Lung ultrasound is a monitoring tool that expands globally in different scenarios, it provides a range of ultrasound parameters that represent lung tissue without pathology, and artifacts that will be generated by the presence of pathology will be a great support during the diagnostic exercise for the physician, who should have the opportunity to do an assessment bedside the patient, dynamically, without risk to himself or to the patient. The semiology described for some of the diseases related to the physician involved in perioperative or critical patient management, has been taken from groups of experts who have validated some of these results with standard techniques such as chest radiography or computerized tomography.
El ultrasonido pulmonar es una herramienta de monitoreo que se expande a nivel mundial en diferentes escenarios, ofrece una serie de parámetros ecográficos que representan el tejido pulmonar sin patología, y los artefactos que se van a generar por la presencia de patología, serán un gran apoyo durante el ejercicio diagnostico para el medico tratante, quien debe tener la oportunidad de hacer una evaluación junto al paciente, de forma dinámica, sin riesgos para el o su paciente. La semiología descrita para algunas de las patologías que le competen al medico involucrado en el manejo del paciente critico o durante el perioperatorio, ha sido tomado de grupos de expertos que han validado algunos de estos resultados con técnicas estándar como la radiografía de tórax o la tomografía axial computarizada.
The diagnosis of the various pathologies has evolved in the course of the last century. According to the literature, the anamnesis, the patient's history, the systems review, and a detailed physical examination continue to be the cornerstone for an accurate diagnosis. However, one of the big changes in medical practice has been the use of various paraclinical laboratory or imaging methods, all of which have experienced an accelerated evolution in the last few decades with considerable impact on the morbidity and mortality outcomes, in addition to cost-effectiveness (Fig. 1).1
Ultrasonography has greatly impacted different healthcare areas; however, emphasis should be placed on its value for the emergency, trauma, and more recently perioperative environments. Several authors have shown through their publications how the tool is not only diagnostic, but also it provides for continuous monitoring and has become a must to assist in the management of the critically ill or highly complex patients.
During the last 25 years, lung images have been of great help for the diagnosis, management, and follow up of pulmonary diseases of the critically ill patient, including all the range from conventional X-rays to highly sophisticated technologies such as positron emission tomography or electric impedance tomography. The first paper on the medical use of ultrasound was published during the forties.2 Joyner was the first to describe the usefulness of ultrasound for diagnosing a pleural effusion and since then there have been a growing number of publications on the value of ultrasound for various pulmonary pathologies.3,4
MethodsA literature review on the use ultrasonography in the lungs was conducted from January 1997 until May 2014, based on the following databases: Ovid, Pubmed, ScienceDirect, Springer and the words used to do the search included: “lung”, “ultrasound”, “pleural disease”, “sonography”, and “chest”. The initial search was limited to articles about adults, humans, meta-analyses, reviews, and random articles.
The principal search identified 305 articles, but only the documents describing the ultrasonograpic pulmonary approach, with a sound semiology representation in critical patients were included; this resulted in 35 articles.
Basic ultrasonography foundationsSound is produced by mechanical waves transmitted through longitudinal movements across elastic tissues. Ultrasound comprises waves with frequencies above 20000Hz.5
Ultrasound is an inaudible sound energy used for diagnostic purposes, at a frequency range of 2 and 20MHz. When the transducer contacts the skin, it generates ultrasound pulses; the transducer has piezoelectric crystals on the distal end that change their configuration when exposed to electric power. Electric stimulation makes the crystals oscillate and increases the frequency, converting electrical energy into ultrasound.
