Sustained low efficiency dialysis (SLED) as primary renal replacement therapy (RRT) in acute kidney injury (AKI) is not widely used, despite substantial economical advantages. We evaluated costs and outcome in a 5 year retrospective study on our ICU.
MethodsFrom 2006 to 2010 we selected all patients with the ICD-10 codes N17 and N18 who were treated with SLED on our ICU. Patients with a stay <2 days, an extra-renal indication for dialysis or chronic dialysis were excluded. Variables: number of SLEDs, duration of ICU and hospital stay, ICU and hospital mortality, SAPS II, TISS 28, blood urea and creatinine, C-reactive protein, mechanical ventilation, diagnoses. Long-term outcome was evaluated by sending all discharged patients a questionnaire.
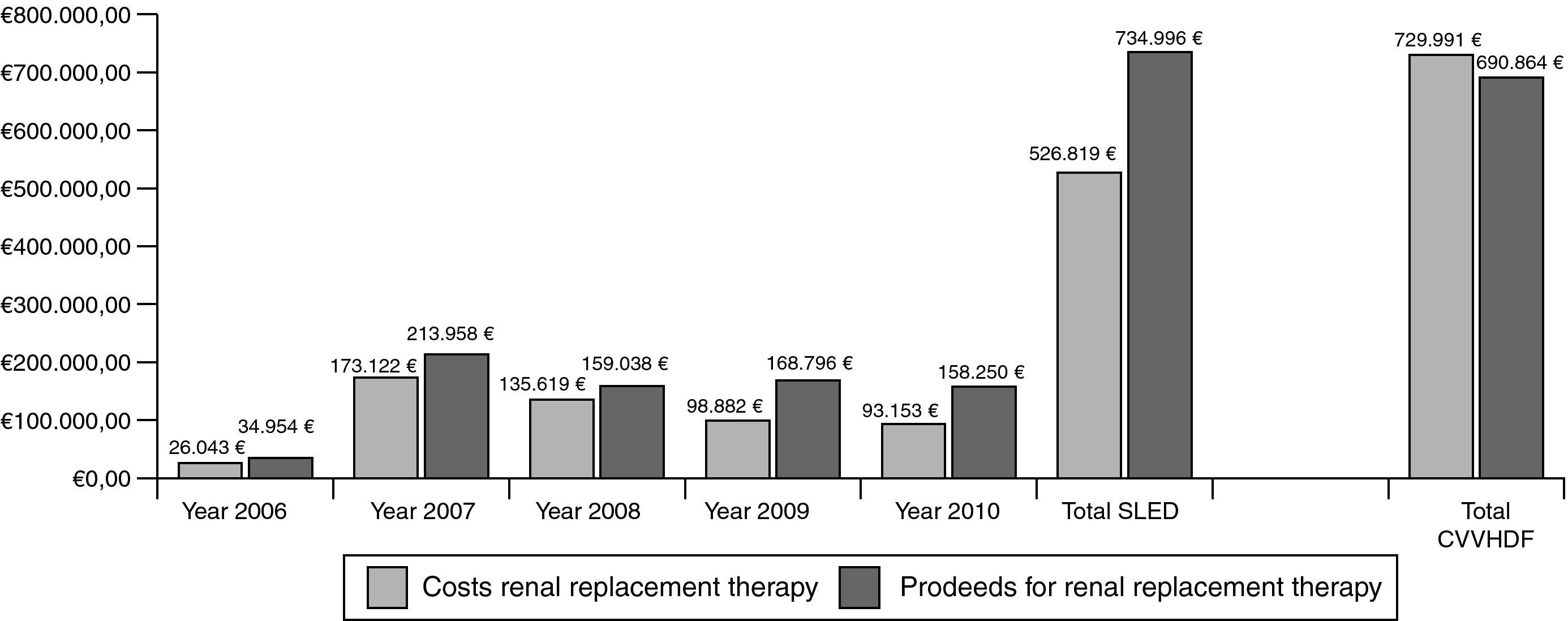
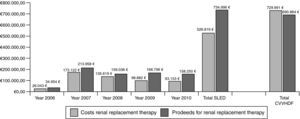
ResultsBetween 2006 and 2010, 3247 SLED-treatments in 421 patients (mean SAPS II: 41 points without GCS) were performed. ICU and hospital mortality in the patients treated only with SLED (n=392) was 34% and 45%, respectively. 71% of all surviving patients had good quality of life and 12% of all discharged patients still needed dialysis. Total costs for SLED were 526.819€ and total proceeds were 734.996€. Assuming also 3247 “CVVHDF-days” for cost comparisons we calculated costs of 722.750€ with proceeds of 690.864€ for CVVHDF.
ConclusionsIn critically ill patients with AKI SLED is an effective RRT, with short- and long-term outcome being comparable to outcome data with CVVHDF. Based on our cost–proceeds analysis SLED seems to be the preferable renal replacement therapy.
La diálisis sostenida de baja eficiencia (sustained low efficiency dialysis [SLED]) como terapia primaria de reemplazo renal en la lesión renal aguda no está muy extendida, a pesar de sustanciales ventajas económicas. Nosotros evaluamos los costos y los resultados en un estudio retrospectivo de 5 años en nuestra unidad de cuidados intensivos (UCI).
