Recent pharmacodynamic and clinical analyses have suggested that proton pump inhibitors (PPIs) may weaken the antiplatelet effects of clopidogrel by involving CYP (cytochrome P450) 2C19 and 3A4. Although acute coronary syndrome (ACS) is common in hemodialysis patients and percutaneous coronary intervention (PCI) is widely performed, little attention has been paid to this point in hemodialysis patients.
Patients and methodsFifty-nine hemodialysis patients (including 28 concomitant users of clopidogrel and PPIs) who had coronary angiography (CAG) were examined in this study. Baseline and follow-up drug use was assessed. Primary outcomes were PCI or coronary artery bypass grafting (CABG) after first CAG.
ResultsIn univariate analysis, observation periods and platelet count between concomitant users (CU: patients with clopidogrel and PPIs) and non-concomitant users A (NCUA: patients with only clopidogrel) and gender, mean age and distribution of multivessel coronary disease between CU and non-concomitant users B (NCUB: NCUA and taking ticlopidine) were significantly different (p<0.05). The cumulative incidence of PCI or CABG after first CAG between CU and NCUA was significantly different (p=0.038 log-rank test) while that between CU and NCUB was not significantly different (p=0.112, log-rank test). The relative hazard (HR) associated with concomitant use of clopidogrel and PPIs for PCI or CABG after CAG between CU and NCUA or CU and NCUB was 1.63 and 1.55 (95% confidence interval (CI): 1.12–2.45, 1.07–2.30), respectively. The other significant factors for PCI or CABG after CAG between CU and NCUA, or NCUB were diabetes mellitus as a cause of renal failure (HR: 1.58, 1.56, CI: 1.08–2.34, 1.06–2.33, respectively).
ConclusionIn hemodialysis patients with serious coronary heart disease treated with clopidogrel and PPIs, the corresponding point estimate for serious cardiovascular events may increase.
En análisis farmacodinámicos y clínicos recientes se ha sugerido que los inhibidores de la bomba de protones (IBP) pueden debilitar los efectos antiagregantes plaquetarios del clopidogrel a través de la participación de las isoenzimas CYP (sistema del citocromo P450) 2C19 y 3A4. Aunque los síndromes coronarios agudos (SCA) son frecuentes en pacientes sometidos a hemodiálisis, y los procedimientos, como la intervención coronaria percutánea (ICP), son de uso difundido, apenas se ha prestado atención a este problema en pacientes sometidos a hemodiálisis.
Pacientes y métodosEn el presente estudio se examinó a 59 pacientes sometidos a hemodiálisis (incluidos 28 usuarios concomitantes de clopidogrel e IBP) en los que se había efectuado una angiografía coronaria (AGC). Se valoró el uso de los fármacos en el período basal y durante el seguimiento. La variable principal analizada fue la ICP o cirugía mediante bypass de la arteria coronaria (CBAC) tras la primera AGC.
ResultadosEn el análisis univariante, los períodos de observación y el recuento de plaquetas entre usuarios concomitantes (UC: pacientes tratados con clopidogrel e IBP) y usuarios no concomitantes A (UNCA: pacientes sólo tratados con clopidogrel) y el sexo, edad media y distribución de la enfermedad coronaria de múltiples vasos entre UC y usuarios no concomitantes B (UNCB: UNCA y tratados con ticlopidina) fueron significativamente diferentes (p<0.05). Entre pacientes UC y UNCA, la incidencia acumulativa de ICP o CBAC tras la primera AGC fue significativamente diferente (p=0.038, prueba del log rank), al contrario que entre pacientes UC y NUCB (p=0.112, prueba del log-rank). El riesgo relativo (RR) asociado al uso concomitante de clopidogrel e IBP para ICP o CBAC tras AGC entre pacientes UC y NUCA o UC y NUCB fue de 1.63 y 1.55 (intervalo de confianza [IC] del 95%: 1.12–2.45, 1.07–2.30), respectivamente. Entre pacientes UC y NUCA, o NUCB, el otro factor significativo para la ICP o CBAC tras AGC fue la diabetes mellitus como causa de insuficiencia renal (RR: 1.58, 1.56, IC: 1.08–2.34, 1.06–2.33, respectivamente).
ConclusiónEn pacientes con coronariopatía grave sometidos a hemodiálisis, tratados con clopidogrel e IBP, la estimación punto correspondiente de acontecimientos cardiovasculares graves puede aumentar.
Acute coronary syndrome (ACS) is a major cause of death in chronic hemodialysis patients.1 Treatment with clopidogrel in addition to aspirin reduces recurrent cardiovascular events following hospitalization for ACS in patients treated either medically or with percutaneous coronary intervention (PCI).2,3 Post-PCI patients on these dual antiplatelet agents are often given proton pump inhibitors (PPIs) with the hope of reducing gastrointestinal bleeding.4 Moreover, it is considered that PPIs are safer than histamine H2 receptor antagonists since plasma concentration of the active form is unchanged in the low glomerular filtration condition.5
Clopidogrel is a pro-drug which is mainly metabolized to its active form by hepatic cytochromes P-450 (CYP) 2C19 and 3A4.6 PPIs, i.e. omeprazole, lansoprazole and rabeprazole, which can be clinically utilized in Japan, are also metabolized to their inactive form by CYP2C19 and 3A4.6 Recent reports indicated that these PPIs may attenuate the benefits of clopidogrel in patients with ACS.7,8 In addition, the recent in vitro studies on post-PCI patients showed that omeprazole reduced the effect on platelet function of clopidogrel.9 However, the clinical relevance of this drug interaction is controversial10 and little attention has been given to this problem in chronic hemodialysis patients. In the present study, the influence of concomitant use of clopidogrel and PPIs on prognosis was assessed in hemodialysis patients.
