Exercise can induce a transient increase in urinary protein excretion. The aim of this study was to determine the effects of different intensities of aerobic exercise on proteinuria in hypoxia and normoxia conditions in young football players.
MethodsTen eligible male football players (age: 18±0.5 years, weight: 64.42±3.64kg, height: 174±4cm) enrolled in the study. They performed six sessions of aerobic activities consisting of 30-min running with three different intensities of 55, 70 and 85% of maximal heart rate in both hypoxia and normoxia conditions. Pre- and post-exercise urine samples were taken to measure total protein, albumin, β2-microglobulin and creatinine. All data analyses were performed using the SPSS v.16 and analysis of variance and t-paired tests were also applied.
ResultsBy increasing exercise intensity, the protein content of urine increased particularly after achievement of 70% maximal heart rate (MHR). However, urinary β2-microglobulin significantly increased at 85% of MHR.
ConclusionIt seems that heavy activities can induce tubular proteinuria (β2-microglobulinuria) while mild to moderate intensity exercises may result in glomerular proteinuria (albuminuria) which is significantly evident at intensity of 70% of MHR. It is concluded that aerobic exercise at the height of 2500m above sea may have no effect on proteinuria, compared to lower elevations.
El ejercicio puede inducir un incremento transitorio de la excreción urinaria de proteínas. El objetivo del presente estudio fue determinar los efectos de diferentes intensidades de ejercicio aeróbico sobre la proteinuria en condiciones de hipoxia y normoxia en futbolistas jóvenes.
MétodosSe reclutaron en el estudio 10 futbolistas de sexo masculino (edad: 18±0,5 años; peso: 64,42±3,64kg; estatura: 174±4cm). Efectuaron seis sesiones de actividades aeróbicas que consistieron en una carrera de 30min con tres intensidades diferentes del 55, 70 y 85% de la frecuencia cardíaca máxima en condiciones tanto de hipoxia como de normoxia. Se obtuvieron muestras de orina pre y postejercicio para la determinación del contenido total de proteínas, albúmina, β2-microglobulina y creatinina. Todos los análisis de los datos se efectuaron usando el programa SPSS v.16 y también se aplicaron un análisis de la varianza y pruebas de la t para datos emparejados.
ResultadosCon el incremento de la intensidad del ejercicio, el contenido total de proteínas aumentó, en particular tras alcanzar el 70% de la frecuencia cardíaca máxima (FCM). No obstante, los valores de β2-microglobulina urinaria aumentaron significativamente con el 85% de la FCM.
ConclusiónParece ser que las actividades físicas extenuantes pueden inducir una proteinuria tubular (β2-microglobulinuria), mientras que el ejercicio de baja o moderada intensidad puede dar lugar a una proteinuria glomerular (albuminuria), que es evidente significativamente con una intensidad del 70% de la FCM. Se concluye que el ejercicio aeróbico, practicado a una altura de 2.500 m por encima del nivel del mar, podría carecer de efectos sobre la proteinuria, comparado con menores elevaciones.
Large proteins, such as globulins and albumin cannot pass through the glomerular membrane (GM) and therefore, are detected in very small amounts in urine, unless glomerular damage results in glomerular proteinuria.1 Although, low molecular weight proteins including β2-microglobulin easily pass through the GM; however, effective tubular reabsorption leads to their scanty urinary levels.1,2 Proteinuria can be increased following heavy physical activities.3 Sport related proteinuria (SRP) was first reported in soldiers who had hard physical exertion in 1878.4 Renal hemodynamic and glomerular membrane permeability alterations are considered as major causes of SRP.5 The intensity of exercise has a determinant role on the pattern of proteinuria, so that moderate and heavy exercises can induce glomerular and mixed (glomerulotubular) type proteinuria, respectively.6,7 Exercise decreases the renal blood flow (RBF) in proportion to its intensity which finally results in decreased glomerular filtration rate (GFR). Whereas, decline of GFR is smaller than RBF reduction, the filtration fraction increases and large proteins can more easily pass through the GM. It is suggested that sympathetic stimulation and endocrine systems, through the adrenergic induced renal artery narrowing and renin–angiotensin system, play an important role in this process.7–10 In addition, loss of capillary wall negative charge due to variations of renal sialic acids and resultant reduced glomerular electrostatic resistance may justify part of more passage of macromolecules through the GM.7,8,11
Alyea and Parish reported different degrees of proteinuria following either contact or noncontact sport activities including football playing.12 Furthermore, proteinuria may be influenced by a variety of environmental factors such as high altitude-induced hypoxia. While the effects of hypoxia on proteinuria have attracted the attention of a few researchers, the effect of low oxygen pressure at altitudes is not well established yet. Some researchers have suggested significant increase in proteinuria under hypoxia conditions13 while others have not.14
The purpose of the present study was to investigate the effects of different intensities of aerobic exercise on proteinuria in both hypoxia and normoxia conditions in young football players.
