MZ twins, though arising primarily from a single zygote and considered genetically identical, will not ever be absolutely the same. Post-fertilization events such as chromosomal mosaicism, skewed X-inactivation and imprinting mechanisms, as well as other epigenetic mechanisms are responsible for the putative differences between MZ twins. Numerous discordant MZ twins have been reported in the literature, including discordance for malformations or genetic diseases, lateral asymmetry, discrepant growth and intrauterine death of the co-twin. This discrepancy for the disorder may be valuable in the analysis of the effect of a disease upon gene expression or on phenotype variation, and have long-term implications. With today's whole-genome sequencing technologies and increasing evidence for genetic and epigenetic differences in some MZ twins, the understanding of these differences is a whole new field of research.
We reviewed the genotypic and phenotypic differences between MZ twins and discuss ten main reasons for being different.
Si bien se considera que los gemelos monocigóticos surgen de un solo cigoto y son genéticamente idénticos, nunca lo son absolutamente. Ciertos procesos posfecundación, como el mosaicismo cromosómico, los mecanismos de inactivación sesgada del cromosoma X e impronta genética, así como otros mecanismos epigenéticos son responsables de las supuestas diferencias entre los gemelos monocigóticos. En la literatura se han publicado numerosos ejemplos de gemelos monocigóticos «discordantes». Esta discrepancia puede resultar valiosa en el análisis del efecto de una enfermedad sobre la expresión genética o la variación fenotípica, y tener implicaciones a largo plazo. Con las tecnologías de secuenciación genómica actuales y el creciente cuerpo de evidencia sobre las diferencias genéticas y epigenéticas en algunos monocigóticos gemelos, la comprensión de estas diferencias se ha convertido en un nuevo campo de investigación.
Revisamos las diferencias genotípicas y fenotípicas existentes entre gemelos monocigóticos y examinamos las 10 razones principales para ser diferente.
Monozygotic (MZ) twins are indeed very much alike but almost never “identical”. However, genotypic as well as phenotypic differences between MZ twins have been poorly explained by measurable genetic or environmental discordance.1–3
Therefore, the new paradigm is not one of ‘nature versus nurture’, but of a complex and dynamic interaction between genetic, epigenetic and environmental factors that act in concert to establish the final phenotype.4 The purpose of this review is to describe the ten reasons that make each MZ twin both phenotypically and/or genetically different.
Understanding the origin of monozygotic twinningZygosity is the one that reflects the type of conception, i.e., whether twins arise from one or two fertilized eggs (MZ or dizygotic (DZ), respectively), whereas chorionicity refers to the type of placentation. Why MZ twinning does occur in humans is not clear.5 According to Machin2 post-zygotic events would not only precede the twinning event, but may actually trigger it. If two different cell clones exist within one early zygote, the differences between the clones may be sufficient to cause mutual ‘recognition and repulsion’, resulting in the ‘splitting’ into two embryos.6 Another exciting theory is the one proposed by Boklage: he suggests that monozygotic and dizygotic twinning events arise from the same embryogenic mechanism, which is completely innovative since previous theories attribute totally different mechanisms for producing MZ (origin in a single zygote) or DZ twins (origin in two ova fertilized by two spermatozoa).7
MZ twins comprise about a third of all spontaneous twins. According to Corrner’ hypothesis which was never proven, since no such evidence exists of a split in observations from in vitro fertilization, dichorionic diamniotic MZ twins (18–36%) have separate membranes and placentas and result from abnormal splitting at the 2 cell stage to morula (day 0–3); monochorionic diamniotic twins (∼80%) separate at the inner cell mass, after the chorion was formed (day 4–7); monochorionic monoamniotic twins (2–4%) are believed to split at the late blastocyst stage (day 7–14) and conjoined twins (2/10,000) are assumed to split around day 14 resulting in the incomplete division of the embryonic disk.8,9
The recognition of zygosity in dichorionic MZ twins is based on physical similarities what may prove inaccurate.8,10 In liked-sex dichorionic twins, we are blind to zygosity in about 44% of the twins. Despite observations of DZ monochorionic twins, the current belief is that all monochorionic twins are MZ. Biochemical characteristics such as blood type, enzyme polymorphisms and HLA types have also been used to classify zygosity. Nevertheless, DNA “fingerprinting” (quantitative fluorescence polymerase chain reaction (QF-PCR) is the gold standard for defining zygosity. However, we must bear in mind that due to vascular placental connections in the placenta of MC twins DNA may be exchanged (chimerism).8
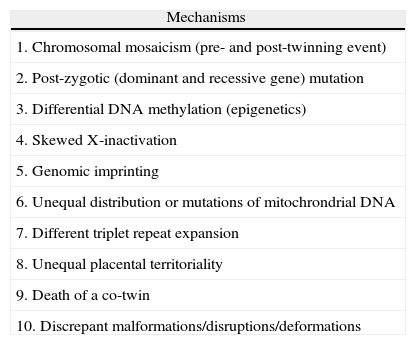
Mechanisms of discordanceThere are several mechanisms of phenotypic discordance in MZ twins available in the literature (Table 1).