These waves travel through the body interacting with the underlying tissues and can be reflected, absorbed, or attenuated, depending on their acoustic impedance. Finally, these waves are processed and turn into a scale of gray images displayed on the screen of the device.6
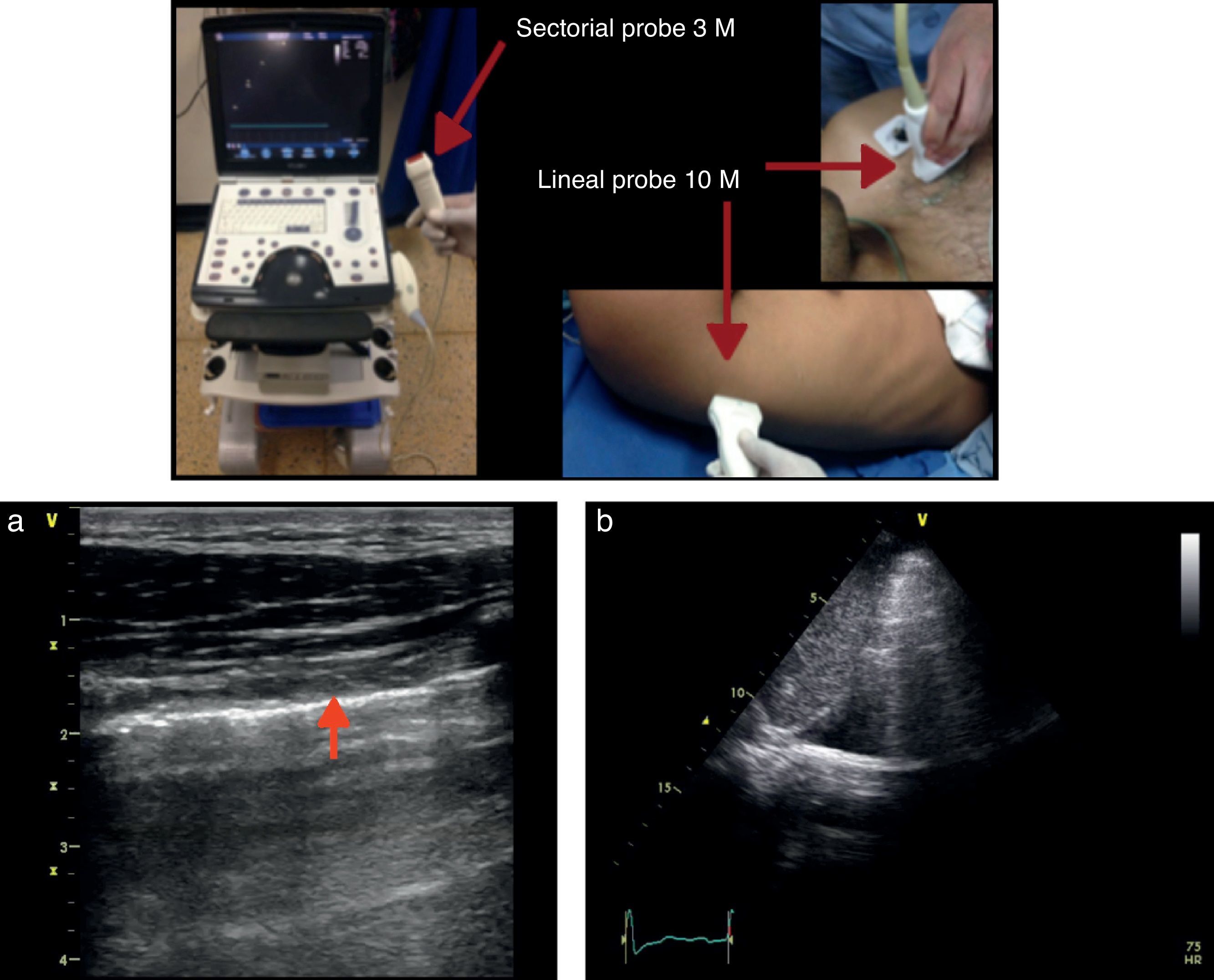
There are different types of transducers that vary across the scale of frequency waves as needed. The curved transducer produces conic shape images ranging from 2 to 5MHz and is usually used for deep tissues (abdomen, pelvis, and in obstetrics). It is characterized by its low resolution and high penetration. The lineal transducer for soft tissues produces rectangular images, ranging from 7 to 15MHz, typically generating high resolution but low penetration images, used for superficial tissues and mostly for peripheral nerve blocks.
Advantages of ultrasoundTransferring critically ill patients to the operating room or to the imaging suite may be quite challenging. Braman et al. describe these transfer-associated complications,7,8 and avoiding these transfers is a clear advantage of this technique, since the examination can be performed by the patient's bedside, and may be repeated as many times as needed during the day, with no added risks for patients or operators. It is worth noting that for some groups, ultrasound has become a routine monitoring tool for the evaluation of critically ill patients. The poor quality images from portable chest X-rays taken in the OR, the post anesthesia care unit, and even in the ICU, are mainly due to technical failures associated with inadequate penetration, rotated projections, inadequate inspiration, etc. These failures represent a waste of time, a waste of resources, and repeated irradiation. Some authors have also underlined the poor value of routine chest X-rays to assist in the diagnostic and therapeutic approach in the ICU.9–11
Other advantages have been described by Peris et al.11 with the regards to the efficacy of pulmonary ultrasound as a diagnostic and monitoring tool in the ICU. Its routine application is associated with 26% (P<0.001) less chest X-rays and 47% (P<0.001) less chest CTs, hence reducing the radiation, the exposure to contrast media, and the transfer of patients to other units, in addition to estimated savings of around twenty seven thousand (27000) Euros during the 6 months of the trial.12
In summary, pulmonary ultrasound offers major advantages, including being a non-invasive procedure, the ability to do the exam at the patient's bedside, low cost, no risk of ionizing radiation, potentially reproducible, and it can be done in a short period of time.12,13
Equipment and probesAny transducer may be used to do a pulmonary ultrasound since at low frequencies the resolution is low but penetrates greater depths. Therefore, low frequency transducers are better for the evaluation of deep structures, for the parenchyma, for consolidations or effusions. In contrast, the excellent resolution of high frequency transducers makes them more suitable to visualize the pleura. Every case must be individualized and all parameters such as adequate depth and gain should be adjusted to optimize the quality of the image.Liechtenstein14 claims that a state-of-the-art machine is not always needed: his recommendation is: “A 5MHz transducer to be able to work between 1 and 17cm, preferably micro convex, a small size ultrasound machine, optimal quality image, fast turn-on time, no need for Doppler, harmonic, or complex filters and of course, reasonable price.”
It is important to consider a few limitations when applying this monitoring or diagnostic tool, since probably its validity depends on the experience and the level of clinical training. The publication by Gargani describes some of these limitations including the individual patient's chest wall characteristics, obesity, the presence of subcutaneous emphysema, and wound dressings that may alter the propagation of ultrasound waves.15 However, Gargani concludes that lung ultrasound is a tool that enables the clinician to guide the diagnosis of a hypoxemic patient, focusing on the clinical characteristics.