Materials and methodsSubjectsThe study sample consisted of 10 young male soccer players whose physical characteristics are presented in Table 1. The participants had playing experience in the premier league at the Fars province of Iran, in the last three years. After announcing call in the premier league and elaborating the study purposes, 10 eligible (based on medical history, examinations and laboratory tests) soccer players who had informed consent were selected. The subjects were otherwise healthy and non-smokers. There were no history of renal or other organ disorders and surgical or medical treatment during prior 6 months. They had regular practice at least 3 days per week. All data, including demographic information, were collected by a questionnaire.
Exercise programTwo days before the initiation of the study, aerobic power of the players was measured, using Bruce test on treadmill, and thereafter they met in the first exercise session.15 The exercise program was composed of six sessions of aerobic exercise consisting 30-min running on treadmill with three different intensities (55, 70, and 85% of maximal heart rate) in two distinct conditions (normoxia and hypoxia). Maximal heart rate (MHR) was calculated by the equation of 208−(0.7×age).16 The sessions were interfered with 48-h resting periods and to avoid misleading results, the order of sessions was chosen on a random basis. Indeed, the exercise sessions were conducted either in normoxia [partial pressure of inhaled oxygen (PIO2) 141mmHg] or in normobaric hypoxia (PIO2 117mmHg). Hypoxia was obtained by reduction to 15.5% of the inspired oxygen fraction. This corresponds to a PIO2 equivalent to that at 2500m height.17 During the study, the players were accommodated in a camp and were asked to rest, avoid further physical activity and refuse taking meals enriched of fat, proteins, and caffeine, at nights before sampling days. They should drink enough water before and after each exercise session to avoid dehydration. All protocols were approved by the Graduate Council of Faculty of Physical Education and Sport Sciences of Islamic Azad University.
Urine samples and analysesUrine samples were taken before and 20min after each exercise session. The samples were measured for main proteinuria indices including: total protein (TP), albumin (glomerular proteinuria), β2-microglobulin (tubular proteinuria) and creatinine. Urinary total protein; albumin; and β2-microglobulin were assayed, using American Diasorin Kit (with a sensitivity of 1μg/dL; 3mg/L; and 0.12mg/L), by Elisa Comasi-blue (Brad Ford); immunoturbidometric; and chemiluminescense methods, respectively.
Statistical methodsThe Kolmogorov-Smirnov test was applied for testing each variable's normality. Continuous variables were presented as “mean±SD” if normally distributed. Analysis of variance (ANOVA) with repeated measurements for the evaluation of variability among the six exercise sessions was used. In case of observing significant difference, in order to reduce errors, the paired-samples t-test with Bonferroni amendment was also utilized. Dependent t-test was applied to check the variability within each of the exercise sessions. All analyses were performed with SPSS, version 16.0 (SPSS Inc., Chicago, IL, USA). P-values <0.05 were considered to be significant.
ResultsTable 1 shows physical characteristics of the study population. The mean values of pre- and post-exercise urinary total protein, albumin, β2-microglobulin and protein/ creatinine ratio (after ending of 6 exercise sessions) have been summarized in Tables 2 and 3.