MétodosDesde 2006 hasta 2010 seleccionamos todos los pacientes con los códigos ICD-10 N17 y N18 que fueron tratados con SLED en nuestra UCI. Fueron excluidos los pacientes con una estancia de menos de 2 días, una indicación extrarrenal para diálisis o la diálisis crónica. Las variables fueron: el número de SLED, la duración en la UCI y la estancia hospitalaria, la mortalidad hospitalaria y en la UCI, SAPS II, TISS 28, la urea y la creatinina séricas, la proteína C reactiva, la ventilación mecánica y los diagnósticos. El resultado a largo plazo se evaluó mediante el envío de un cuestionario a todos los pacientes dados de alta.
ResultadosEntre 2006 y 2010 se llevaron a cabo 3.247 tratamientos de SLED en 421 pacientes (media de SAPS II: 41 puntos sin GCS). La mortalidad en la UCI y el hospital de los pacientes tratados solo con SLED (n = 392) fue del 34 y del 45%, respectivamente. El 71% de todos los pacientes que sobrevivieron tenían buena calidad de vida y el 12% de todos los pacientes dados de alta aún necesitaban diálisis. Los costos totales de SLED fueron de 526.819 D, y el producto total, de 734.996 D. Si se considera 3.247 «días de hemodiafiltración venovenosa continua [HDFVVC]» para las comparaciones de costos, se calcularon los costos en 722.750 D con el producto de 690.864 D para HDFVVC.
ConclusionesEn los pacientes críticamente enfermos con lesión renal aguda la SLED es una eficaz terapia de reemplazo renal con resultados a corto y largo plazo que son comparables a los datos de los resultados de HDFVVC. En base a nuestro análisis de costo-beneficios, SLED parece ser la terapia preferible de reemplazo renal.
With an incidence of more than 35% in the critical ill patient AKI is the most frequent organ complication of a basic illness.1,2 AKI is an independent risk factor for mortality within the hospital3,4 and may be a precursor of the multiple organ system failure MODS.5
Around 5–6% of all patients with an AKI in the ICU need a renal replacement therapy.6,7 Continuous renal replacement therapy (continuous veno-venous hemofiltration or hemodiafiltration CVVH(DF)) is still believed to provide better hemodynamic stability in case of a MODS and more physiological serum levels for urea, creatinine and potassium over the time. On the other hand intermittent renal replacement therapies (hemodialysis HD, sustained low efficiency dialysis SLED) need less intense anticoagulation and provide more opportunities for the patient's mobilization and other interventions.
Evidence-based guidelines concerning timing, modality and termination of renal replacement therapy are currently not available. Two randomized controlled studies comparing continuous (CVVHDF) with discontinuous (SLED, hemodialysis) techniques did not find any advantage or disadvantage of either technique even in septic ICU patients.8,9 In addition more intense treatment modalities did not translate in better patient outcome regardless whether a discontinuous (3 times vs. 6 times per week9) or a continuous (25ml/kg bw vs. 40ml/kg bw effluent10 and 20ml/kg bw vs. 35ml/kg bw effluent9) replacement therapy was used.
However, one striking advantage of all intermittent therapies is the fact that the costs per treatment are substantially lower compared to continuous therapies.11–13 We provide SLED as standard renal replacement therapy in our unit since fourth quarter of 2006. After 5 years of using SLED the present study evaluates three main questions:
- (1)
Are the outcomes (ICU and hospital mortality) of our patients treated with SLED comparable to the published data?
- (2)
How are the long-term survival, quality of life and adherence to dialysis?
- (3)
How is the cost–proceeds ratio over the past 5 years?
From 2006 to 2010 we selected all patients with the ICD-10 diagnoses N17 or N18 who were treated with SLED or CVVHDF on our ICU. We excluded all patients with a hospital stay <2 days or with an extra-renal indication for dialysis or with pre-existing chronic dialysis.
MethodsFollowing variables were extracted from the chart: number of SLED treatments, number of CVVHDF treatment days, duration of ICU and hospital stay, ICU and hospital mortality, SAPS II and TISS 28, blood urea and creatinine, C-reactive protein, mechanical ventilation, diagnoses. All data were entered in MS Excel 2007®.
As a part of our routine quality management we evaluated the long-term outcome including persistent dialysis dependency of all discharged patients. The survey was done by sending all discharged patients a questionnaire with the following questions (see Appendix 1):
- (1)
In which condition according to the Glasgow Outcome Scale has the patient been discharged?14
- (2)
If the patient had died meantime, after which time period after discharge did that happen? (The answer was given by the closest family members.)
- (3)
Has a follow-up treatment with dialysis dependency been necessary after discharging? (Various options for response in terms of duration according to RIFLE criteria ‘LOSS’ and ‘ESKD’.)
- (4)
In a free text field the patients could fill in some suggestions and comments.
In the envelope with the questionnaire a stamped envelope for return was also added. To those patients not answering to the first survey, the same questionnaire was sent again after six months. All returning questionnaires were also entered into MS Excel database.
Extracorporeal circuit, hemofiltration solutions and anticoagulantsIntermittent renal replacement therapy was performed as SLED with the Genius® system, a mobile single pass batch dialysis system. The system is described elsewhere.15 For those patients not at risk for bleeding unfractionated heparin was used, in all others anticoagulation was achieved by sodium citrate. A loading dose of 1000U was given to the tubing system at the beginning of dialysis treatment, followed by a continuous infusion between 400 and 800U/h. Several boluses were necessary when clotting occurred in the extracorporeal circuit. Sodium citrate was administered by a Ca2+ target level between 0.3 and 0.45mmol/l in the venous arm of the extracorporeal circuit. The mean treatment period for SLED was 10h (range: 6–20h).