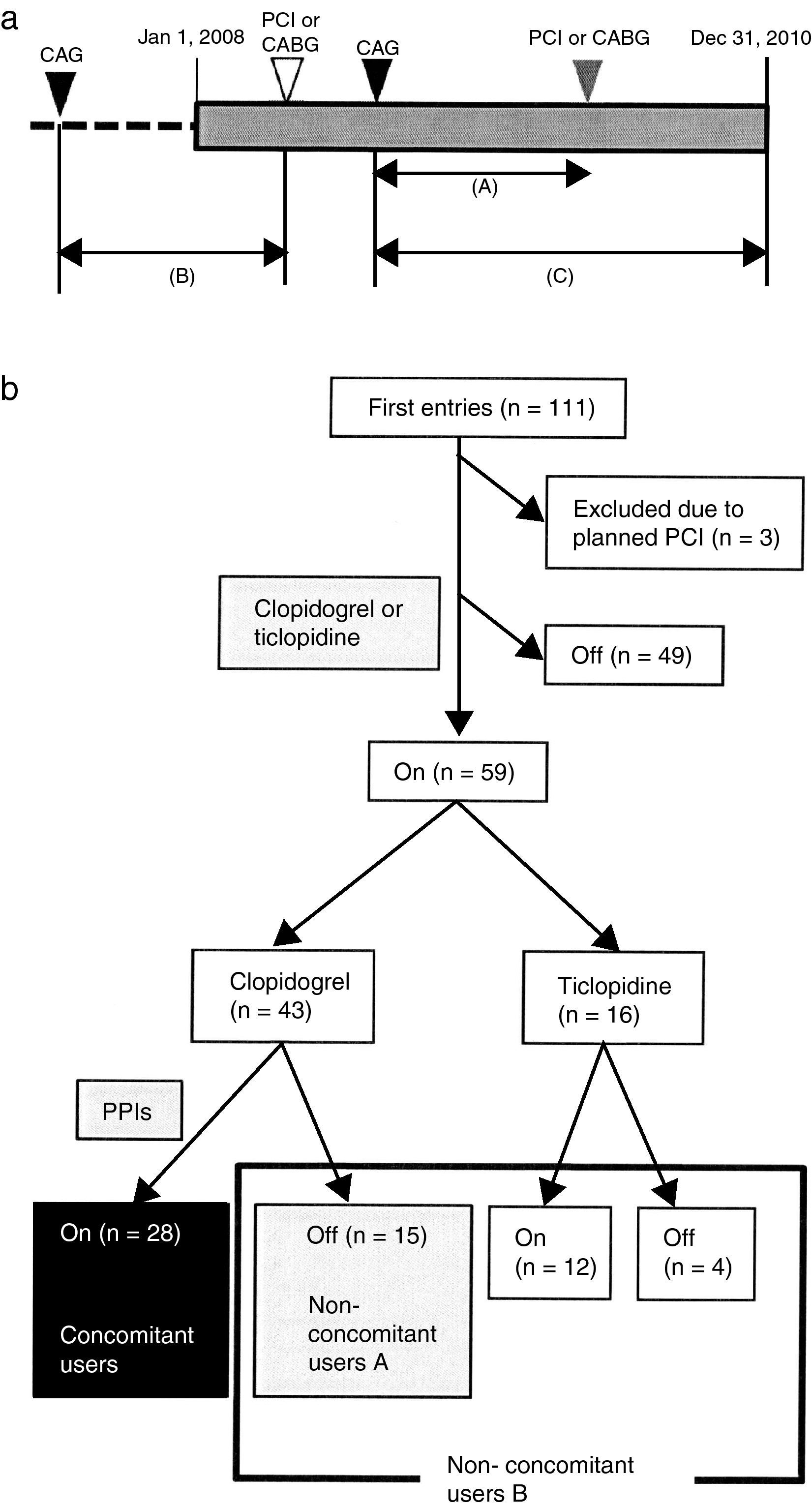
Patients and methodsA retrospective open cohort study was performed from computed medical records in the Juntendo University Hospital. The chronic hemodialysis patients who underwent CAG, PCI or CABG from January 1, 2008 to December 31, 2010 were identified. After CAG, the dates of re-hospitalization due to severe cardiovascular diseases (CVD) until December 31, 2010 were investigated. If there was no record of re-hospitalization, these patients were considered as censored cases. The date of first CAG was retrospectively retrieved for each patient who had a medical record of PCI or CABG. The duration from first CAG to PCI or CABG was determined as the observation period (Fig. 1a).
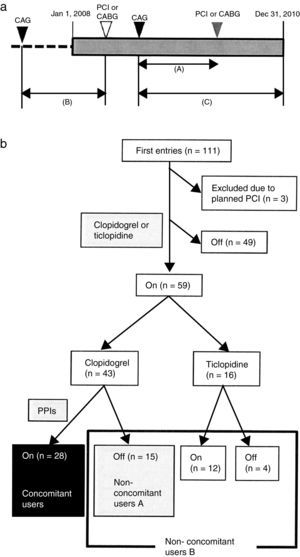
(a) Schematic demonstration of study design. Retrospective open cohort study using computed medical records of Juntendo University Hospital to identify who received coronary angiography (CAG), percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) from January 1, 2008 to December 31, 2010. Primary outcome was PCI or CABG after first CAG. Each observation period was calculated for patients who reached the primary outcome (shown as (A)). Patients who underwent only PCI or CABG from January 1, 2008 to December 31, 2010 were examined for their first CAG retrospectively (shown as (B)). Patients without events after CAG were considered as censored cases (shown as (C)). (b) Grouping of patients. One hundred and eleven patients underwent CAG during the observation period. The number of clopidogrel and PPIs concomitant users was 28 (black box). Non-concomitant users A (n=15) included patients who took clopidogrel without any PPIs (gray box). Non-concomitant users B (n=31) were the patients who were non-concomitant users A and were treated with ticlopidine with or without PPIs (gray and white box surrounded by black line).