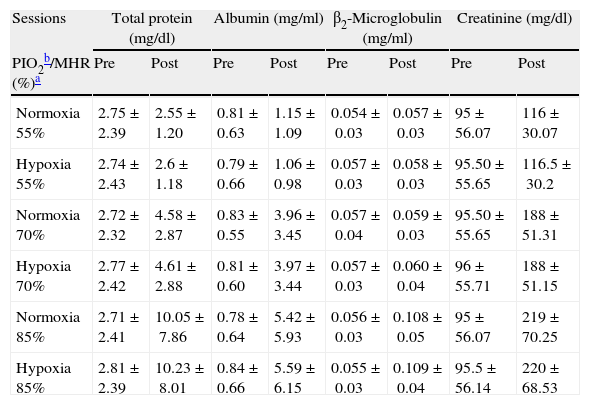
Pre- and post-exercise urinary proteins following three different intensities of aerobic exercise in normoxia and hypoxia conditions.
| Sessions | Total protein (mg/dl) | Albumin (mg/ml) | β2-Microglobulin (mg/ml) | Creatinine (mg/dl) | ||||
| PIO2b/MHR (%)a | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| Normoxia 55% | 2.75±2.39 | 2.55±1.20 | 0.81±0.63 | 1.15±1.09 | 0.054±0.03 | 0.057±0.03 | 95±56.07 | 116±30.07 |
| Hypoxia 55% | 2.74±2.43 | 2.6±1.18 | 0.79±0.66 | 1.06±0.98 | 0.057±0.03 | 0.058±0.03 | 95.50±55.65 | 116.5±30.2 |
| Normoxia 70% | 2.72±2.32 | 4.58±2.87 | 0.83±0.55 | 3.96±3.45 | 0.057±0.04 | 0.059±0.03 | 95.50±55.65 | 188±51.31 |
| Hypoxia 70% | 2.77±2.42 | 4.61±2.88 | 0.81±0.60 | 3.97±3.44 | 0.057±0.03 | 0.060±0.04 | 96±55.71 | 188±51.15 |
| Normoxia 85% | 2.71±2.41 | 10.05±7.86 | 0.78±0.64 | 5.42±5.93 | 0.056±0.03 | 0.108±0.05 | 95±56.07 | 219±70.25 |
| Hypoxia 85% | 2.81±2.39 | 10.23±8.01 | 0.84±0.66 | 5.59±6.15 | 0.055±0.03 | 0.109±0.04 | 95.5±56.14 | 220±68.53 |
Values are mean±SD.
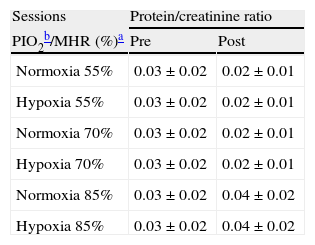
Pre- and post-exercise protein/creatinine ratios following three different intensities of aerobic exercise in normoxia and hypoxia conditions.
| Sessions | Protein/creatinine ratio | |
| PIO2b/MHR (%)a | Pre | Post |
| Normoxia 55% | 0.03±0.02 | 0.02±0.01 |
| Hypoxia 55% | 0.03±0.02 | 0.02±0.01 |
| Normoxia 70% | 0.03±0.02 | 0.02±0.01 |
| Hypoxia 70% | 0.03±0.02 | 0.02±0.01 |
| Normoxia 85% | 0.03±0.02 | 0.04±0.02 |
| Hypoxia 85% | 0.03±0.02 | 0.04±0.02 |
Values are mean±SD.
This study revealed significant elevation of post-exercise urinary total protein, albumin, and β2-microglobulin levels, compared to basal values, at the ending of six exercise sessions (P=0.015, 0.019, and 0.043, respectively). Indeed, protein content (total protein and albumin) of the urine significantly correlated to the exercise intensity when 70% of MHR was achieved and thereafter. However, urinary β2-microglobulin significantly increased only at 85% of MHR. Moreover, no significant difference was detected between hypoxia and normoxia conditions at 55% (P=0.273), 70% (P=0.081), and 85% (P=0.081) of MHR.
