Hypertensive disorders of pregnancy, intrauterine growth restriction (IUGR) and preterm delivery are pathologies associated with significant fetal and maternal morbidity and mortality. This study evaluates the usefulness of false positive results for trisomy 21 prenatal screening and isolated changes in biochemical screening markers for the identification of increased risk for those pregnancy complications.
MethodTwo case–control studies were performed: the association of those pathologies with a false positive screening (Integrated or Double) and the distribution of the biochemical markers, PAPP-A, free beta-hCG and alpha-Fetoprotein (AFP), in affected and unaffected pregnant women. The population included all the 4224 pregnant women who underwent prenatal screening for trisomy 21 in Hospital S. Francisco Xavier, Lisbon, between March 2003 and August 2007.
ResultsThe association was significant (P<0.05) between IUGR and false positives for Integrated and 2nd trimester screening and between IUGR and isolated changes in the three biochemical markers. Isolated changes of PAPP-A and AFP were significantly associated with preterm delivery and hypertensive disorders of pregnancy, respectively.
ConclusionScreening false positive results or isolated changes in biochemical screening markers are surrogate markers of increased risk for placental pathology. This information is available before 20 weeks of gestation and can be used in follow-up.
La hipertensión del embarazo, el retraso del crecimiento intrauterino (RCIU) y el parto prematuro son enfermedades asociadas a morbimortalidad maternofetal significativa. Este estudio evalúa la utilidad de los falsos positivos y de los cambios aislados en los marcadores bioquímicos del cribado prenatal de la trisomía 21 para la identificación de un mayor riesgo para estas enfermedades.
MétodoSe hicieron 2 estudios caso-control: uno de la asociación entre los falsos positivos por el cribado (integrado y del segundo trimestre) y aquellas enfermedades y otro de la distribución de los marcadores bioquímicos, PAPP-A, fracción libre de la beta-hCG y alfa-fetoproteína (AFP), en mujeres embarazadas afectadas y no afectadas. La población de estudio incluyó todos los 4.224 cribados realizados en el Hospital S. Francisco Xavier (Lisboa), entre marzo de 2003 y agosto de 2007.
ResultadosLa asociación fue significativa (p<0,05) entre el RCIU y los falsos positivos del cribado integrado y del segundo trimestre y entre el RCIU y los cambios aislados de los 3 marcadores bioquímicos. Los cambios aislados de PAPP-A y AFP se asociaron significativamente con el parto prematuro y la hipertensión del embarazo, respectivamente.
ConclusiónLos falsos positivos en el cribado o cambios aislados en los marcadores bioquímicos son marcadores indirectos de un mayor riesgo de efermedad placentaria. Esta información está disponible antes de las 20 semanas de gestación y puede ser utilizada en el seguimiento del embarazo.
Prenatal screening for trisomy 21 is based on the calculation of the risk associated with maternal age and several biochemical and ultrasound markers. Detection rate reaches 85–90%, but this is achieved with 3–5% false positive rates.1,2
Previous studies3–5 showed an increased incidence of pregnancy complications with direct or indirect involvement of the placenta in false positive screenings. This agrees with the association between placental dysfunction and changes in maternal levels of screening biochemical markers: hCG increases in response to placental hypoxia,6 there is an association between reduction of PAPP-A and fetal growth retardation7,8 and transfer of AFP to the maternal blood is impaired by decreased placental function.9,10
Complications of pregnancy with placental involvement are important factors for fetal and maternal morbidity and mortality. Preterm delivery is the leading cause of perinatal death in Western world, with an incidence between 9 and 12%.11,12 Incidence of IUGR, the second leading cause of perinatal death that accounts for 53% of preterm fetal deaths and 26% of stillbirths at term,13 varies between 2–5%12 and 11%.13 Incidence of hypertensive disorders of pregnancy varies between 5 and 10%11,14 and, in its presentation with proteinuria (preeclampsia), accounts for 17% of maternal deaths15 and is strongly associated with IUGR and preterm delivery.11,15
On the other hand, preventive strategies for preeclampsia and IUGR show good results when adopted early15–18 but screening methods for these conditions are not satisfactory. Risk factors for preeclampsia are unspecific and uterine artery Doppler velocimetry shows good results but only when it is performed after 20 weeks of gestation.19 Uterine artery Doppler velocimetry at 14 weeks of gestation identifies a group at risk for IUGR20 but early prevention appears to be effective only when associated with other risk factors.13 Methods suggested for early detection of preterm delivery are often complex and have limited results.11
Specific biochemical markers, especially for hypertensive disorders of pregnancy and IUGR, have been studied but all have problems of appliance to clinical practice, like the need for additional blood samples, complexity and cost.21–23
In this context, an eventual association between false positive results or isolated changes of biochemical markers in trisomy 21 screening and obstetric complications with placental involvement may be helpful in early recognition of those situations: information is available before 20 weeks of pregnancy and involves no additional blood samples or costs.