(Epi)genetic mechanisms that may cause MZ twin discordance.
| Mechanisms |
| 1. Chromosomal mosaicism (pre- and post-twinning event) |
| 2. Post-zygotic (dominant and recessive gene) mutation |
| 3. Differential DNA methylation (epigenetics) |
| 4. Skewed X-inactivation |
| 5. Genomic imprinting |
| 6. Unequal distribution or mutations of mitochrondrial DNA |
| 7. Different triplet repeat expansion |
| 8. Unequal placental territoriality |
| 9. Death of a co-twin |
| 10. Discrepant malformations/disruptions/deformations |
MZ twins may have a different chromosomal composition (heterokaryotypic twins). All possible combinations of karyotypes observed in twins can be attributed to the unequal allocation of the abnormal cells to each twin: abnormal/normal, mosaic/mosaic, abnormal/mosaic, and normal/mosaic,11,12 including discordant sex phenotype with mosaicism 46XY/45X, Turner's syndrome in female twins with mosaicism 46XX/45X, trisomy 21 and trisomy 13. There are a few case reports of MZ twins with discordant phenotype and rare partial chromosomal anomalies including 22q11 deletion,13 7q syndrome,14 monosomy 2115 and partial trisomy 1. Trisomy 11p has already been reported, either associated with a non-specific clinical pattern or with the Beckwith–Wiedemann syndrome (BWS) when the additional region cause paternal disomy. MZ pairs discordant for 45X emerging from 47XXY, 47XXX, 46XY and 46XX zygotes have also been reported.
If the mitotic error occurs before the twinning event, a mosaic will be present in both fetuses with different distribution of the two cell lines between the twins; if the mitotic error occurs after the twinning event, the mosaic will be present in only one twin.11 Blood mosaicism may also be present in normal twins, as a result of interfetal anastomoses, and therefore, karyotyping in MZ twins that are discordant for some fetal abnormality should be performed in amniocytes rather than in fetal blood.11
Different gender phenotypes are mostly attributed to loss of the Y chromosome in only one of the 46XY MZ twins, mostly an inv dup(Y) or alternatively, due to an assumed mutation in a sex-determining gene (for example, the SRY gene). Complete sex discordance, or one male and one intersexual individual would depend on the timing of the event. Sex discordance could also occur in one of 46XX twins if a somatic mutation in a recessive or X-linked gene results in masculinization of the external genitalia (adrenogenital syndrome).16
MZ twins with concordant chromosomal aberration and discordant phenotypes have also been described and may be explained by differences in blood flow, blood chimerism, confined placental mosaicism, mosaicism with different proportions of abnormal cells and differences in epigenetic control as recently postulated for MZ twins with chromosome 22q11 deletion.13
Post-zygotic gene mutationThere are reports on MZ discordance for conditions transmitted by:
-post-zygotic mutations that have been observed rather frequently in several autosomal disease of dominant inheritance, such as spondylocostaldysostosis, oral–facial–digital syndrome, Sotos syndrome, Cornelia de Lange syndrome, Aicardi syndrome, Gerstmann–Strausler–Scheinker syndrome and William syndrome, thanatophoric dysplasia and especially for neurofibromatosis and tuberous sclerosis.
-post-zygotic recessive gene mutations that are an extremely rare event. Walker described discordant MZ twins with cleft palate and retinoblastoma. Recently, we described a pair of MZ twin girls, in which one had arthogriposis and the other did not. This may be attributed to random cleavage or separation or inactivation of mitochondrial DNA.