DiscussionAlthough, in the absence of renal diseases, SRP is considered as a benign and reversible disorder but there is little information regarding the effects of different intensities and duration of various exercises on renal performance in long term.18–20 Poortmans and Vancalck reported excretion of proteins (TP and albumin) even after short strenuous exercise and other researchers also confirmed this finding.20,21
This study showed a positive correlation between exercise intensity and SRP (glomerular type) which was significant at the level of 70% and 85% of MHR. Poortmans and Labilloy also reported that the post-exercise proteinuria is more related to activity intensity.22 This conclusion was then confirmed by other researchers.18–20,22,23 Indeed, light aerobic activity at 55% of MHR had also increased albuminuria in our study, but insignificantly. It has been suggested that following light physical activities, increased proteinuria may observe in some unhealthy such as diabetic and sedentary people which confirms our study findings on healthy sportsmen.22
Furthermore, exercise intensity had a determinant role in increasing levels of urinary β2-microglobulin (tubular proteinuria) at 85% of MHR which resulted in mixed (glomerulotubular) proteinuria. Poortmans and Vancalck and Montelpare et al. reported that periodic physical load increases β2-microglobulinuria which indicated that post-exercise proteinuria has also a tubular origin.20,24 Exercise induced β2-microglobulinuria has been accompanied with increasing blood lactate and consecutively decreasing blood PH that may change glomerular permeability and also prevent tubular absorption.25–27 On the other hand, under resting conditions more than 95% of filtered proteins are reabsorbed by proximal tubular cells and converted to amino acids. Exercise induced amino acid overload can prevent protein reabsorption as a result of absorption capacity completion.28
Interestingly, in the present study, hypoxia conditions equivalent to an altitude of 2500m above sea level caused a slight increase in post-exercise proteinuria which was not statistically significant. This finding is opposite to Winterborn et al. study that indicates significant increase in albuminuria, in close relation to hypoxia degree, following the climbing to high elevations.13 This confliction may be attributed to the different target altitudes of two studies (3000m above sea level vs. 2500). However, Soylu et al. could not also find significant proteinuria at high altitudes (1200m above sea). They suggested that the study should be performed at higher altitudes.14 Hypoxia induced proteinuria may be related to increased capillary permeability along with the effects of hypoxia resulting in a larger filtration load.13 Hypoxia is a common pathway in various renal diseases that results in increased glomerular permeability and eventually proteinuria.29–31 Another explanation for hypoxia induced proteinuria is the prevention of tubular reabsorption of urinary proteins. This hypothesis is empowered by the presence of low molecular weight proteins such as β2-microglobulin, which are completely reabsorbed in proximal tubule under normal conditions.13 In Pines’ study, different altitudes were applied (up to 5890m) which the mean protein urine concentration in morning specimens was over 100mg/100ml after climbs during the first 12 days but fell to 15mg/100ml during subsequent climbs. They suggested that adaptation of kidneys to new environmental conditions may have a contributory role in such finding. Indeed, proteinuria was a definite response to height and not to exercise, for the most strenuous and exhausting periods of the trip were at heights up to 3000m.32 From the above, it is postulated that proteinuria may appear at least at the height of 3000m above sea level, as some references define that high altitude begins at 2400m (8000ft) above sea level.33 This issue needs more extensive investigation regarding target altitude, duration and frequency of hypoxia exposure and continuity of proteinuria during exposure.
ConclusionIt seems that the amount and the pattern of sport related proteinuria closely correlate to exercise intensity. Light to moderate exercises can induce glomerular proteinuria while heavy exercise may result in mixed proteinuria with the glomerular fraction predominance. Different intensities of exercise with a PIO2 equivalent to that at 2500m altitude could not significantly change the amount and type of post-exercise proteinuria in our study. We recommend more researches surrounding the hypoxia induced proteinuria of different altitudes, particularly at elevations higher than 2500m above sea.
In addition, it is suggested that young professional athletes (particularly heavy exercises) carefully consider their dietary protein content and have a regular referral for urine examinations. However, post-exercise proteinuria could not be a limiting factor for physical activities.
Conflict of interestThe authors have no conflict of interest to declare.