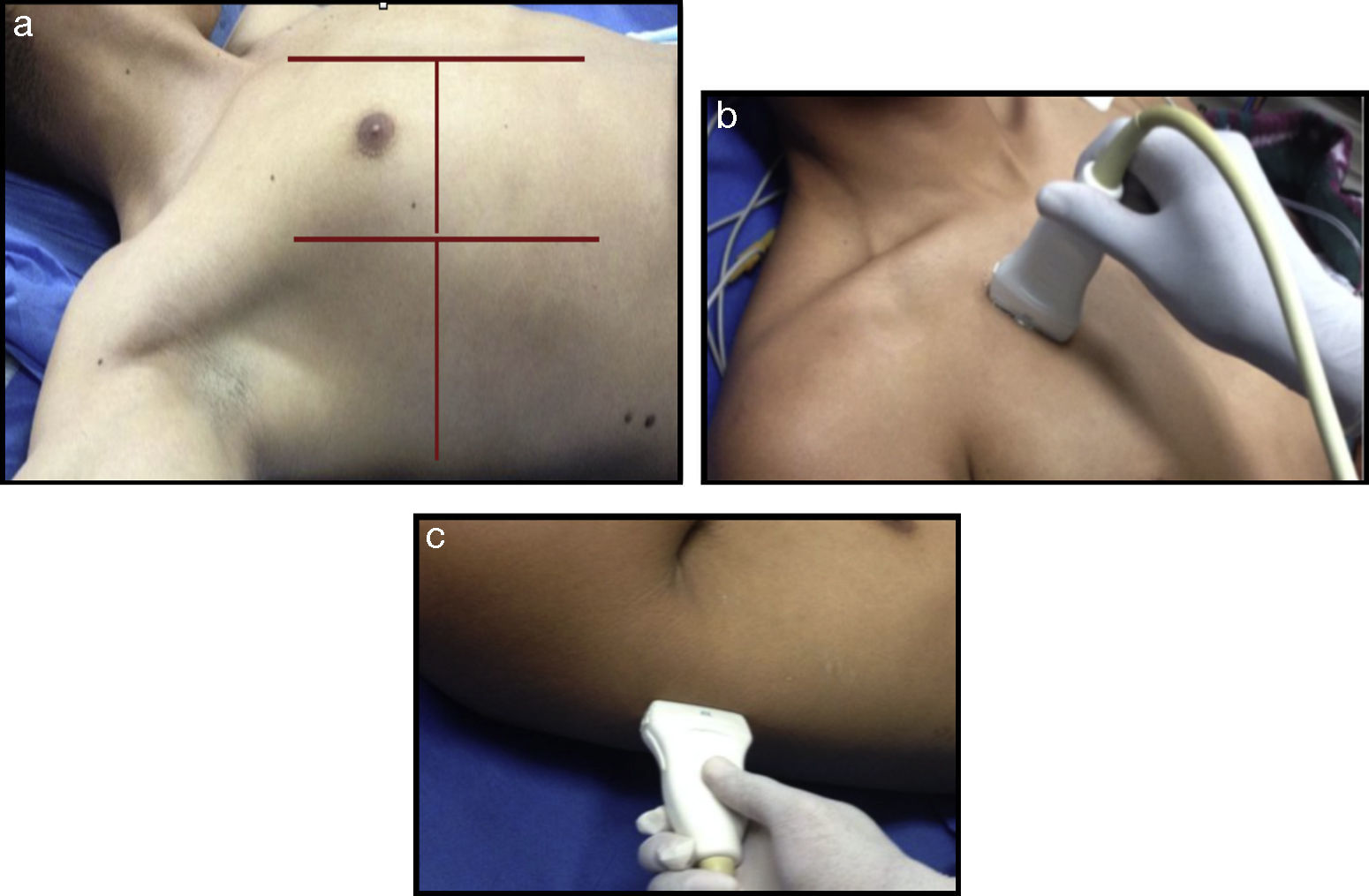
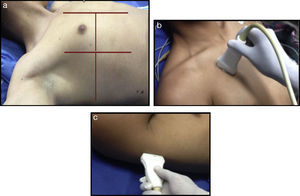
Evaluating the lung with the use of ultrasonographyWhen considering an ultrasonography evaluation of the lung, the recommendation is to try to identify the pleura, the pleural space, the diaphragm and the lung parenchyma.16 The patient should be in decubitus supine position and a comprehensive evaluation should take from 10 to 15min. Under unstable conditions however, when pleural involvement or intrathoraxic fluid is suspected, a 5-min ultrasound scan may be done initially and once the patient is stabilized, proceed with a more detailed evaluation. There are different options in terms of the areas for evaluating the lung using ultrasound. The current approach according to the recommendations of the International Liaison Committee on Lung Ultrasound for the International Consensus Conference on Lung Ultrasound in 2012 is to divide the hemithorax into four quadrants (Fig. 2).16,17
A comprehensive examination of the lung involves the assessment of each individual intercostal space, while a simplified approach consists of scanning and interpreting the each area (Fig. 3).
(a) Ultrasound examination of the lung by regions – International Liaison Committee on Lung Ultrasound (ILC-LUS) for the International Consensus Conference on Lung Ultrasound.17 (b, c) The transducer should be perpendicular to the ribs as illustrated. The projection obtained in b is usually recommended for the evaluation of pleural movement and c is ideal to identify the presence of pleural fluid.