Regardless of whether Genius90® or CVVHDF treatment was performed each package always contained the extracorporeal circuit with hoses and filter, the ingredients for preparing the dialysis fluid, a suitable syringe for application of anticoagulants and a bottle of sodium chloride 0.9% (1l) for filling the tubing system.
The price for RRT depended on the number of applications per year. The cost were calculated by the number of packages that had been used the year before and included all disinfectants for the devices, rent of equipment, maintenance costs and all services by Fresenius®.
Continuous renal replacement therapy was performed as CVVHDF with the multiFiltrate®. If CRRT was used, exchange volume was about 25ml/kg/h. Unfractionated heparin or sodium citrate was administered as described previously.
StatisticsTo evaluate the economical impact of our renal replacement therapies following variables were extracted from our material supply department: number of sets for SLED and CVVHDF (filter, hoses and syringe for anticoagulation), drugs (heparin, citrate), substitution solutions as well as ultrafiltration bags. These data were also entered in MS Excel.
All data are presented as mean±SD, if not otherwise noted. Data are presented for each year and for the whole five years.
Short- and long-term outcomes were evaluated for all patients treated solely with SLED. For these patients SAPS II and TISS at admission were compared with the outcome as described in the questionnaire. In addition, the relationship between SAPS II and TISS and the individual outcome were summarized for each year investigated.
All presented amounts of money include value added tax with 16% in 2006 and 19% since 2007. Economical data were compared as average sum per year of the costs and proceeds of one procedure as well as total sum of costs and proceeds after five years.
