Twin pregnancies are at increased risk of complications and adverse outcome compared to singletons. A significant contribution to higher morbidity and mortality seen in monochorionic twins is made by cardiac complications.
Abnormal cardiac development in monochorionic pregnancies can be divided into primary structural cardiac lesions (structural cardiac anomalies unrelated to the existence of TTTS) and acquired cardiac lesions (any cardiac anomaly considered to be associated with TTTS).
The etiology of primary structural congenital heart disease in these twins is poorly understood. Both environmental and genetic factors influence this figure.
Acquired cardiac lesions are the consequence of hemodynamic anomalies which can occur as a result of TTTS. These cardiac anomalies are present primarily in recipient twin and include biventricular hypertrophy and diastolic dysfunction as well as right ventricular outflow tract obstruction and pulmonic stenosis.
This review will focus on anomalies of cardiac development in monochorionic twin pregnancies.
Los embarazos gemelares tienen un mayor riesgo de complicaciones y de desenlaces adversos que los simples. Las complicaciones cardíacas contribuyen de forma significativa a la mayor morbimortalidad observada en los gemelos monocoriónicos.
El desarrollo cardíaco anormal en los embarazos monocoriónicos se puede dividir en lesiones cardiacas estructurales primarias (anomalías cardíacas estructurales no relacionadas con la existencia de STFF) y en lesiones cardíacas adquiridas (cualquier anomalía cardíaca que se considere asociada con STFF).
La etiología de la cardiopatía congénita estructural primaria en estos gemelos es poco conocida. Tanto los factores genéticos como los ambientales influyen en estas cifras.
Las lesiones cardíacas adquiridas son consecuencia de anomalías hemodinámicas que pueden ocurrir por la existencia de un síndrome STFF. Dichas anomalías cardíacas están presentes principalmente en el gemelo receptor e incluyen la hipertrofia de los dos ventrículos y la disfunción diastólica, así como la obstrucción del tracto salida de ventrículo derecho y la estenosis pulmonar.
Esta revisión se centrará en el desarrollo de anomalías cardíacas en los embarazos gemelares monocoriónicos.
Approximately one-third of twin pregnancies are monozygotic. If the zygote divides within the first 72h after fertilization, two embryos, two amnions, and two chorions will develop and a dichorionic diamniotic twin pregnancy evolves. If the division of the embryonic mass occurs between the fourth and eighth day, a monochorionic diamniotic twin pregnancy will result. About eight days after fertilization, the chorion and the amnion have already differentiated and the division results in two embryos with a common amnionic sac, that is, a monochorionic monoamniotic twin pregnancy. Conjoined twins will result if the twinning process is initiated later.
Twin pregnancies are at increased risk of complications and adverse outcome compared to singletons. Chorionicity rather than zygosity determines the risk to the pregnancy and deeply influences the perinatal course. A significant contribution to this higher morbidity and mortality is made by the cardiac complications of being a monochorionic twin. This includes an increased risk of structural congenital heart disease (CHD) and also of acquired cardiac complications associated with the twinning process.
The incidence of structural CHD is 8:1000 live births.1 The incidence of heart disease is higher than this in twins, particularly in monochorionic twins.2 A proportion of these defects are acquired as a result of the altered hemodynamics in the recipient twin associated with twin-to-twin transfusion syndrome (TTTS).3
Near 95% of monochorionic twins are diamniotic and have a 15% chance of developing TTTS.4
Abnormal cardiac development in monochorionic pregnancies can be divided into primary structural cardiac lesions (structural cardiac anomalies unrelated to the existence of TTTS) and acquired cardiac lesions (any cardiac anomaly considered to be associated with de development of TTTS).
This review will focus on abnormalities of cardiac development in monochorionic twin pregnancies.
Materials and methodsWe conducted a bibliographic review of published English literature on MEDLINE (PubMed) from the last 25 years using the medical terms “monozygotic twins”, “congenital heart defect” and “twin-to-twin transfusion syndrome”.
We selected the more relevant papers and their references.
Results and discussionPrimary structural cardiac anomalies in monochorionic twinsThe incidence of structural CHD in monochorionic twins is considerably higher than in the general population. The etiology of primary structural congenital heart disease in these twins is poorly understood. Both environmental and genetic factors influence this figure. Theories suggested to explain the increased risk for structural CHD in MC twins include the possibility that the twinning process itself may increase the incidence of CHD, by the unequal division of the inner cell mass, disturbance of laterality and by phenotypic variability of the same genome resulting in discordant cardiovascular anatomy.5 A genetic cause has now been established for many forms of CHD, and further developments in molecular genetics may lead to an even clearer understanding. Recently, microarrays and next-generation sequencing emerged as new approaches for detection of genomic alterations in patients with congenital defects and selection of candidate genes.6,7
When considering CHD in monochorionic twins, primary structural cardiac anomalies must be distinguished from acquired cardiac manifestations that result from hemodynamic changes.