When considering the basic principles of physics, the behavior of ultrasound beams at an interphase such as that of the healthy lung parenchyma, Harrison's book “Principles of Internal Medicine”18 2001, states that “ultrasound images are not useful to assess the lung parenchyma” and only produce artifacts.
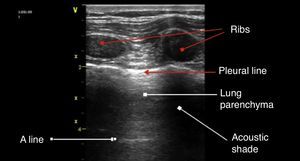
However, this becomes an advantage in terms of its potential application, based on the echographic signs of a healthy lung parenchyma, since these artifacts considered to be suggestive of a normal condition, are the hallmark of a disease-free status that can be identified in a practical and expeditious manner. The bi-dimensional (B-mode) ultrasound is used initially with the transducer perpendicular to the ribs so that the screen image depicts two costal ridges, the pleura, and lung tissue in the middle.19
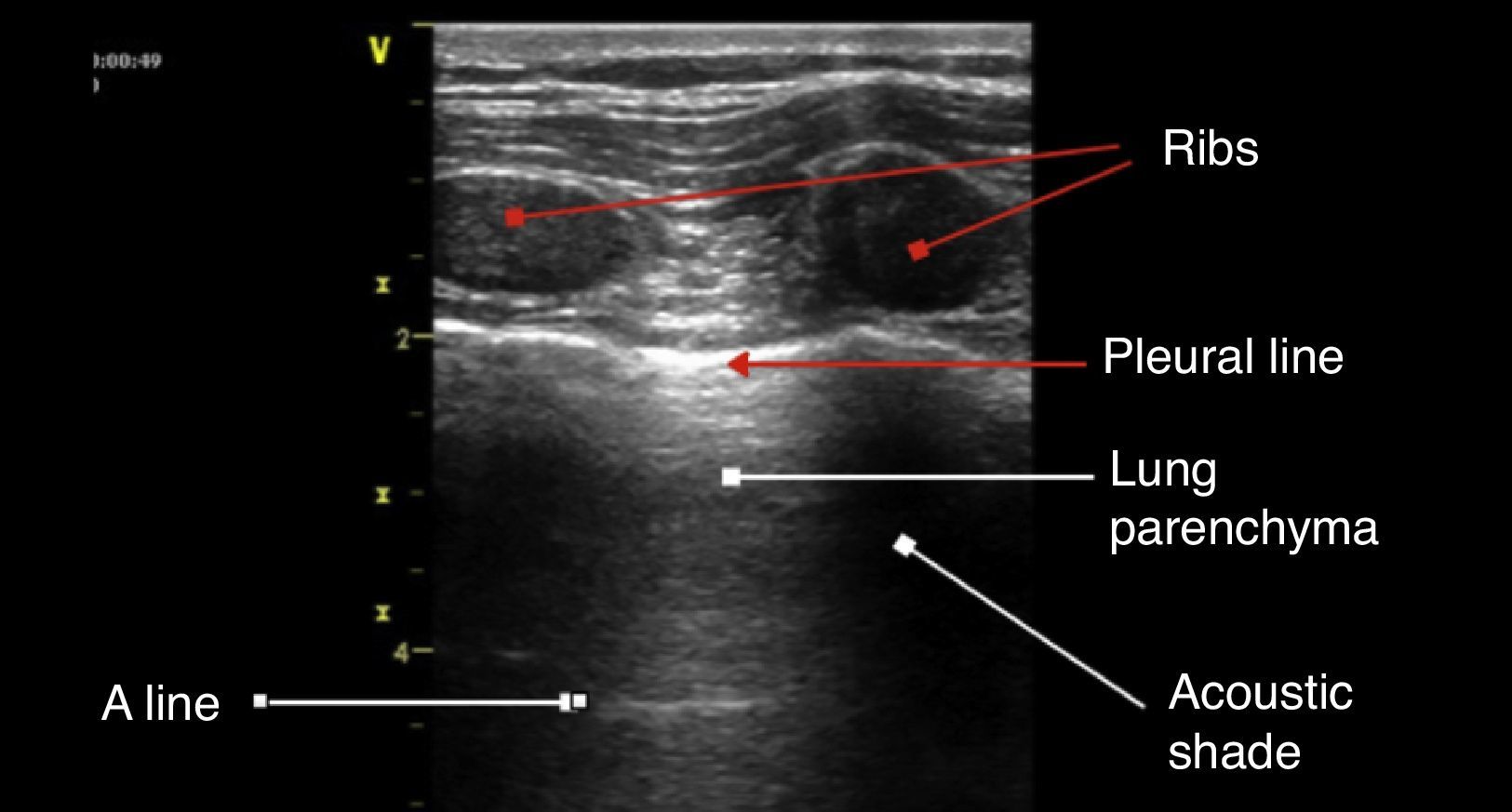
The anatomic description in Fig. 4 is based on the identification of bone structures that generate an acoustic shadow beneath them; it is between these two areas that the pleura can be seen. The pleural line is the first hyperechoic structure to be identified. Usually it is impossible to differentiate the two pleural layers using the 5MHz transducers. A typical sign of an ultrasound evaluation is the line of intersection of the parietal pleura with the visceral pleura, generated with each breathing cycle; this is called lung sliding and is associated with the breathing cycle movements.20,21 Murphy further specifies that the absence of this sign is characteristic of other pathological conditions such as pleural adhesions, selective bronchial intubation, consolidation, or pulmonary atelectasis.22
A linesThese are artifacts resulting from the gas interphase of the lung parenchyma, characterized by lineal horizontal hyperechoic, static images, that reappear at regular intervals.23 The separation is due to the reflection of the ultrasound waves from the skin to the pleura; as the ultrasound beams penetrate deeper, it takes them longer to return to the transducer (Fig. 4). A lines are not suggestive of any particular pathology.