ResultsPatients510 patients were identified of which 83 patients were excluded from further investigations according to our predefined criteria. The majority of these excluded cases (n=46) were already dependent upon dialysis. In addition, 30 patients had a shorter stay than two days in the hospital. In 7 patients dialysis was done for extra-renal indications: intoxication by lithium (n=4), hypercalcemia by hyperparathyroidism (n=1), hyperpotassemia with malignant arrhythmias (n=1) and extracorporeal rewarming after accidentally hypothermia (n=1).16
Thus, 427 patients remained for our cost-benefit analysis. There were 266 medical patients (62%) and 161 surgical patients (38%). In the medical patients, 211 patients suffered from a respiratory or cardio-vascular disease. 41 patients were treated due to a nephrological disease and in 14 patients an illness of the upper abdominal organs was presented. In the surgical patients, 124 patients underwent abdominal, 8 patients thoracic and 2 patients general surgery. 21 patients were admitted to trauma or orthopedic surgery. In 6 patients gynecological procedures were performed. Regarding to dialysis modality 392 patients were treated solely by SLED with an average of 8 treatments (range: 1–133). 6 patients received CVVHDF with a mean treatment period of 3.4 days. In 29 cases both treatment modalities, SLED and CVVHDF, were used.
Mechanical ventilation was required in 347 patients (81%); in 221 patients (52%) a percutaneous tracheostomy was performed. Average ventilation time was 382h (±390).
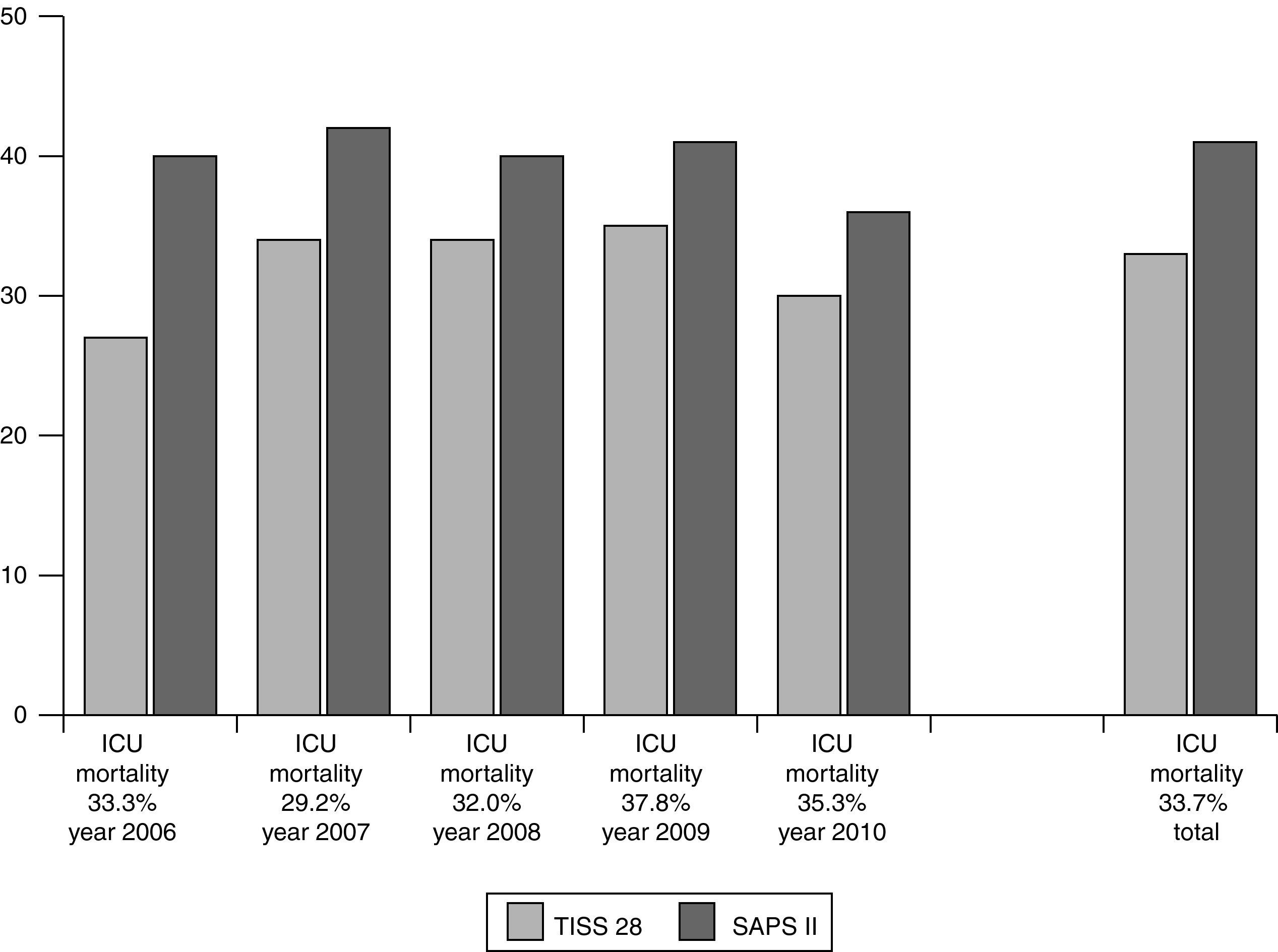
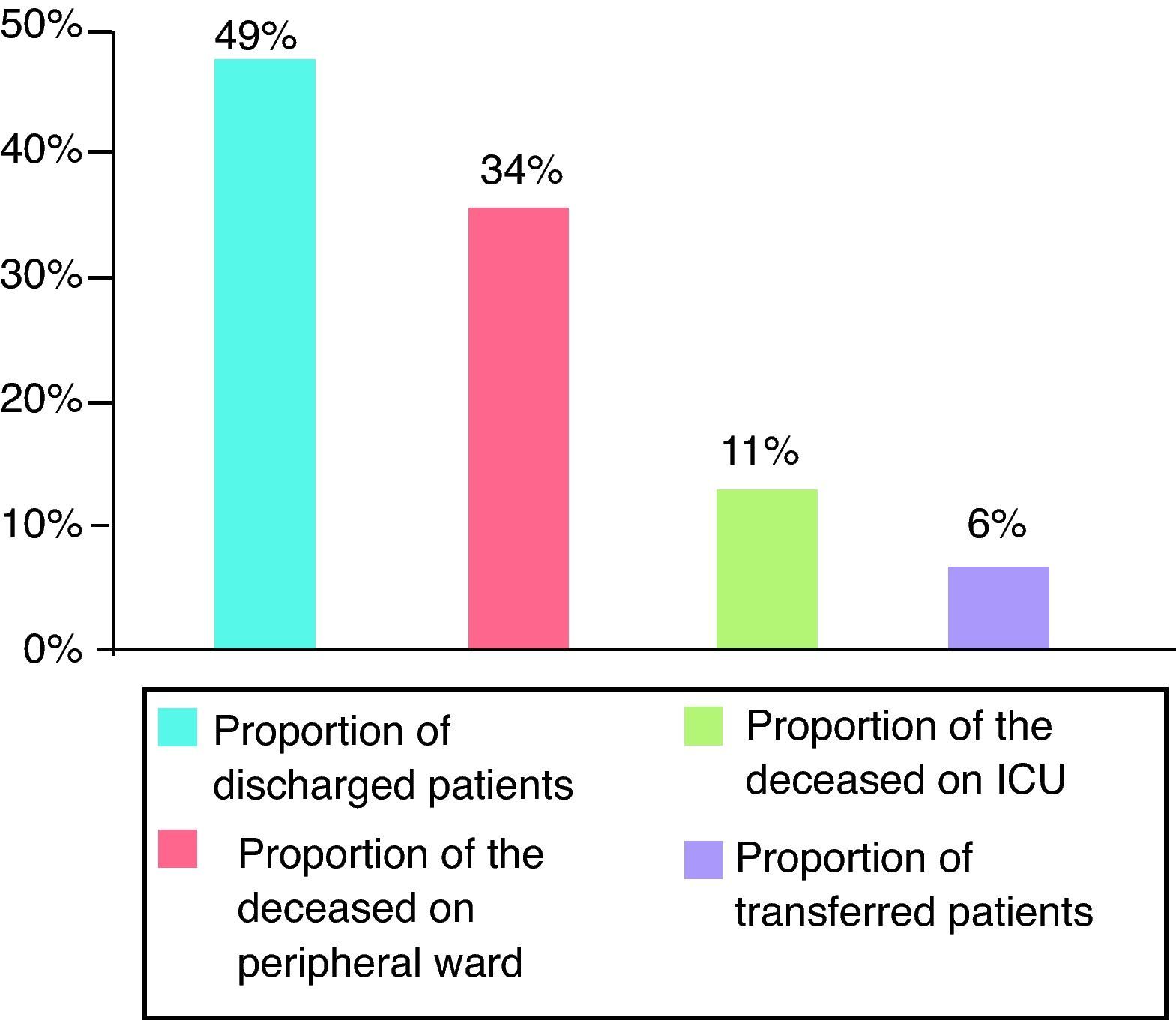
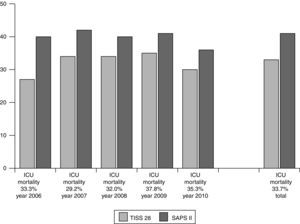
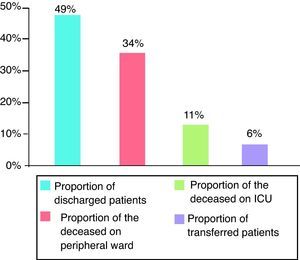
For evaluation of short- and long-term outcomes only the patients treated solely with SLED were considered (n=392). Mean SAPS II score was 41 points (±11), without inclusion of Glasgow Coma Scale because analgosedation was performed in the majority of our study population. Mean TISS 28 was 33 points (±11) (Fig. 1). 192 patients could leave the hospital to their previous living situation or into a rehabilitative facility. 132 patients died in our ICU and another 44 patients in the peripheral ward. 24 patients had been transferred for medical reasons into other hospitals (Fig. 2). The mean duration of ICU stay was 16 days (±16); mean hospital stay was 28 days (±23).