Initially, a total of 111 patients were enrolled in this study. Three patients were excluded since re-hospitalization had already been planned when CAG was performed. Forty-nine patients dropped out of the study because of no administration of clopidogrel or ticlopidine. The remaining 59 patients (51 males/8 females) were classified into three groups. The patients who used both clopidogrel and PPIs were concomitant users (CU) (n=28) and those who took clopidogrel without PPIs were non-concomitant users A (NCUA) (n=15). The patients who were administered ticlopidine with or without PPIs and who belong to NCUA composed non-concomitant users B (NCUB) (n=31) (Fig. 1b).
Baseline and follow-up medications, gender, age, cause of renal failure [diabetes mellitus (DM) or non-diabetes mellitus (non-DM)], hemoglobin level, platelet count, serum low density lipoprotein (LDL)-cholesterol, calcium corrected by albumin, phosphorus, aspartate aminotransferase (AST), aminotransferase (ALT) and the distribution of diseased coronary arteries were also examined from computed medical records of the Juntendo University Hospital.
Statistical analysisContinuous variables were reported as mean values±standard deviations (SD) and categorized variables as frequencies. Intergroup univariate analysis was performed with the independent t-test and chi-square test. The cumulative incidence of PCI or CABG after the first CAG among patients who belonged to each category (CU and NCUA or NCUB) was plotted using the Kaplan–Meier method. The differences between the groups were assessed by the log-rank test. Multivariable statistical analysis of the groups was performed using clinical parameters and the patients’ condition including the concomitant use of clopidogrel and PPIs using the Cox proportional hazard analysis. Before performing Cox proportional hazard analysis, multiple regression analysis was performed using same variables and for those whose F-values were over 1.8 were selected Akaike's information criterion (AIC)11 was calculated in each combination of variables and the combination of variables which showed minimum AIC was used for Cox proportional hazard analysis. All statistical calculations were performed using JMP 7 (SAS Institute Inc., Cary, NC, USA). p<0.05 was considered to be statistically significant.
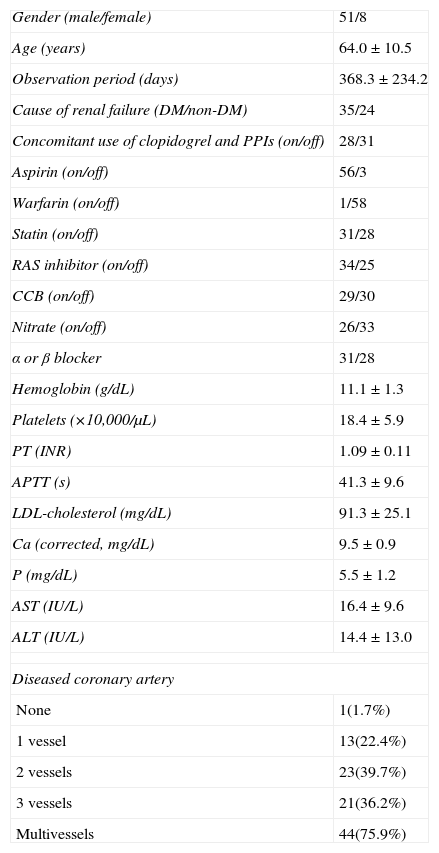
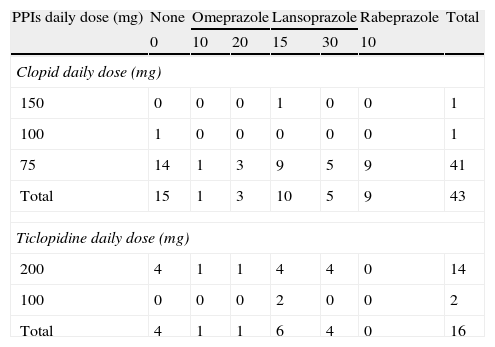
ResultsBaseline characteristicsThe baseline characteristics of the 59 hemodialysis patients who underwent CAG are outlined in Table 1. Detailed drug administration of the patients including distribution of kinds and doses of antiplatelet agents and PPIs are summarized in Table 2. Although over 95% of the patients (41/43) were administered 75mg of clopidogrel daily, the kinds and doses of PPIs were variable (Table 2).
Baseline characteristics of the study population (n=59).
| Gender (male/female) | 51/8 |
| Age (years) | 64.0±10.5 |
| Observation period (days) | 368.3±234.2 |
| Cause of renal failure (DM/non-DM) | 35/24 |
| Concomitant use of clopidogrel and PPIs (on/off) | 28/31 |
| Aspirin (on/off) | 56/3 |
| Warfarin (on/off) | 1/58 |
| Statin (on/off) | 31/28 |
| RAS inhibitor (on/off) | 34/25 |
| CCB (on/off) | 29/30 |
| Nitrate (on/off) | 26/33 |
| α or β blocker | 31/28 |
| Hemoglobin (g/dL) | 11.1±1.3 |
| Platelets (×10,000/μL) | 18.4±5.9 |
| PT (INR) | 1.09±0.11 |
| APTT (s) | 41.3±9.6 |
| LDL-cholesterol (mg/dL) | 91.3±25.1 |
| Ca (corrected, mg/dL) | 9.5±0.9 |
| P (mg/dL) | 5.5±1.2 |
| AST (IU/L) | 16.4±9.6 |
| ALT (IU/L) | 14.4±13.0 |
| Diseased coronary artery | |
| None | 1(1.7%) |
| 1 vessel | 13(22.4%) |
| 2 vessels | 23(39.7%) |
| 3 vessels | 21(36.2%) |
| Multivessels | 44(75.9%) |
Abbreviations: DM, diabetes mellitus; RAS, renin–angiotensin system; CCB, calcium channel blockers; LDL, low density lipoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Distribution of kinds and doses of PPIs among the patients.
| PPIs daily dose (mg) | None | Omeprazole | Lansoprazole | Rabeprazole | Total | ||
| 0 | 10 | 20 | 15 | 30 | 10 | ||
| Clopid daily dose (mg) | |||||||
| 150 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| 100 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| 75 | 14 | 1 | 3 | 9 | 5 | 9 | 41 |
| Total | 15 | 1 | 3 | 10 | 5 | 9 | 43 |
| Ticlopidine daily dose (mg) | |||||||
| 200 | 4 | 1 | 1 | 4 | 4 | 0 | 14 |
| 100 | 0 | 0 | 0 | 2 | 0 | 0 | 2 |
| Total | 4 | 1 | 1 | 6 | 4 | 0 | 16 |
Upper table shows the combination of kinds and doses of clopidogrel and PPIs and the lower shows that of ticlopidine and PPIs.