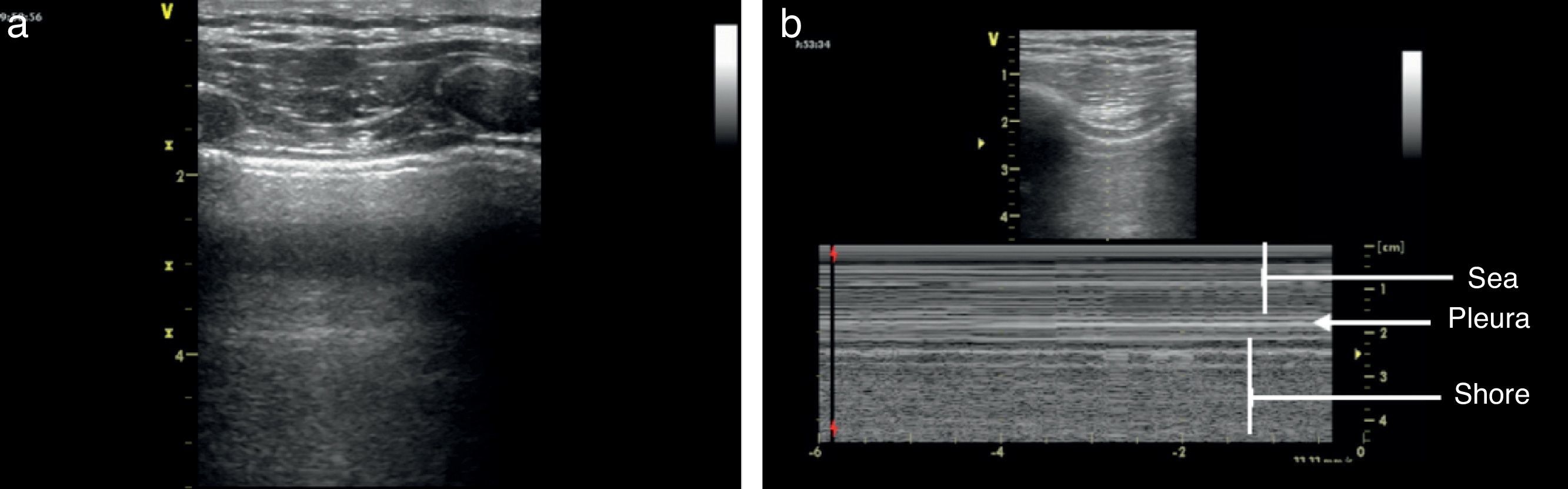
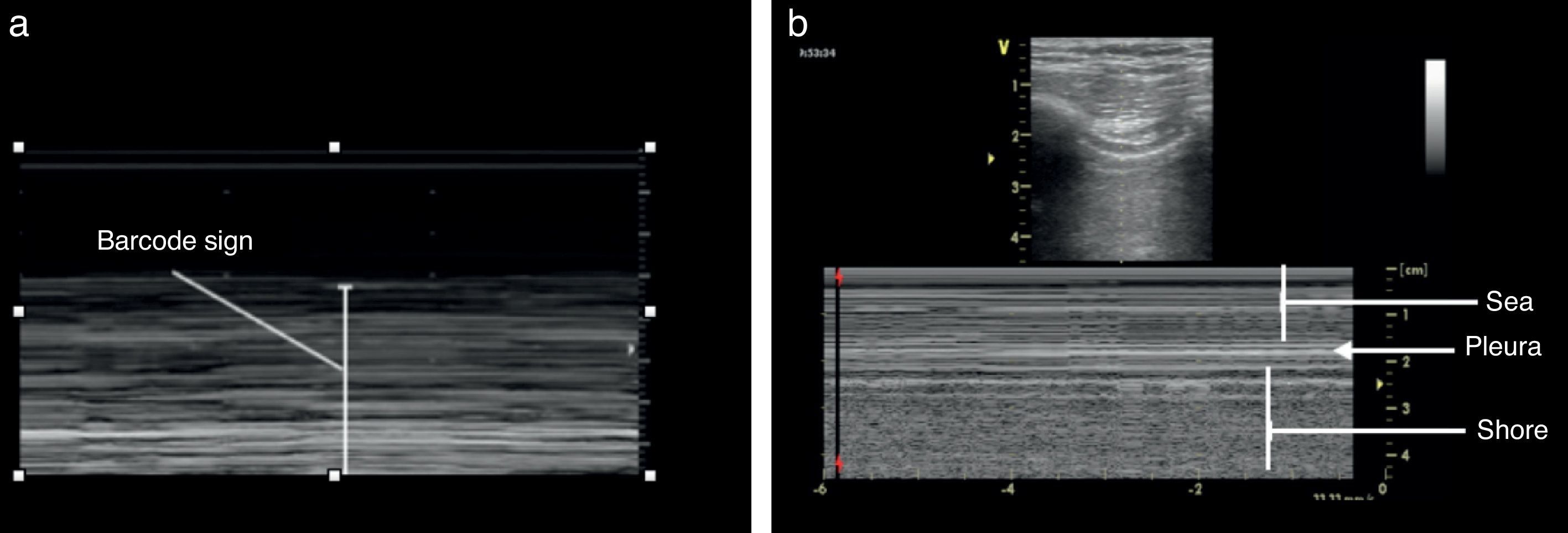
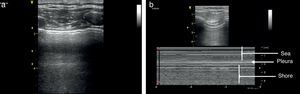
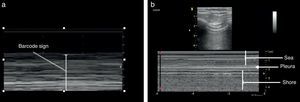
Once the image is identified using the transducer's B mode, the cursor should then be placed over the pleural line between the two ribs and then proceed to change over to mode M. It is important to keep in mind the relationship of the structures previously identified: the subcutaneous cell tissue, and the muscular tissue (corresponding to a lineal static pattern). Then the pleural line should be identified – a hyperechogenic line that separates the lung tissue – that is identified distally on the screen in relationship to the probe. In the absence of pathology, it is characterized by a granular homogeneous pattern corresponding to the air movement generated by each breathing cycle under the pleura; this sign is called the “sea-shore” sign. Always do a contralateral lung ultrasound to compare the result (Fig. 5).24
We may then conclude that a normal lung ultrasound pattern in mode B is comprised by pleural sliding and lines A, and in mode M, the characteristic is the sea-shore sign.