On September 2011 and March 2012 we contacted all 216 survivors of our study group through mail. 52 letters could not be delivered because the person had moved without providing a forwarding address.
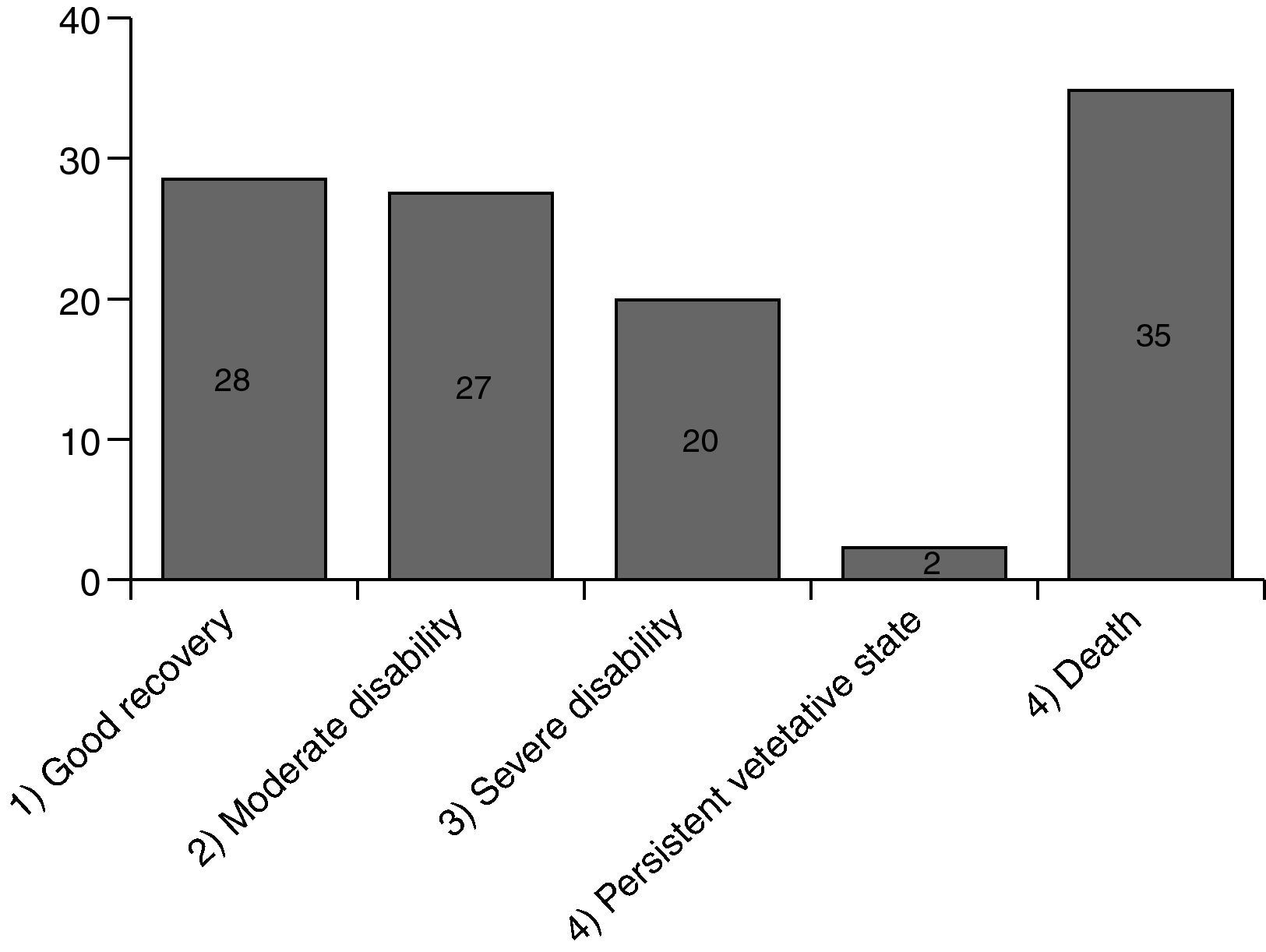
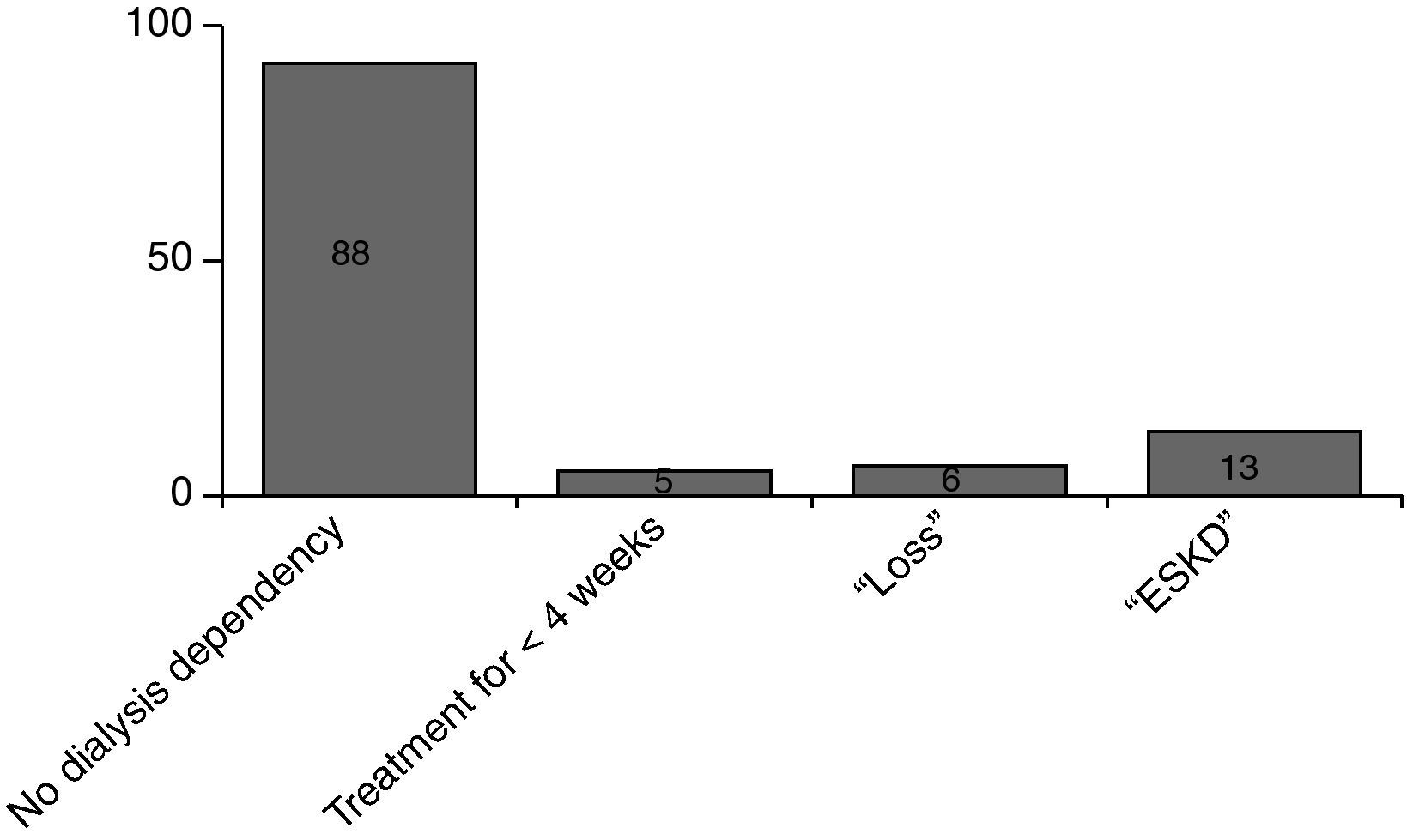
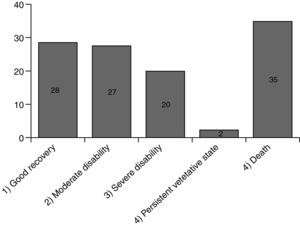
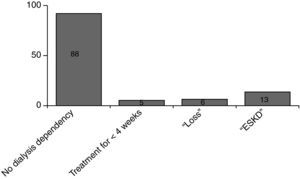
Fig. 3 shows the results. A total of 112 responses were sent back. 77 (69%) of them were still alive, 35 (31%) had been died meanwhile. 28 patients (36%) had recovered well from their disease (GOS 1), another 27 patients (35%) had some health restrictions but with preserved self-sufficiency of daily activities of life (GOS 2). 20 patients (26%) had been discharged with serious disability and are in need of personal assistance (GOS 3). 2 patients (3%) are 24h dependent on care (GOS 4). 88 patients have been discharged without dialysis dependency, 13 patients had persistent need of dialysis treatment (Fig. 4).17
Cost–proceeds ratioThe price of one set of extracorporeal circuit for SLED amounted to 182.12€ in 2006. After three years, the price was reduced to 127.52€. From 2006 to 2010 total costs for extracorporeal circuits were 518.431€ for 3247 treatments. The most frequently used anticoagulant for SLED was heparin with a number of 2941 applications. The price for one vial of sodium heparin (25,000U) was 2.24€. Calculated heparin consumption was 10,000U per treatment and total cost amounted to 2.635€. Sodium citrate was applied 306 times. The price for one infusion bottle of sodium citrate 1M (250ml) was 9.40€ and it was applied by a volumetric infusion pump. Calculated citrate consumption was 500ml per treatment and total cost amounted to 5.753€. Thus, during 2006–2010 the overall costs for SLED treatment including all cost factors amounted to 526.819€.