Acquired cardiac anomalies in monochorionic twinsAcquired cardiac lesions are the consequence of hemodynamic abnormalities which can occur as a result of TTTS.
Pathophysiology of twin-to-twin transfusion syndromeThe pathophysiology of this disease is not fully understood, but the presence of vascular anastomoses connecting both fetal circulations at the level of the placenta is mandatory for its development.
Virtually all monochorionic diamniotic placentas have anastomoses, yet not all monochorionic diamniotic twins develop TTTS. These anastomoses can be superficial with a very low resistance and bidirectional flow [the arterioarterial (AA) or venovenous (VV) anastomoses], or deep, with high resistance and unidirectional flow [the arteriovenous (AV) or venoarterial (VA) anastomoses]. AA are more common in non-TTTS placentas than in TTTS placentas,8,9 but it is possible that they must be interpreted as markers, rather than functional determinants, of TTTS as they may reflect a general antiangiogenic condition in TTTS placentas.8 Sebire et al.,10 were the first to hypothesize that TTTS results from a progressive, but asymmetric, reduction of bidirectional AV/VA anastomoses, present in 95–98% of MC twin placentas.11 The AV imbalance, however, does not seem to be larger in TTTS placentas but actually smaller and it correlates with the donor/recipient roles of the twins.
This ultimately results in a volume depleted donor twin, who will show signs of oligouria and oligohydramnios and a volume overloaded recipient twin who will present with polyuria and polyhydramnios.
Nevertheless, there appear to be additional factors beyond placental morphology, such as complex interactions of the renin–angiotensin system (RAS) in the twins involved in the development of this disorder.12–14
Diagnosis and staging of twin-to-twin transfusion syndromeThe diagnosis of TTTS is based on strict sonographic criteria reflecting severe intertwin fluid discordance. It requires the presence of a monochorionic diamniotic pregnancy and the presence of oligohydramnios (defined as a maximal vertical pocket of 2cm) in one sac, and of polyhydramnios (a maximal vertical pocket of 8cm) in the other sac.
The disease is currently staged using the TTTS staging system developed by Quintero et al.15 in 1999, and is based on sonographic findings. The TTTS Quintero staging system includes 5 stages, ranging from mild disease with isolated discordant amniotic fluid volume to severe disease with demise of one or both twins. Stage I represents the most benign form of TTTS with polyhydramnios of the recipient and oligohydramnios of the donor, with its bladder still visible. In stage II the donor is “stuck” and its bladder is no longer visible. Stage III describes forms with severely abnormal Doppler flow patterns in the umbilical artery of the donor or venous abnormalities in the recipient, or both. Stage IV is characterized by fetal hydrops and stage V describes fetal demise of one or both twins. Although the Quintero staging system estimates the severity of TTTS, it disregards the cardiac involvement of the disease that may be present even at its earlier stages.16
Several screening models have been proposed to predict the occurrence of TTTS prior to 18 weeks based on the appearance of early hemodynamic compromise.17,18 The pioneering work of our group disclosed preliminary evidence that abnormal flow in the DV at 11 to 13+6 weeks’ gestation in singletons is associated with increased risk of chromosomal abnormalities19 and cardiac defects20 and that it represents a manifestation of cardiac dysfunction/strain.21 This rationale was applied, with promising results, to MC twins that eventually developed TTTS.22–24 Improved screening for TTTS could facilitate timely therapeutic treatment and thus improve outcomes.
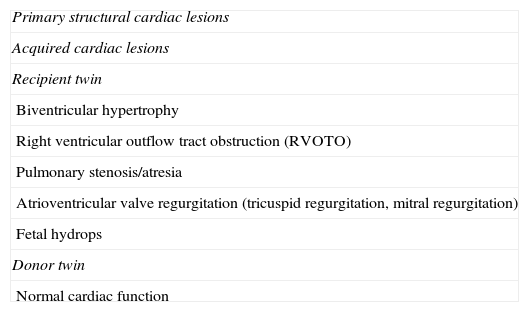
Acquired cardiac anomalies in recipient twinThe functional cardiac anomalies that complicate TTTS occur primarily in recipient twins. Volume overload causes increased pulmonary and aortic velocities, cardiomegaly, and atrioventricular valve regurgitation (Table 1). Over time, recipient twins can develop progressive biventricular hypertrophy and diastolic dysfunction as well as poor right ventricular systolic function that can lead to functional right ventricular outflow tract obstruction (RVOTO) and pulmonary stenosis.4,25 The development of right ventricular outflow obstruction, observed in close to 10% of all recipient twins, is likely multifactorial, a consequence of increased preload, afterload, and circulating factors such as renin, angiotensin, endothelin, and atrial and brain natriuretic peptides.26–28
Cardiac morbidity in monochorionic twins.