Pathological lung semiologyThe following semiology has been described and validated by several authors using CAT-scan as the gold standard.
The objective is to understand the various artifacts generated by ultrasound beams over the lung tissue that looses its normal aeration. Artifacts change when they come across a different air-fluid mix and this finding helps the clinician in making a diagnosis.4,25 So any air loss such as in the case of consolidation or atelectasis, results in a typical solid tissue image while increased fluids as compared to air such as in pulmonary edema, render a totally different image.26,27 Liechtenstein puts it in simple terms and describes the pleural effusion as pure fluid; the alveolar concentration has more fluid than air; the interstitial syndrome more air than fluid; and pneumothorax as pure air.14 These US traits become tools to identify and diagnose particular syndromes that may define an emergency or an evolving hypoxemia at the patient's bedside.20,28
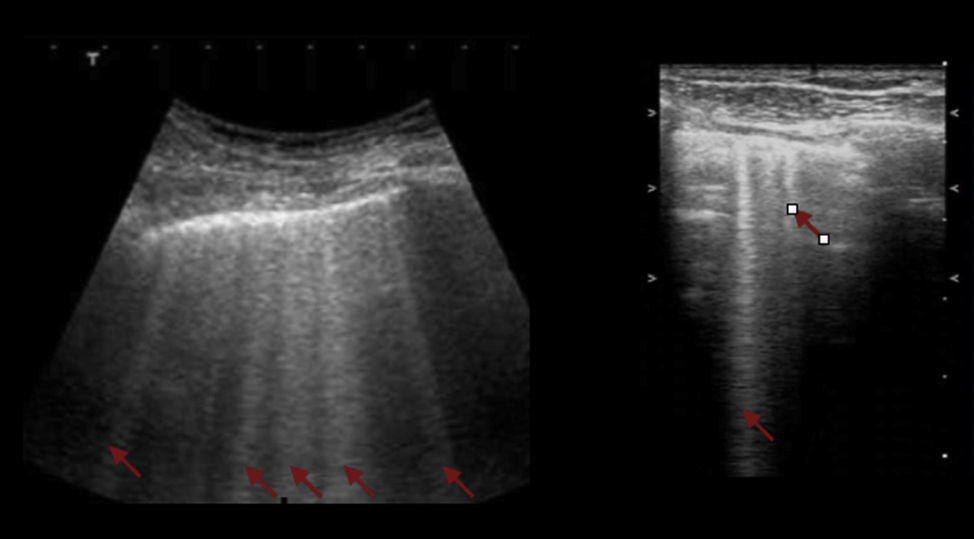
B linesAnother physical phenomenon to consider is the relationship of fluids and gases to gravity. Gases are on top, while fluids are below; however, when both mix together, the B lines or comet tail artifact develops. These were initially described in the 80s, but it was really Liechtenstein who described them based on ultrasonographic findings with CAT images.29,30
These B lines are images that should meet 7 characteristics: fluid-air artifacts in the shape of a comet tail; begin at the pleural line; hyperechoic; well defined; disseminated toward the end of the screen; erase the A lines; and move along with pleural slide when it is present.
The occurrence of more than 3 B lines is indicative of alveolar – interstitial syndrome.