The hospital proceeds of one treatment with SLED were 247.90€ in 2006 and declined over the years to 221.02€ in 2010. The complete hospital proceeds of SLED during the evaluation period were 734.996€.
For direct cost comparisons between the intermittent and the continuous renal replacement therapies we assumed also 3247 “CVVHDF-days”. From 2006 to 2010 we performed 119 CVVHDF treatment days and total costs including extracorporeal circuits, hemofiltration solution bags and anticoagulation therapy amounted to 26.754€. Thus, the mean cost for one treatment day with CVVHDF was 224.82€. Based on this value we extrapolated total costs of 729.991€. The hospital proceeds for one CVVHDF treatment day were negotiated each year by the hospital administration and amounted to 212.77€ for the five years examined. Based on that value we extrapolated total proceeds of 690.864€. Thus, the costs of a continuous treatment are substantially higher mainly because of the large demand of hemofiltration substitution solutions (Fig. 5).
DiscussionWe found that in patients treated solely with SLED, ICU and hospital mortality (34% and 45%) as well as 1-year mortality (55%) were comparable to the published data with CVVHDF.8–10 The long-term outcome shows that 71% of all surviving patients have good quality of life (no need in practical support for daily activities of life). 12% of all discharged patients were long-term dependent on dialysis. The cost–proceeds ratio over the past 5 years demonstrates a total positive profit margin of 208.177€, when SLED was used.
Short-term outcomeOur ICU and hospital mortality in those 392 patients treated solely by SLED was 33.7% and 44.9%, respectively. By comparison, a cornerstone study in 2008 did investigate the influence of the intensity of renal replacement therapy on mortality in the ICU.9 In that study depending on the patient's condition different modalities of RRT (including SLED) have been compared, either with an intensive (IRRT for 6 times per week and CRRT at 35ml/kg bw effluent) or a less intensive mode (IRRT for 3 times per week and CRRT at 20ml/kg bw effluent). The primary endpoint of this trial was mortality on day 60. Mortality was 53.6% and 51.5%, respectively, for intensive and less intensive mode of treatment and did not differ between both modalities. Similar results were achieved in a second cornerstone study in 2009.10 Two study groups received different levels of intensity of continuous venovenous hemodiafiltration. Primary endpoint was mortality on day 90 and it was 44.7% in each group. Thus, since our ICU and hospital mortality with SLED treatment is in the same range, the renal replacement therapy modality seems to play no major role in short-term mortality.
In our database CRP levels in the deceased patients (median 128mg/dl at the time of death) are remarkable higher compared with the surviving patients (median 45mg/dl at the time of discharge). This observation strongly suggests that in the deceased patients an active infection has prevailed, which might be the leading cause of death in critically ill patients with multi organ dysfunction.18 In this context AKI has gained importance since it might induce some immune-suppression thereby enhancing a persistent infection.19
One critical point in evaluating the outcome of AKI patients is the question, when to start renal replacement therapy in the course of the illness. A survival benefit for an early onset of RRT at the critically ill patient was demonstrated in a retrospective study of 1999 in 100 adult trauma patients who were treated with CRRT for AKI. The patients were divided into two groups: an “early” starter-group or “late” starter-group, based upon BUN-level < or >60mg/dl prior to CRRT initiation. Survival rate was significantly higher in the “early” starter-group than in the control group (39% vs. 20%).20 A prospective clinical trial in cardiac surgical patients yielded similar results: the authors randomized 64 patients either to an early (urine output <100ml/8h) or a late start (urea level ≥30mmol/l) of continuous venovenous hemofiltration. Hospital mortality within the “early” and “late” group was 22% vs. 43%, respectively (p<0.05).21 Thus, both studies suggested a survival benefit for patients who were treated “early”.
In addition, the introduction of the RIFLE criteria, which classify the severity of renal failure on the increase of serum creatinine, the decrease of glomerular filtration rate or the decrease of urine output, yields to an earlier diagnosis of acute kidney injury with consecutive earlier start of treatment.17 Currently a target urea of 150mg/dl yields no difference in mortality between an intense and a less intense therapy.9 The annual comparison of our laboratory chemical examinations in our patients showed a decline of urea level at the time of the initiation into dialysis (from 176mg/dl in 2006 to 131mg/dl in 2010), which is in line with published data.20,21 However, mortality did not decrease over the years. Thus, current target values of blood urea might not be a suitable marker for initiation and maintenance of renal replacement therapy.
Long-term survival, quality of life and renal recoveryIn our study, 112 of 216 discharged patients responded, 77 patients are still alive and of those a portion of 28 (36%) belongs to GOS 1 with a good recovery. 55 patients (71%) belong to GOS 1 and 2 with self-sustaining of daily activities of life. 35 patients died after discharge (GOS 5). Longest survival after discharge was 58 months by an 84-year-old male patient who classified himself to GOS 2 without dialysis dependency.