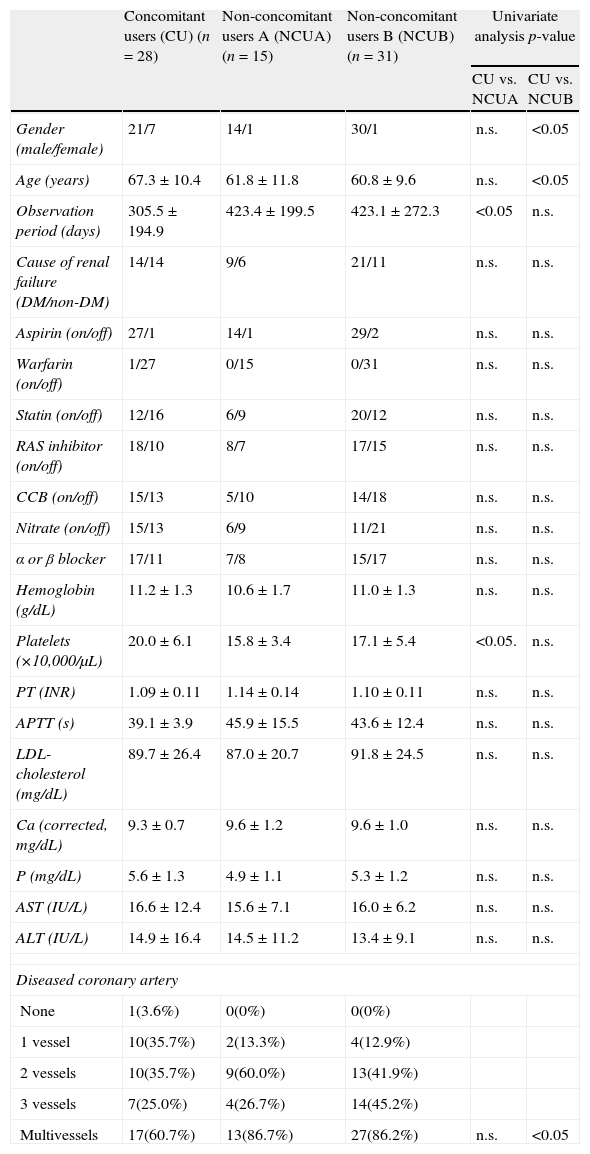
The univariate intergroup analysis concerning the patients’ gender, age, observation period, cause of renal failure, additional drug usage (aspirin, statins, renin–angiotensin system (RAS) inhibitor, calcium channel blocker (CCB) and nitrate), hemoglobin level, platelet count, serum low density lipoprotein (LDL)-cholesterol, serum calcium, serum phosphate, aspartate aminotransferase (AST), alanine aminotransferase (ALT) levels and multivessel coronary artery disease between CU and NCUA or NCUB revealed that the observation period and platelet count were significantly different between CU and NCUA and gender, age and multivessel coronary artery disease were significantly different between CU and NCUB (p<0.05) (Table 3).
Comparison of baseline characteristics between the concomitant users and non-concomitant users A or B.
| Concomitant users (CU) (n=28) | Non-concomitant users A (NCUA) (n=15) | Non-concomitant users B (NCUB) (n=31) | Univariate analysis p-value | ||
| CU vs. NCUA | CU vs. NCUB | ||||
| Gender (male/female) | 21/7 | 14/1 | 30/1 | n.s. | <0.05 |
| Age (years) | 67.3±10.4 | 61.8±11.8 | 60.8±9.6 | n.s. | <0.05 |
| Observation period (days) | 305.5±194.9 | 423.4±199.5 | 423.1±272.3 | <0.05 | n.s. |
| Cause of renal failure (DM/non-DM) | 14/14 | 9/6 | 21/11 | n.s. | n.s. |
| Aspirin (on/off) | 27/1 | 14/1 | 29/2 | n.s. | n.s. |
| Warfarin (on/off) | 1/27 | 0/15 | 0/31 | n.s. | n.s. |
| Statin (on/off) | 12/16 | 6/9 | 20/12 | n.s. | n.s. |
| RAS inhibitor (on/off) | 18/10 | 8/7 | 17/15 | n.s. | n.s. |
| CCB (on/off) | 15/13 | 5/10 | 14/18 | n.s. | n.s. |
| Nitrate (on/off) | 15/13 | 6/9 | 11/21 | n.s. | n.s. |
| α or β blocker | 17/11 | 7/8 | 15/17 | n.s. | n.s. |
| Hemoglobin (g/dL) | 11.2±1.3 | 10.6±1.7 | 11.0±1.3 | n.s. | n.s. |
| Platelets (×10,000/μL) | 20.0±6.1 | 15.8±3.4 | 17.1±5.4 | <0.05. | n.s. |
| PT (INR) | 1.09±0.11 | 1.14±0.14 | 1.10±0.11 | n.s. | n.s. |
| APTT (s) | 39.1±3.9 | 45.9±15.5 | 43.6±12.4 | n.s. | n.s. |
| LDL-cholesterol (mg/dL) | 89.7±26.4 | 87.0±20.7 | 91.8±24.5 | n.s. | n.s. |
| Ca (corrected, mg/dL) | 9.3±0.7 | 9.6±1.2 | 9.6±1.0 | n.s. | n.s. |
| P (mg/dL) | 5.6±1.3 | 4.9±1.1 | 5.3±1.2 | n.s. | n.s. |
| AST (IU/L) | 16.6±12.4 | 15.6±7.1 | 16.0±6.2 | n.s. | n.s. |
| ALT (IU/L) | 14.9±16.4 | 14.5±11.2 | 13.4±9.1 | n.s. | n.s. |
| Diseased coronary artery | |||||
| None | 1(3.6%) | 0(0%) | 0(0%) | ||
| 1 vessel | 10(35.7%) | 2(13.3%) | 4(12.9%) | ||
| 2 vessels | 10(35.7%) | 9(60.0%) | 13(41.9%) | ||
| 3 vessels | 7(25.0%) | 4(26.7%) | 14(45.2%) | ||
| Multivessels | 17(60.7%) | 13(86.7%) | 27(86.2%) | n.s. | <0.05 |
Differences between concomitant users and each group of non-concomitant users are shown as CU versus NCUA/CU versus NCUB.