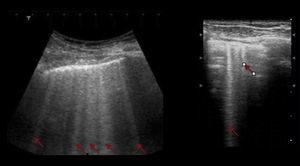
Interstitial syndromeThe interstitial syndrome involves a set of pathologies (pulmonary edema, infectious processes) than must always be analyzed within the clinical context characterized by the presence of certain US signs that assist in making a diagnosis and that are generated by the thickening of the interstitial space. The ultrasound evaluation typically describes the presence of B lines, a pattern usually associated with larger air volumes and less fluid (Fig. 6). These B lines or comet tail signs should be visible on the different projections except for the lower view in the intercostal space, immediately above the diaphragm. In this location, the B lines are not indicative of pathology.20,28,31
The AP views should be used since in the dorsal view the effect of gravity may be an explanation for the presence of those lines. More than 3 B lines are correlated with chest X-ray findings in over 93% of the cases and in 100% with TAC. It should be noted however that ultrasound does not differentiate alveolar fluid from pus, nor whether it is infiltrative or fibrotic tissue. Therefore, a clinical approach should be adopted to support the ultrasonographic findings.4,21
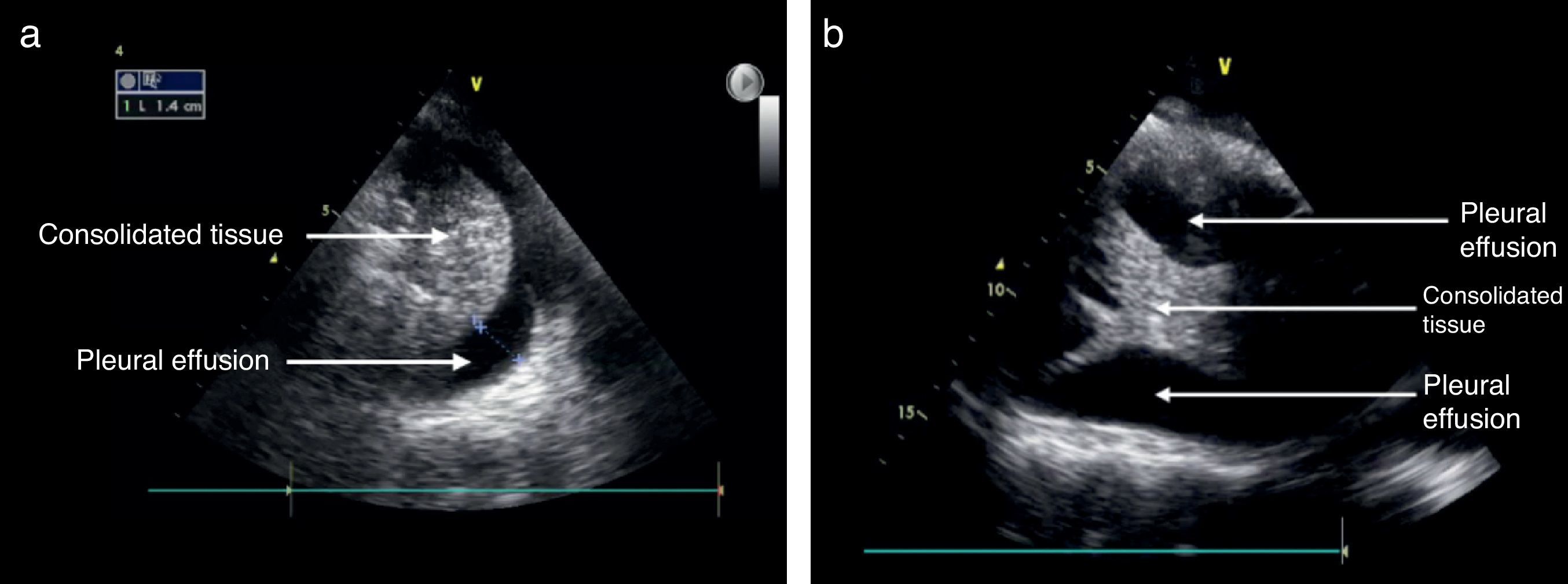
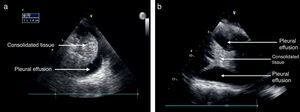
Pleural effusionThe recommended site for the probe is the posterior axillary line, scanning the different intercostal spaces to establish the extent of the effusion. The lung ultrasound may then estimate the effusion volume and mark or guide the puncture site for drainage or analysis. The position of the patient should also be standardized for measurement purposes. Usually the inclination is between 0° and 15°.21 As mentioned before, the pleural effusion is mostly a fluid phase with an anechoic appearance in the ultrasound image (Fig. 7). It is important to identify this fluid collection above the diaphragm and the recommendation is to evaluate other semiology signs to actually determine the presence of fluid inside the pleural space.20,28,31 The M mode should be used and the dimension of “sinusoidal” sign in the anechoic area changes with the breathing cycle.
The information from the US examination may also assist in identifying the type of fluid present inside the pleura. In large effusions you may find some areas of consolidation or even atelectasis due to lung tissue compression. In some cases membranes or irregular mobile segments may be seen, and these are referred to as “Plankton” sign, more often associated with hemothorax or empyema. Some images may show septa that are also associated to these conditions.