This study evaluates the association between false positive results for trisomy 21 screening or isolated changes of PAPP-A, free beta-hCG or AFP and hypertensive disorders of pregnancy, IUGR or preterm delivery events.
MethodsTwo case–control studies were performed. The first one compares the incidence of obstetric complications with placental involvement in false positive screening results and equal number of controls. The second compares the distribution of the biochemical markers’ values, expressed as multiples of the median (MoM), in pregnant women of the first study who developed obstetric complications and equal number of controls. Cases and controls were matched by type of screening, maternal age (within 2 years) and weeks of gestation at the time of blood collection.
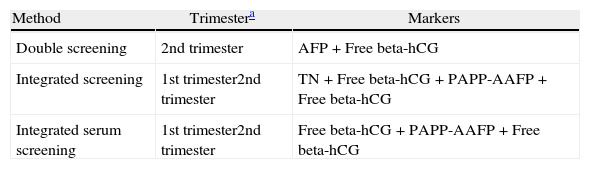
Studies included all pregnant women who underwent screening for trisomy 21 at Hospital S. Francisco Xavier, Lisbon, between March 2003 and August 2007. In this period 4224 complete screenings were made, of which 54% were Integrated screening and 24% were Integrated serum screening. Integrated screening was the first choice and Integrated serum screening was performed when NT measurement was not available. When gestational age exceeded the 13+6 weeks at time of first evaluation, 2nd trimester test was performed. Screening methods used were detailed in Table 1.
Screening methods in use at Hospital S. Francisco Xavier for trisomy 21.
| Method | Trimestera | Markers |
| Double screening | 2nd trimester | AFP+Free beta-hCG |
| Integrated screening | 1st trimester2nd trimester | TN+Free beta-hCG+PAPP-AAFP+Free beta-hCG |
| Integrated serum screening | 1st trimester2nd trimester | Free beta-hCG+PAPP-AAFP+Free beta-hCG |
Conditions studied included hypertensive disorders of pregnancy with and without proteinuria (HTA), preterm delivery (PTD) and intrauterine growth restriction (IUGR), identified by searching hospital diagnostic database, which uses the International Classification of Diseases, 9th Revision (ICD-9) codes.
Biochemical assays were done with TRACE technology (BRAHMS Kryptor Systems) and risk was estimated with ALPHA software (Logical Medical Systems Ltd). Gestational age is estimated by ultrasound measurement of the crown-rump length (CRL). Screening was considered positive for an adjusted risk equal to or greater than 1:300 (at the expected date of delivery).
For statistical analysis of data non-parametric tests was used, the chi square test with Yates correction for continuity for the first study and the U test of Mann and Whitney for the second study. P<0.05 was defined as cut-off for statistical significance.
ResultsMaternal demographic factors of the study groups are displayed in Tables 2 and 3.