Abbreviations: CU, concomitant users; NCUA, non-concomitant users A; NCUB, non-concomitant users B; NS, not significant; DM, diabetes mellitus; RAS, renin–angiotensin system; CCB, calcium channel blockers; LDL, low density lipoprotein; PT, prothrombin time; INR, international normalized ratio; APTT, activated thromboplastin time; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
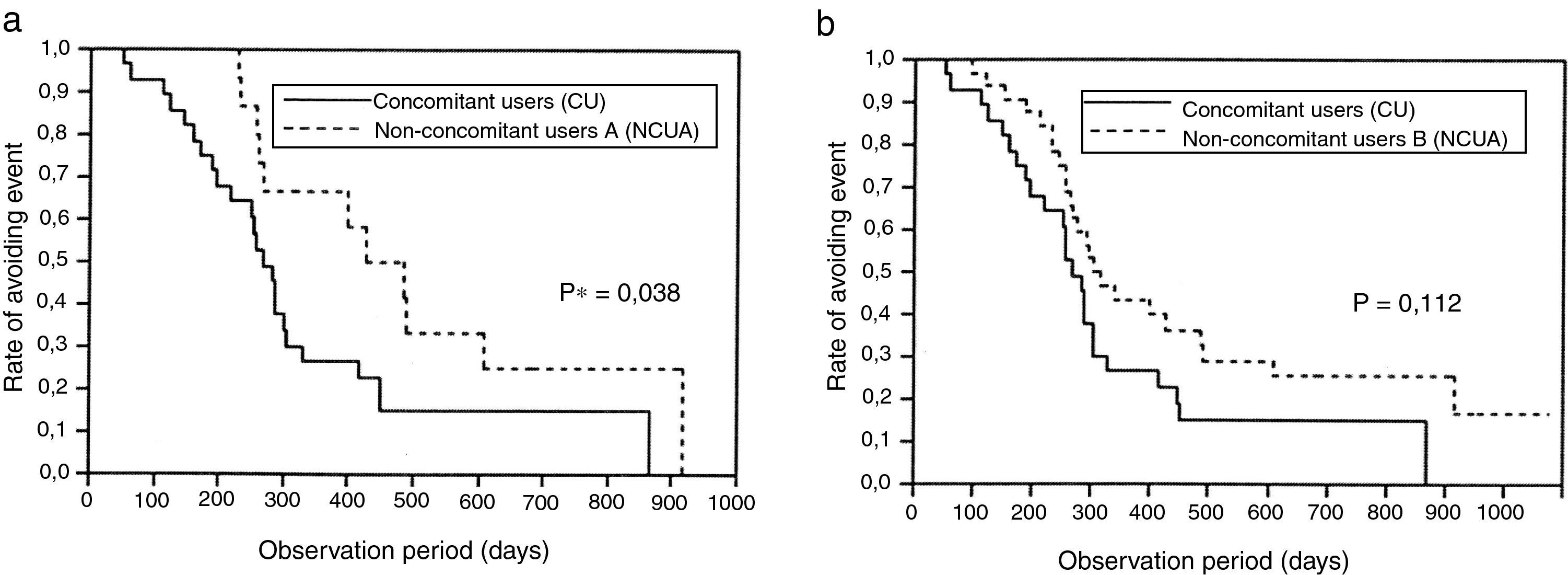
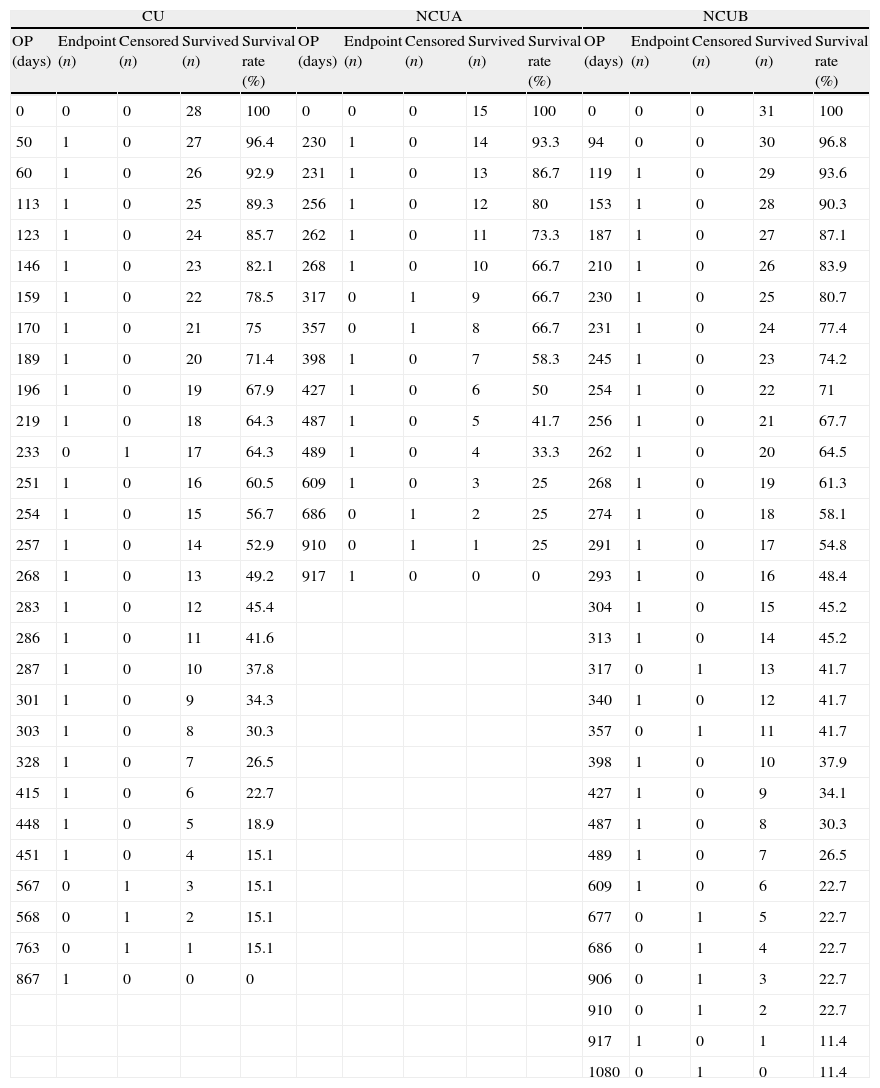
While Kaplan–Meier survival analysis showed that there were no significant differences between CU and NCUB (p=0.112, log-rank test) (Fig. 2b), significant difference between CU and NCUA was observed (p=0.038, log-rank test) (Fig. 2a). The number of the patients at risk and survival rate in any point of time is also shown (Table 4).
(a) Survival analysis between clopidogrel and PPIs concomitant users and non-concomitant users A. A significant difference was observed (p=0.038, log-rank test). (b) Survival analysis between clopidogrel and PPIs concomitant users and non-concomitant users B (p=0.112, log-rank test).
Detail data of Kaplan–Meier survival analysis.
| CU | NCUA | NCUB | ||||||||||||
| OP (days) | Endpoint (n) | Censored (n) | Survived (n) | Survival rate (%) | OP (days) | Endpoint (n) | Censored (n) | Survived (n) | Survival rate (%) | OP (days) | Endpoint (n) | Censored (n) | Survived (n) | Survival rate (%) |
| 0 | 0 | 0 | 28 | 100 | 0 | 0 | 0 | 15 | 100 | 0 | 0 | 0 | 31 | 100 |
| 50 | 1 | 0 | 27 | 96.4 | 230 | 1 | 0 | 14 | 93.3 | 94 | 0 | 0 | 30 | 96.8 |
| 60 | 1 | 0 | 26 | 92.9 | 231 | 1 | 0 | 13 | 86.7 | 119 | 1 | 0 | 29 | 93.6 |
| 113 | 1 | 0 | 25 | 89.3 | 256 | 1 | 0 | 12 | 80 | 153 | 1 | 0 | 28 | 90.3 |
| 123 | 1 | 0 | 24 | 85.7 | 262 | 1 | 0 | 11 | 73.3 | 187 | 1 | 0 | 27 | 87.1 |
| 146 | 1 | 0 | 23 | 82.1 | 268 | 1 | 0 | 10 | 66.7 | 210 | 1 | 0 | 26 | 83.9 |