| Primary structural cardiac lesions |
| Acquired cardiac lesions |
| Recipient twin |
| Biventricular hypertrophy |
| Right ventricular outflow tract obstruction (RVOTO) |
| Pulmonary stenosis/atresia |
| Atrioventricular valve regurgitation (tricuspid regurgitation, mitral regurgitation) |
| Fetal hydrops |
| Donor twin |
| Normal cardiac function |
Although cardiovascular disorders in recipients may result from increased preload caused by to chronic hypervolemia, it is the increased afterload29 resulting from increased arterial resistance and pressure that has been identified by many as a key factor in the pathogenesis of cardiomyopathy.30–33 As such, in about half the cases, the heart is enlarged as a result of hypertrophy rather ventricular dilatation34 and the thickened, dysfunctional myocardium causes alterations in ventricular filling. So, frequently, diastolic functional impairment is present before systolic dysfunction35 and, by prolonging relaxation time, is more important than systolic dysfunction in compromising fetal circulation and producing hydrops.
Failure of the right ventricle, through diastolic dysfunction, can be demonstrated in the ductus venosus by reduced forward blood flow with the atrial contraction. As the dysfunction progresses, the two diastolic waveforms fuse and the Doppler inflow pattern regresses to that typically seen in the first trimester, supporting the notion that RVOTO may be caused by the diminished forward blood flow through the right side of the heart.16
Systolic functional impairment may also occur, with a considerable decrease in the shortening fraction in about 30% of the recipients,4,16 and predominantly at the level of the right ventricle.27 Right ventricular hypertrophy may develop progressively, leading to acquired “congenital” pulmonary stenosis or even pulmonary atresia.36,37 Also, tricuspid regurgitation occurs in about 30–50% of recipients16,38,39 but is severe in only half of these.16,27,38 Mitral regurgitation, on the other hand, is much less frequent (6–14% of cases),16,38 yet usually severe (9%).16,34 Finally, chronic pressure overload and the increased shear stress associated with TTTS may cause calcification of the aorta and pulmonary artery, with hyperplasia of the intima and media, in the absence of valvular disease.4 Changes also take place in the coronary arteries, which favor supply to the overloaded right ventricle.33
Moreover, as growth of fetal cardiac structures is dependent on the blood flow streaming through them, persistent ventricular dysfunction can lead to secondary anatomic changes.40
The cardiovascular response to TTTS contributes to the poor outcome of recipient twins while recipients with normal cardiac function have improved survival.
Acquired cardiac anomalies in donor twinIn contrast to these changes in recipient twins, donor twins with TTTS tend to have normal cardiac function. Placental vascular resistance increases in the donor who is hypovolaemic with oligohydramnios and usually growth retarded but does not develop the acquired structural cardiac anomalies frequently seen in the recipient.
As a consequence of hypovolaemia in the donor twin, the RAS is activated with up-regulation in the donor and down-regulation in the recipient.4,31 Arterial compliance is reduced in the donor with possible long-term effects including hypertension and renal damage. It is suggested that these changes in the RAS system may be crucial in the pathogenesis of TTTS by aggravating the oligohydramnios, increasing arterial resistance, contributing to placental dysfunction and thus to intrauterine growth restriction.
Echocardiographic features in monochorionic twinsScreening for congenital heart disease with fetal echocardiography is warranted in all monochorionic twins as the prevalence of congenital cardiac anomalies has been reported to be 2% in otherwise uncomplicated monochorionic gestations and 5% in cases of TTTS, particularly among recipient twins.29
Once a TTTS is fully installed, echocardiographic findings tend to progress over time, with worsening ventricular hypertrophy and systolic dysfunction, which can ultimately lead to fetal hydrops and intrauterine fetal demise.
Van Mieghem et al.40 have shown that changes in cardiac function are already present well before the actual development of TTTS. As such, about 30% of fetuses with moderate amniotic fluid discordance not fulfilling the criteria of TTTS but ultimately progressing to the syndrome show an increased myocardial performance index.34 The “Quintero system” does not comprise echocardiographic features. Stage I encompasses a wide and heterogeneous variety of cases and this has recently led to questions being raised regarding the need for expectant management of these cases. Myocardial performance anomalies have been described, particularly in recipient twins, including those with only stage I or II TTTS.41
In an attempt to provide a more pathophysiologic classification of TTTS, different groups have suggested to use new staging systems that are mainly based on the severity of cardiac dysfunction in the recipient fetus.34 While this approach has some benefits, the models have not yet been prospectively validated.
ConclusionThe angioarchitecture of monochorionic placentas determines occurrence of TTTS and eventual cardiac involvement. Screening for congenital heart disease with fetal echocardiography is warranted in all monochorionic twins, initially to establish normal anatomy and then to check for any evolving lesions or cardiac dysfunction in pregnancies complicated by TTTS.33 Effective early screening for TTTS allows more precisely grade the severity of disease, improves decision making for treatment, and sets a prognosis for possible late sequelae in childhood or beyond.16
Further scientific evidence is necessary to determine the prognostic value of cardiac profiling in the early diagnosis of TTTS.
Conflict of interestThe authors declare that there are no conflicts of interest.