Maternal demographic factors in first study case and control women (by type of screening).
| Cases (57) | Controls (57) | |
| Integrated test | ||
| Median age at expected date of delivery (years) | 33.5 | 33.4 |
| Median gestational age at test (weeks+days) | 15+3 | 15+3 |
| Primiparity (%) | 56.4 | 57.9 |
| Cases (60) | Controls (60) | |
| Serum integrated test | ||
| Median age at expected date of delivery (years) | 33.7 | 33.6 |
| Median gestational age at test (weeks+days) | 15+2 | 15+3 |
| Primiparity (%) | 58.9 | 56.8 |
| Cases (62) | Controls (62) | |
| 2nd trimester screening | ||
| Median age at expected date of delivery (years) | 34.0 | 34.0 |
| Median gestational age at test (weeks+days) | 16+4 | 16+4 |
| Primiparity (%) | 60.2 | 58.3 |
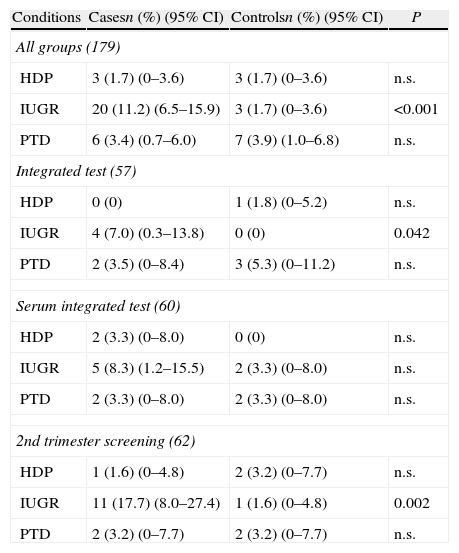
The 179 false positive results in the group of pregnant women who underwent screening for trisomy 21 were included in the first study (Table 4). Regarding all groups, difference between cases and controls was statistically significant in frequency of IUGR. Difference in frequency of IUGR was also significant for Integrated screening and 2nd trimester screening but not for the Integrated serum screening. No differences in frequency of preterm delivery or hypertensive disorders of pregnancy were found.
Frequency of obstetric complications in false positive results for trisomy 21 screening (all groups and by type of screening).
| Conditions | Casesn (%) (95% CI) | Controlsn (%) (95% CI) | P |
| All groups (179) | |||
| HDP | 3 (1.7) (0–3.6) | 3 (1.7) (0–3.6) | n.s. |
| IUGR | 20 (11.2) (6.5–15.9) | 3 (1.7) (0–3.6) | <0.001 |
| PTD | 6 (3.4) (0.7–6.0) | 7 (3.9) (1.0–6.8) | n.s. |
| Integrated test (57) | |||
| HDP | 0 (0) | 1 (1.8) (0–5.2) | n.s. |
| IUGR | 4 (7.0) (0.3–13.8) | 0 (0) | 0.042 |
| PTD | 2 (3.5) (0–8.4) | 3 (5.3) (0–11.2) | n.s. |
| Serum integrated test (60) | |||
| HDP | 2 (3.3) (0–8.0) | 0 (0) | n.s. |
| IUGR | 5 (8.3) (1.2–15.5) | 2 (3.3) (0–8.0) | n.s. |
| PTD | 2 (3.3) (0–8.0) | 2 (3.3) (0–8.0) | n.s. |
| 2nd trimester screening (62) | |||
| HDP | 1 (1.6) (0–4.8) | 2 (3.2) (0–7.7) | n.s. |
| IUGR | 11 (17.7) (8.0–27.4) | 1 (1.6) (0–4.8) | 0.002 |
| PTD | 2 (3.2) (0–7.7) | 2 (3.2) (0–7.7) | n.s. |
HDP: hypertension disorders of pregnancy; IUGR: intrauterine growth restriction; PTD: preterm delivery; CI: confidence interval.
In first study cases and controls there were 42 pregnant women with one or more of the studied conditions, 29 among false positives and 13 in the control group (Table 5). IUGR was the most frequent condition found (23 cases). Differences in distribution of biochemical markers concentration values in maternal blood, expressed as MoM, were statistically significant between cases and controls for IUGR and preterm delivery with PAPP-A, for IUGR alone with beta-hCG free and for IUGR and hypertensive disorders of pregnancy with AFP.
