depth parameters. The upper part shows the systolic blood pressure (SBP) and the heart rate (HR) with basal pre-induction values and every 10min for the first TIC infusion hour. In the middle, the tele-expiratory CO2 (ETCO2) and the oxygen inspired fraction (FiO2); the dividing lines represent the median. The lower section shows the state entropy (SE) and response entropy (RE); the dividing lines represent the accepted anesthetic depth range. The data are expressed as median [IQR]*.](https://static.elsevier.es/multimedia/22562087/0000004200000004/v1_201409200219/S2256208714000522/v1_201409200219/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