| 159 | 1 | 0 | 22 | 78.5 | 317 | 0 | 1 | 9 | 66.7 | 230 | 1 | 0 | 25 | 80.7 |
| 170 | 1 | 0 | 21 | 75 | 357 | 0 | 1 | 8 | 66.7 | 231 | 1 | 0 | 24 | 77.4 |
| 189 | 1 | 0 | 20 | 71.4 | 398 | 1 | 0 | 7 | 58.3 | 245 | 1 | 0 | 23 | 74.2 |
| 196 | 1 | 0 | 19 | 67.9 | 427 | 1 | 0 | 6 | 50 | 254 | 1 | 0 | 22 | 71 |
| 219 | 1 | 0 | 18 | 64.3 | 487 | 1 | 0 | 5 | 41.7 | 256 | 1 | 0 | 21 | 67.7 |
| 233 | 0 | 1 | 17 | 64.3 | 489 | 1 | 0 | 4 | 33.3 | 262 | 1 | 0 | 20 | 64.5 |
| 251 | 1 | 0 | 16 | 60.5 | 609 | 1 | 0 | 3 | 25 | 268 | 1 | 0 | 19 | 61.3 |
| 254 | 1 | 0 | 15 | 56.7 | 686 | 0 | 1 | 2 | 25 | 274 | 1 | 0 | 18 | 58.1 |
| 257 | 1 | 0 | 14 | 52.9 | 910 | 0 | 1 | 1 | 25 | 291 | 1 | 0 | 17 | 54.8 |
| 268 | 1 | 0 | 13 | 49.2 | 917 | 1 | 0 | 0 | 0 | 293 | 1 | 0 | 16 | 48.4 |
| 283 | 1 | 0 | 12 | 45.4 | 304 | 1 | 0 | 15 | 45.2 | |||||
| 286 | 1 | 0 | 11 | 41.6 | 313 | 1 | 0 | 14 | 45.2 | |||||
| 287 | 1 | 0 | 10 | 37.8 | 317 | 0 | 1 | 13 | 41.7 | |||||
| 301 | 1 | 0 | 9 | 34.3 | 340 | 1 | 0 | 12 | 41.7 | |||||
| 303 | 1 | 0 | 8 | 30.3 | 357 | 0 | 1 | 11 | 41.7 | |||||
| 328 | 1 | 0 | 7 | 26.5 | 398 | 1 | 0 | 10 | 37.9 | |||||
| 415 | 1 | 0 | 6 | 22.7 | 427 | 1 | 0 | 9 | 34.1 | |||||
| 448 | 1 | 0 | 5 | 18.9 | 487 | 1 | 0 | 8 | 30.3 | |||||
| 451 | 1 | 0 | 4 | 15.1 | 489 | 1 | 0 | 7 | 26.5 | |||||
| 567 | 0 | 1 | 3 | 15.1 | 609 | 1 | 0 | 6 | 22.7 | |||||
| 568 | 0 | 1 | 2 | 15.1 | 677 | 0 | 1 | 5 | 22.7 | |||||
| 763 | 0 | 1 | 1 | 15.1 | 686 | 0 | 1 | 4 | 22.7 | |||||
| 867 | 1 | 0 | 0 | 0 | 906 | 0 | 1 | 3 | 22.7 | |||||
| 910 | 0 | 1 | 2 | 22.7 | ||||||||||
| 917 | 1 | 0 | 1 | 11.4 | ||||||||||
| 1080 | 0 | 1 | 0 | 11.4 | ||||||||||
The number of patients and survival rate of each group in any point of time is shown. Abbreviations: CU, concomitant users; NCUA, non-concomitant users A; NCUB, non-concomitant users B; OP, observation period.