EpigeneticsEpigenetic phenomena are characterized by “modifications in gene expression that are controlled by heritable but potentially reversible changes in DNA methylation and/or chromatin structure”.4 Epigenetic mechanisms are dynamic processes that are influenced by the developmental stage, tissue type, environmental factors, and stochastic events.4
According to Wong et al.,17 MZ twins reared together (MZT) are similar to MZ twins reared apart (MZA), for a large number of traits, suggesting that stochastic events, and not specific environmental effects, may be a more important cause of phenotypic differences.
Differences in DNA methylation seem to occur more frequently in MZ twins and may influence susceptibility to bipolar disorder and schizophrenia. Different DNA methylation patterns in relation to specific schizophrenia candidate genes, such as the dopamine D2 receptor gene or the catechol-O-methyltransferasegene, have been implied in the discordance for schizophrenia in MZ twins.18
More recently, Bianchi and coworkers19 pointed out to alternative methods for evaluating in vivo genetic differences in MZ twins, such as cell-free fetal DNA (ff DNA) in maternal blood, mRNA in amniotic fluid and RNA single nucleotide polymorphism (SNP) allelic ratio analysis. Therefore, prenatal gene expression investigation in vivo may open a new field for prenatal twin research.
Skewed X-inactivationX-inactivation (or Lyonization) refers to a process of inactivation of one of the X chromosomes in females to achieve dosage compensation of X-linked genes with males. In the majority of cases X-inactivation is random with both X chromosomes in a female having an equal chance of being inactivated by methylation. However, in a minority of individuals, for reasons not yet fully understood, X-inactivation can be ‘skewed’ or ‘non-random’, that is, the X chromosome of one parental origin is preferentially “switched off” with unequal allocation of the blastomeres to each twin.9 A random or quasi-random X-inactivation pattern will most likely occur in the twin receiving a larger number of blastomeres. Consequently the X-chromosome discordance may result from the total lack of expression of a given X-linked gene in a male and from a nullisomy phenotype in a female with the first allele silenced by X-inactivation and the second by aberrant methylation.5
Skewed X-inactivation has been identified in numerous MZF pairs discordant for several X-linked conditions such as fragile X-syndrome,20 Fabry's disease, Rett's syndrome,21 Duchenne muscular dystrophy,22 color blindness,23 Hunter's disease, G6PD deficiency, Aicardi's syndrome, autoimmunity, X-linked immunodeficiencies,24 Lesch–Nyhan disease, and hemophilia.
Finally, the highly similar patterns of X chromosome inactivation among monochorionic twins may indicate that X chromosome inactivation occurs before the twinning event in this subgroup of MZ twins.
Genomic imprintingGenomic imprinting is a phenomenon in which one of two alleles, from maternal or paternal origin, is inactivated by DNA methylation. Imprinting is initiated during gametogenesis and transmitted to embryos via mature male and female gametes. Appropriate imprinting of the two gametes is critical and implicated in prenatal growth, development and differentiation, behavior and human disease.5
Discordance between MZ twins has been reported for several diseases where genomic imprinting is suspected or implicated, such as BWS.25 May well be that unequal splitting of the inner cell mass results in differential methylation between the two cell masses. These parent-of-origin effects, together with the finding of paternal uniparental disomy of chromosome 11p15 in 20% of BWS cases, suggests that abnormal genomic imprinting might play an important role in the etiology of BWS. It has been postulated that loss of imprinting itself caused twinning. This can explain the higher rate of twins in BWS.26
Discordance for hyperinsulinemic hypoglycemia27 and Russell–Silver syndrome28 has also been reported and although these are likely to be heterogeneous conditions, it is possible that imprinted genes play nonetheless an important role.
Mitochondrial DNA mutationsUnbalanced mitochondrial DNA (mtDNA) allocation or mtDNA mutations can determine phenotypic variability for common Mendelian disorders. Heteroplasmy is the phenomenon in which wild-type mtDNA coexists with mutated mtDNA. MZ twin pairs discordant for several mitochondrial conditions have been reported, such as Leber's disease, chronic progressive external ophthalmoplegia, neurofibromatosis type I, adrenoleukodystrophy and obesity.
Repeat expansion disordersDifferent number of triplets or differential activation of copy genes have been implicated in the variable expression of diseases such as Steinert's myotonic dystrophy in MZ twins. Still there are few data concerning repeat expansion disorders in twins.