Quality of life can be characterized by different variables, but from the point of view of patients and their relatives one main aspect is the self-sustaining of daily activities of life. In a large retrospective study of 2002 a survey on 301 patients with CRRT in AKI was performed after hospital discharge.22 Postdischarge information was available for 267 patients. 77% of patients assessed their current health status as good to excellent, and 57% were self-sustaining, comparable to our results.
Based on the data currently available there is no difference in terms of hospital mortality between IHD and CRRT.8–10 However, there exists some variation in long-term survival according to different patient populations. 1-year mortality varies between 57% and 64%.23,24 Our patient population is comparable to the previous published studies (SAPS II 41 points ±11) and the evaluation of our questionnaires shows an estimated one year mortality rate of 55%.
Long-term renal outcome can also be assessed in terms of ESKD rate. Since long adherence to dialysis imposes a considerable amount of cost on the health care system, any difference in ESKD between the various dialysis modalities would be of importance. Only a few studies focus on long-term renal function after ICU-RRT with inconsistent results.25,26 One representative prospective study from Switzerland demonstrates an ESKD rate of 10.1% at 3 years when continuous renal replacement therapy was performed in critically ill patients.27 The follow-up of our patients treated with SLED revealed an ESKD rate of 12%, i.e. comparable to published data with CRRT.
Cost–proceeds ratioIn our analysis of the years 2006 to 2010 the price for one treatment with SLED was 162.25€ on average and the reimbursement according to the catalogue of per-case fees was 226.36€ on average, yielding a positive contribution margin of 64.11€ per treatment. Calculated daily cost for one treatment with CVVHDF was 224.82€ on an average and reimbursement was 212.77€ on an average, yielding a negative contribution margin of 12.05€ per treatment day. Thus, compared to CVVHDF, SLED yields a substantial positive contribution margin.
There is one multicenter study from 2010 that describes the cost difference between CRRT and IRRT among 53 centers from 23 countries.11 In fact, compared to IRRT, CRRT was 289.60$ (median) per day more expensive. The study investigators found also that costs varied widely by region.
To make a Genius90® device ready for dialysis it takes approximately 20min, which is considerable shorter compared to CVVHDF. Accordingly, there is a high acceptance toward the use of SLED among the medical and care staff,28 because the device requires a shorter instruction briefing and it is easy to handle. In our study staff costs have not been taken into account because in Germany the personnel planning for the ICU does not consider any renal replacement therapy.
A further advantage of an intermittent renal replacement system such as the SLED is the substantial reduction of anticoagulation drugs. For example, the use of heparin for adequate anticoagulation in a SLED group was one-fifth compared to a CVVH group (median of 4000IE/d vs. 21,100IE/d).29 Thus, bleeding complications should be by far less using SLED compared to CRRT and might be even more less with sodium citrate.30,31
SummaryTo evaluate whether or not the use of SLED in AKI yields comparable results to published data with CRRT, we retrospectively analyzed our patients treated from 2006 to 2010. During that period we performed 3247 SLED treatments in 421 patients. ICU and hospital mortality in these patients treated solely with SLED (n=392) was 34% and 45%, respectively. Cumulative 1-year and 3-year mortality was 55% and 60%, respectively. Long-term outcome evaluation revealed a good quality of life in 71% of the survivors. 13 patients were on permanent dialysis dependency. Total costs and proceeds for SLED treatment amounted to 518.431€ and 734.996€, respectively. Thus, in terms of outcome SLED is at least comparable to standard CVVHDF. In terms of costs SLED seems to be the preferred mode of renal replacement therapy.
FundingNone
Conflicts of interestT Neuenfeldt has no conflicts of interest.
HB Hopf has been a lecturer in workshops sponsored by Fresenius®.
Questionnaire for the outcome after treatment with renal replacement therapy
- 1.
Please choose in which state of health you or rather your dependant has been discharged from our hospital.
- ∘
Good recovery
- ∘
Moderate disability/physical handicap with self-sustaining in daily activities of life
- ∘
Serious disability/physical handicap with some need in personal assistance for daily activities of life
- ∘
Heavy or full care dependency, persistent vegetative state
- ∘
Deceased
- ∘
- 2.
If your dependant has unfortunately been passed away meantime, please choose in which timescale that has happened after discharge from our hospital.
- ∘
Within 3 months
- ∘
Within 6 months
- ∘
Within 12 months
- ∘
Within ………… months/years
- ∘
- 3.
Have you or rather your dependant been treated in a dialysis center after discharge from our hospital?
- ∘
No
- ∘
Yes, for a limited period of <4 weeks
- ∘
Yes, for a limited period between 1 and 3 months
- ∘
Yes, more than 3 months
- ∘
Yes, but dialysis dependency had already been prior to hospital stay
- ∘
- 4.
If you have any requests, suggestions or criticism you would like to send us, please use the free area down below.
Thank you once again for all your help!
Please cite this article as: Neuenfeldt T, Hopf H-B. Diálisis sostenida de baja eficiencia en una unidad de cuidados intensivos interdisciplinarios: un análisis costo-beneficio a 5 años. Rev Colomb Anestesiol. 2013;41:88–96.