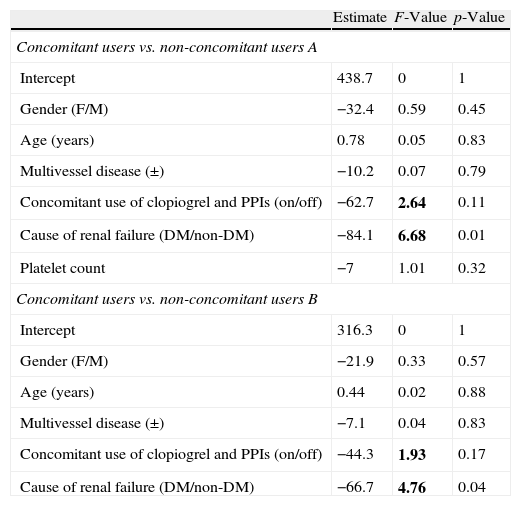
Multiple regression analysis was performed to determine the variables for Cox proportional hazard analysis between CU and NCUA, or NCUB. According to univariate analysis, gender, age, multivessel coronary disease, concomitant use of clopidogrel and PPIs, DM as a cause of renal failure, platelet count was participated between CU and NCUA.
Concomitant use of clopidogrel and PPIs, and DM as a cause of renal failure were selected since each F-value was above 1.8 and other variables were excluded (Table 5, upper). Similar analysis was performed between CU and NCUB and concomitant use of clopidogrel and PPIs, and DM as a cause of renal failure were also selected as possible variables for Cox proportional hazard analysis (Table 5, lower).
Multiple regression analysis.
| Estimate | F-Value | p-Value | |
| Concomitant users vs. non-concomitant users A | |||
| Intercept | 438.7 | 0 | 1 |
| Gender (F/M) | −32.4 | 0.59 | 0.45 |
| Age (years) | 0.78 | 0.05 | 0.83 |
| Multivessel disease (±) | −10.2 | 0.07 | 0.79 |
| Concomitant use of clopiogrel and PPIs (on/off) | −62.7 | 2.64 | 0.11 |
| Cause of renal failure (DM/non-DM) | −84.1 | 6.68 | 0.01 |
| Platelet count | −7 | 1.01 | 0.32 |
| Concomitant users vs. non-concomitant users B | |||
| Intercept | 316.3 | 0 | 1 |
| Gender (F/M) | −21.9 | 0.33 | 0.57 |
| Age (years) | 0.44 | 0.02 | 0.88 |
| Multivessel disease (±) | −7.1 | 0.04 | 0.83 |
| Concomitant use of clopiogrel and PPIs (on/off) | −44.3 | 1.93 | 0.17 |
| Cause of renal failure (DM/non-DM) | −66.7 | 4.76 | 0.04 |
Multiple regression analysis was performed to determine the variables for Cox proportional hazards analysis. Each variable whose F-value was above 1.8 was selected for calculation of Akaike's information criterion (AIC) (bold). Upper: CU vs. NCUA. Lower: CU vs. NCUB.
Abbreviations: CU, concomitant users; NCUA, non-concomitant users A; NCUB, non-concomitant users B; PPIs, proton pump inhibitors; DM, diabetes mellitus.
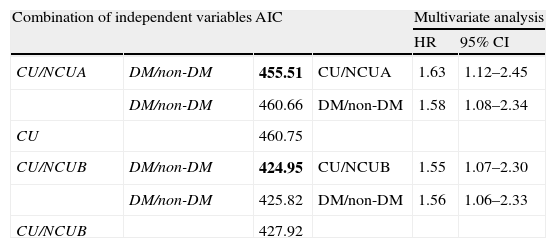
Akaike's information criterion (AIC) was calculated in each combination of possible variables (Table 6, upper left and lower left). The minimum AIC was calculated by the combination of concomitant use of clopidogrel and PPIs and DM as a cause of renal failure between CU and NCUA, or NCUB (Table 6, upper left and lower left). This result indicates that both variables should be used for the best model in Cox proportional hazard analysis.