Fetal growth and placental territorialityUp to 21% of monochorionic twin pregnancies are complicated by severe birth weight discordance (BWD) even in the absence of TTTS. This discordant birth weight in MZ twins can be the result of unequal territory sharing9 or inequitable allocation of stem cells to each twin (larger clone sizes from fewer precursor cells in one twin).10
Velamentous cord insertion (VCI) occurs rarely in singleton placentas (2%) and is far more common in dichorionic (7%) and particularly in monochorionic twin placentas (12%). This high incidence of VCI in monochorionic twin placentas seems to result from a “battle” for space between each twin's placental shares (trophotropism). It is currently associated with a smaller placental mass and lower birth weights.29 In our recent study, these findings were significantly replicated in a larger series in which the presence of VCI in one twin was significantly associated with small for gestational age (SGA) and BWD.30
Single fetal deathMany initial twin pregnancies are lost or converted into singleton pregnancies. Theoretically, singletons that started as MZ twins are at risk for congenital anomalies that are frequent in MZ twins.31 A “vanishing” twin may result from an early fetal demise, often in the first trimester. These anomalies are probably caused by acute hemodynamic changes via vascular anastomoses in monochorionic twins. If one fetus dies in utero, the surviving co-twin is at increased risk of major morbidity such as cerebral palsy, learning disabilities and damage to end-organs.31,32 The ‘hemodynamic imbalance theory’ states that the placental anastomoses allow transfer of blood from the surviving twin to the dead co-twin giving rise to periods of hypoperfusion, hypotension and acute fetal anemia, resulting in neurological damage.32,33
According to Ong et al.32 fetal death occurring after the first trimester increases the odds of intrauterine death of the co-twin and neurological abnormality among survivors. Furthermore, this seems to be 6 and 4 times higher in monochorionic compared with dichorionic pregnancies.
The effect of timing of intrauterine death on outcome and prognosis of the survivor is still controversial.31,33
Discrepant malformations/disruptions/deformationsCongenital anomalies occur more frequently in MZ twins (prevalence 5 times higher than in the general population) and may be concordant or discordant.1 Some malformations, such as conjoined and acardiac twins, as well as fetus papyraceous, are related in some way to the twinning process itself.34 Malformations, including cloacal anomalies, neural tube defects and congenital heart defects, occur when normal development fails. Many of these malformations are likely to be multifactorial. It is still unclear how these discordant events happen, however, frequently, the apparently unaffected co-twin has also a manifestation of the same disorder, but a mild one. This requires that the predisposition for the malformation is present in both twins.9 Extrophy of the cloaca, holoprosencephaly, cyclopia, and anencephaly are early origin malformations frequently discordant in MZ twins. These malformations could be due to the same injury that causes the twining process itself. Discordance has also been described for tracheoesophageal fistula, ventral body wall defects, neural tube defects, cloacaldysgenesis, vertebral anomalies, anal atresia, esophageal atresia with fistula and VATER association (a combination of the above plus renal anomalies). This may result from unequal allocation of blastomeres at the time of separation and/or differences in attachment to the placenta or type of chorion.
Disruptions in MZ twins including limb reduction defects, hemifacialmicrosomia, and amyoplasia, may be the result of shared placental circulation leading to secondary vascular disruptions and sometimes to single intrauterine fetal death.8
Discordance in MZ twins for lateral body wall defect and other forms of “amniotic band syndrome” can be explained by major abnormal blastogenesis.35 The widely accepted “exogenous” theory suggests that early amniotic rupture leads to the formation of pathologic amniotic strands, which then induce nonanatomic fetal abnormalities. The exclusive development of amniotic band syndrome in monozygotic versus dizygotic twin gestations, the description of early amniotic rupture in one sac of a dizygotic twin gestation without subsequent fetal abnormalities, and the paradoxical observations of discordance in monoamniotic and concordance in diamniotic twin gestations, fail to support an “exogenous” etiology for the amniotic band syndrome.35
Finally, clubfeet, dislocated hips and cranial synostosis are deformations associated with constraint and intrauterine crowding that are apparently related to limited space in utero for the affecting one or both fetuses.
ConclusionsMZ twins, though arising from a single zygote and considered genetically identical, will not ever be the same. Genetic, epigenetic and environmental influences will eventually determine both genotypic and phenotypic variation. Discordant MZ twins can be considered a model of immense future potential to study a disease or a genetic defect upon detailed phenotyping. The combination of genetic twin research, databases of twins, pre- and postnatal RNA and DNA complete examination, and functional investigations in twins, namely MZ discordant twins, will contribute more and more to the better understanding of genetic and pathological insights.
Conflict of interest statementNone declared.