Distribution of MoM values for PAPP-A, free beta-hCG and AFP in cases with obstetric complications and matched controls.
| Conditions | Cases (n) | Cases median (range) | Controls median (range) | P | Variation (vs. trisomy 21) |
| PAPP-A | |||||
| HDP | 3 | 0.39 (0.32–0.83) | 1.25 (0.63–2.20) | n.s. | Similar |
| IUGR | 11 | 0.58 (0.23–2.01) | 1.48 (0.67–2.57) | <0.01 | Similar |
| PTD | 9 | 0.54 (0.35–2.48) | 1.44 (0.71–2.30) | <0.05 | Similar |
| Free beta-hCG | |||||
| HDP | 6 | 1.46 (0.65–1.69) | 1.46 (0.62–3.00) | n.s. | Reverse |
| IUGR | 23 | 1.40 (0.27–4.18) | 1.03 (0.22–6.88) | <0.05 | Similar |
| PTD | 13 | 1.35 (0.44–4.33) | 1.77 (0.44–3.34) | n.s. | Reverse |
| AFP | |||||
| HDP | 6 | 0.90 (0.33–1.39) | 1.26 (1.17–1.52) | <0.05 | Similar |
| IUGR | 23 | 0.81 (0.40–2.01) | 1.08 (0.70–2.02) | <0.01 | Similar |
| PTD | 13 | 0.91 (0.40–1.75) | 1.22 (0.49–1.85) | n.s. | Similar |
HDP: hypertension disorders of pregnancy; IUGR: intrauterine growth restriction; PTD: preterm delivery.
Variation in MoM distribution between cases and controls was similar to variation that occurs in trisomy 21, except for free beta-hCG levels in preterm delivery and hypertension disorders of pregnancy events.
DiscussionIt is a limitation of the study not to consider alternative values of cut-off for screening, which could lead to other results, but the aim of the study was to evaluate the usefulness of screening as it is actually used.
Concerning demographics, no differences in parity were found between false positive results and controls, what agrees with evidence that parity does not affect significantly screening performance. In second study, differences between cases and controls in parity, with greater incidence of placental related complications of pregnancy in primiparous, agree with known risk factors for those conditions.
Association between increased risk for IUGR and a false positive screening result (Integrated or 2nd trimester) agrees with the fact that, in IUGR, there is an equally significant association with changes of the three biochemical markers studied (PAPP-A, beta-hCG free and AFP). Preterm delivery association was only with PAPP-A changes and hypertensive disorders of pregnancy were associated only with changes in AFP, and that may explain why no association was found between those conditions and false positive results for trisomy 21 screening.
These results also agree with what we know about the pathophysiology of those conditions. The association between IUGR and changes verified in all biochemical markers agrees with the fact that IUGR may result from multiple placental and fetal causes, all leading to a situation of early utero-placental impairment.12,13 In hypertensive disorders of pregnancy, placental changes arise just before installation of preeclampsia24 and early preeclampsia (prior to 34 weeks) does not exceed 10% of all cases,15 which may explain why observed association was weak. Preterm delivery's weak association with changes in biochemical markers agrees with later occurrence and heterogeneity of the triggering factors (that includes, in addition to abnormalities of placenta, premature rupture of membranes, chorioamnionitis, uterine abnormalities and fetal pathology).
Moreover, variations in biochemical markers are similar to those occurring in trisomy 21, which supports the association with false positive results of screening. PAPP-A, between 10 and 14 weeks of pregnancy, decreases in trisomy 21 and decreases in hypertensive disease of pregnancy,25,26 IUGR25,27,28 and preterm delivery.25,29 Free beta-hCG increases in trisomy 21 and in hypertensive disease during the 2nd trimester of pregnancy.30 Changes in AFP are described in the literature in association with hypertension and IUGR, although some authors describe an increase in maternal serum,31,32 unlike what happens in trisomy 21, and others refer a decrease in hypertensive disorders.9,10
We can therefore conclude that false positives or isolated changes in biochemical markers of screening for trisomy 21 are surrogate markers of increased risk for placental pathology, especially IUGR and, to a lesser degree, hypertensive disorders of pregnancy and preterm delivery. This information is available at an early stage of pregnancy, before 20 weeks and installation of those conditions, when preventive strategies can be effective. So, a false positive screening for trisomy 21 or isolated changes in biochemical markers should alert the obstetrician to a higher risk of occurrence of those conditions.
Ethical disclosuresProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of Data. The authors declare that no patient data appears in this article.
Right to privacy and informed consent. The authors declare that no patient data appears in this article.
Conflict of interestThe author declares no conflict of interest.