The statistical model identification according to Akaike's information criterion (AIC) and multivariable analysis by Cox proportional hazards model.
| Combination of independent variables | AIC | Multivariate analysis | |||
| HR | 95% CI | ||||
| CU/NCUA | DM/non-DM | 455.51 | CU/NCUA | 1.63 | 1.12–2.45 |
| DM/non-DM | 460.66 | DM/non-DM | 1.58 | 1.08–2.34 | |
| CU | 460.75 | ||||
| CU/NCUB | DM/non-DM | 424.95 | CU/NCUB | 1.55 | 1.07–2.30 |
| DM/non-DM | 425.82 | DM/non-DM | 1.56 | 1.06–2.33 | |
| CU/NCUB | 427.92 | ||||
AIC was calculated at each step, using the possible risk factors selected by multiple regression analysis (Table 5). The minimum AIC (bold) was obtained by the combination with diabetes mellitus as a cause of renal failure (DM) and concomitant use of clopidogrel and PPIs. The multivariate analysis by Cox proportional hazard analysis was performed by the both variables.
Upper left: AIC of the CU vs. NCUA. Upper right: relative hazards and 95% CI of the possible risk factors of CU vs. NCUA. Lower left: AIC of the CU vs. NCUA. Lower right: relative hazards and 95% CI of the possible risk factors of CU vs. NCUB.
Abbreviations: HR, hazard ratio; CI, confidence interval.
Cox proportional hazard analysis between CU and NCUA revealed that concomitant use of clopidogrel and PPIs, and DM as a cause of renal failure was independent risk factor for PCI or CABG after CAG in hemodialysis patients (hazard ratio (HR): 1.63, 95% confidence interval (CI): 1.12–2.45; HR: 1.58, 95% CI: 1.08–2.34, respectively) (Table 6, upper right). Between CU and NCUB, each of these factors (concomitant use of clopidogrel and PPIs and DM as a cause of renal failure) could be significantly independent risk for PCI or CABG after first CAG (HR: 1.55, 95% CI: 1.07–2.30; HR 1.56, 95% CI: 1.06–2.33, respectively) (Table 6, lower right).
DiscussionReports about drug interactions between clopidogrel and PPIs showed a possibility to worsen the prognosis of the cornary heart disease patients who are taking both drugs.7–9 While most previous pharmacodynamic studies showed attenuation of clopidogrel activity by PPIs, clinical outcomes by the drug interaction are unclear.10 These findings suggested that drug interactions between clopidogrel and PPIs actually occur. However, their influences for clinical outcomes depend on the patients’ background.
In this study, cumulative incidences of PCI or CABG after first CAG in CU were significantly higher than NCUA (Fig. 2a). Cumulative incidences for PCI or CABG after first CAG between CUA and NCUB were not significantly different although the Kaplan–Meier curve suggested difference (Fig. 2b). The multiple regression analysis suggested that the significantly different variables, gender, age and multivessel coronary disease between CU and NCUB in univariate intergroup analysis were not risk factors for PCI or CABG after CAG in hemodialysis patients (Table 6). The pharmacological effect of this dose is equivalent of 75mg of clopidogrel.12 The 88% (14/16) of the ticlopidine users were administered 200mg daily. The possible reason of this discrepancy is considered as the effect of the long survivor in CU and NCUB and censored cases in both groups in log-rank test.13 The difference between CU and NCUB would be observed if more patients were attended in this study.
Concomitant use of clopidogrel and PPIs was retrieved as an independent risk factor for PCI or CABG after CAG in hemodialysis patients by the Cox proportional hazard analysis. First, we tried multiple regression analysis to examine the reliability of the each clinical variable as the risk of the PCI or CAG. The variables with F-value above 1.8 were selected as possible risk factors. AIC provides a means for comparison among statistical models, a tool for model selection and the preferred model is the one with the minimum AIC value, given a set of candidate models for data.11 Since AIC values of the prognostic factors can be compared across different models,14 we adopted AIC for the next Cox proportional hazard analysis for treatment and prognostic effects with censored survival cases and assumption of constant hazard ratio.
As another independent risk factor, DM as a cause of renal failure was rose by Cox proportional hazard analysis. Because of proatherosclerotic, proinflammatory and prothrombotic states associated with DM, diabetic patients with ACS are at higher risk of subsequent cardiovascular events.15 Similar analysis in Japanese population without renal failure, the independent predictor of high on treat platelet activity (HPR) was concomitant use of thienopyridine derivatives including clopidogrel and PPIs, DM and calcium channel blocker use.16 Another investigation revealed that HPR was more frequently observed in DM compared to non-DM and HPR was independent predictor of myocardial infarction.17
Hemodialysis-specific factors might influence the drug interaction between clopidogrel and PPIs. A report on the pharmacokinetics of clopidogrel given to patients with renal function impairment showed that the plasma concentration–time curve from 0 to 24h (AUC(0–24)) differed between the moderately impaired and severely impaired groups, while platelet aggregation was equally inhibited.18 This finding suggests that the pharmacokinetics of clopidogrel is influenced to some extent by renal function impairment. PPIs are one of the drugs for which it is not necessary to reduce doses for patients with renal impairment since the active form is not excreted in the urine.19 Since plasma protein binding of clopidogrel and PPIs is very high (>90%), only small amounts of both drugs can be eliminated by hemodialysis.20 However, it is possible that their kinetics is changed due to enlargement of the distribution volume (Vd) by edema or metabolic acidosis.21
There are few studies on drug interactions or pharmacokinetic changes under uremic environments.22 Basic pharmacokinetic research and prospective randomized clinical studies will be necessary to solve this problem in the future.
LimitationsThis is a small retrospective cohort study based on data from a single center.
ConclusionSince the corresponding point estimate for serious cardiovascular diseases is possibly increased in hemodialysis patients with coronary heart disease when they are treated with clopidogrel and PPIs, more attention should be paid if concomitant use of these drugs is necessary.
Conflict of interestNone declared.
This study was supported partly by grants from Ministry of Education, Culture, Sports, Science and Technology of Japan and Juntendo University. The authors are grateful to the nurses, medical technicians and secretarial staff in the Blood Purification Center of Juntendo University Hospital.