Hence, US provides a diagnostic tool to differentiate the type of fluid collection present in a pleural effusion.32
Alveolar consolidationThe alveolar consolidation refers to the fluid inside the alveolus. Nevertheless, the consolidation may be due to atelectasis, pneumonia, lung contusion, or a tumor lesion, inter alia.21
Some of the ultrasound findings include the similarity of the consolidated lung tissue to a solid organ tissue (Fig. 7); thus a judicious evaluation should be performed of the various lung segments.28,31
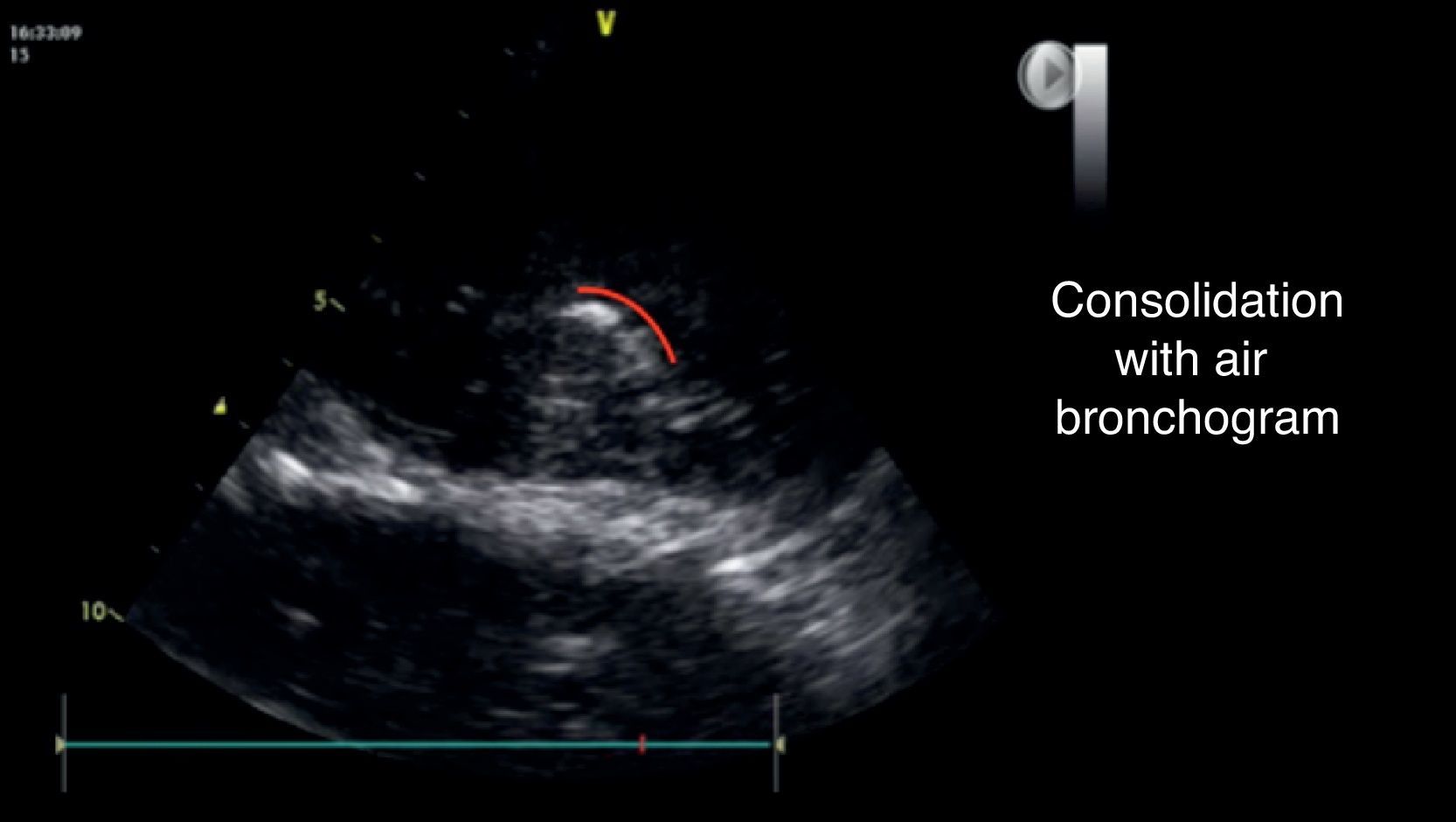
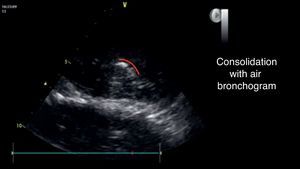
An additional finding that may help to confirm this diagnosis is the presence of the air bronchogram (Fig. 8), that is typically a hyperechogenic image that moves with the breathing cycle.33
PneumothoraxLung ultrasound has proven to be more effective in the diagnosis of pneumothorax, as opposed to plain X-rays34; this fact has been documented in different publications with a negative predictive value of 100% to rule out the presence of pneumothorax.35 The air between the two pleural layers avoids the pleural slide and B lines. In this case only air is present and no fluid, as mentioned before.
The identification of pneumothorax involves anterior views and the patient should be in supine decubitus position. The initial approach is the identification of the pleural slide. The absence of a pleural slide is highly suspicious for pneumothorax and it should be confirmed with B mode to detect a “bar code” sign (Fig. 9).20,31,36
Another finding described is the presence of the “lung dot” with a lung segment producing a normal ultrasound pattern with the bar code in mode M.
ConclusionDespite its limitations, this monitoring technique offers a number of advantages, both for the patient and the treating physician. Training in this technology is not too time-consuming but it does demand knowledge about the basic ultrasound principles and then some hands-on experience on healthy patients before moving on to evaluating some lung pathologies under supervision. Obtaining the images is easy using the technique, but it requires proper knowledge of the semiology components identified and described for a range of conditions. Various authors have validated these tools.
This introduction to the semiology based on the use of ultrasound for pulmonary evaluation helps to a clear understanding of the current protocols designed for the approach and management of the patient with hemodynamic instability, respiratory distress, or hypoxemia, used during the perioperative period, in the ICU or in the emergency environment.
Conflicts of interestThe authors have no conflicts of interest to declare.
FundingThe authors did not receive sponsorship to udertake this article.
Please cite this article as: García-Araque HF, Aristizábal-Linares JP, RuÍz-Ávila HA. Semiología pulmonar por ultrasonido – monitoreo dinámico disponible junto al paciente. Rev Colomb Anestesiol. 2015;43:290–298.