El fracaso renal agudo está asociado a diferentes cuadros clínicos, especialmente los relacionados con sepsis, insuficiencia hepática, accidentes cerebrovasculares e insuficiencia cardíaca aguda descompensada. La hemodiálisis y la hemodialfiltración continuas ofrecen la oportunidad de asistir en la terapia de las condiciones descritas no sólo por su efecto terapéutico en el manejo del volumen extracelular, sino también en el control de la homeostasis de elecrolitos y del balance ácido-base, mediante mecanismos de difusión y convección. Esta revisión pretende mostrar, de un modo didáctico y sucinto, los efectos y beneficios clínicos del uso de las diferentes técnicas de depuración extracorpóreas en las patologías derivadas de la sepsis, la insuficiencia hepática, los accidentes cerebrovasculares y la insuficiencia cardíaca descompensada.
© 2009 SEDYT. Publicado por Elsevier España, S.L. Todos los derechos reservados.
Acute kidney injury is associated with various clinical conditions, especially sepsis, liver disease, stroke and acute decompensated heart failure. Continuous renal replacement therapy is useful in the treatment of these conditions because of its effects on volume management and the control of distinct acid-base and electrolyte abnormalities through diffusion and convection. The present review attempts to comprehensively describe the effects and benefits of distinct extracorporeal techniques in sepsis, liver failure, acute neurological injury, and decompensated heart failure.
© 2009 SEDYT. Published by Elsevier España, S.L. All rights reserved.
Acute kidney injury (AKI) aflicts at least 20-30% of patients admitted to the intensive care unit (ICU) and is associated with a substantial morbidity and mortality1. Continuous renal replacement therapies (CRRT) provide extracorporeal treatment of these hemodinamically unstable, critically ill patients, who are hypercatabolic and fluid overloaded2. The proliferation of modalities of renal replacement therapy (RRT) over the past three decades have provided nephrologist and intensivists with an increasing selection of options for managing renal support3. A recent international survey showed that 80% of patients with AKI in the ICU are currently treated with continues therapies, 17% with intermittent therapies and 3% with peritoneal dialysis or slow continous ultrafiltration4. These novel techniques of renal substitution therapy have permitted a conceptual shift from renal “replacement” to renal “support” therapies5, whereby the strategies to treat AKI have become an integral part of overall critically ill patient management, with “renal” and “nonrenal” applications such as sepsis, acute respiratory distress syndrome (ARDS), acute decompensated heart failure and liver disease. CRRT techniques commonly use three types of depurative mechanisms: convection, diffusion, and membrane adsorption. The simultaneous infusion of replacement fluid permits fluid removal without intravascular volume contraction, metabolic control to almost normal parameters, and removal of large size toxins and cytokines. The role of CRRT in sepsis and multiorgan failure (MOF) can be seen from two major aspects, first from the point of RRT per se and second as an immunomodulatory tool helping to influence the systemic consequences of severe sepsis and septic shock6.
From the liver disease standpoint of view, RRT represents and integral part of the general supportive management of these patients, allowing regulation of acid-base balance, and control of sodium, water, divalent ion fluxes and nutritional support7. In the acute neurological patient, the use of continous modes of renal replacement therapy have been shown to cause fewer surges in intracranial pressure and greater stability of cerebral perfusion pressure than standard intermittent techniques8. Finally, CRRT also represents a potential extracorporeal option to unload the failing heart and restore cardiac and renal function9.
CRRT in the septic patient with multiorgan failure (MOF)Sepsis is characterized by a systemic inflammatory reaction which involves complex interactions between endothelial cells, platelets, leukocytes, coagulation system, and multiple inflammatory mediators. Mortality rates can reach 50% to 70% respectively, once it evolves into severe sepsis, septic shock and MOF10. Severe sepsis and septic shock are associated with AKI in 5-50% of the patients and the risk increases with positive blood cultures and worsening clinical signs of sepsis11. Mortality from septic shock in combination with AKI is 75%. The role of CRRT in sepsis and MOF can be seen from two major aspects, first from the point of RRT and second as an immunomodulatory tool helping to influence the systemic consequences of severe sepsis and septic shock.
The indications for CRRT in sepsis-associated AKI are not really different from the other forms of AKI. However, septic patients in the ICU do not show prominent azotemia when developing AKI. Because of this some authors suggest starting CRRT earlier in order to provide immunomodulation in addition to replacement of renal function. Therefore, other criteria such as prolonged oliguria or severe metabolic acidosis have been suggested as suficient indication to start RRT12. This hypothesis is only supported by retrospective cohort studies13. CRRT is considered the favored RRT modality in patients with septic shock because of better hemodynamic tolerability than intermittent hemodialysis (IHD)14. Although there are some retrospective trials supporting early initiation of continous veno-venous hemofiltration(CVVH) the question of its benefit has only been investigated in one trail that included surgical patients with low incidence of sepsis not showing improve survival15.
Regardings the issue of CRRT dosing, it was assumed that higher treatment doses in sepsis may improve survival16. In his study, Ronco et al compared prescribed CVVH doses of 20,35 and 45 ml/kg/hour and found improved survival in the 35 and 45 ml/kg/hour group as compared with 20 ml/kg/hour group. In the subgroup of patients with sepsis, which accounted for 11-14% per randomized group, there was a trend toward an even further improved survival between the two higher treatment groups. In contrast to these results, two trials published in 2008 do not support this hypothesis. Both trials included at least 60% patients with sepsis. The first one by Tolwani included 200 patients treated with continous veno-venous hemodial filtration(CVVHDF) at two different dosages, 20 and 35 ml/kg/hour. Neither survival nor renal outcomes was significantly different between the two groups17. The second trial, with the participation of our center, the VA/NIH Acute Renal Failure Trial Network included 1124 patients comparing intensified versus standard treatment, 615 patients were treated with CVVHDF at a dose of 20 or 35 m//kg/hour with no influence in the final outcome18. Based on the current evidence it remains unclear whether any dose above 22 ml/kg/hour provides additional benefit in patients with sepsis.
Because of the introduction of continous hemofiltration in the ICU, the idea of clearing inflammatory mediators from patients with sepsis has become an ulterior goal in intensive care medicine. High flux membranes with a cut-off of 30-40 kD should be capable of eliminating significant amounts of inflammatory mediators including chemokines and cytokines by convection, but this has raised the question whether the amount of removal is of clinical significance considering the high turnover rate of this mediators. A study using CVVH at filtration rates of up to 2.6 l/hour showed lack of effect of convection on serum levels of several cytokines including interleukin (IL)-1B, IL-1ra, IL-6, IL-8, IL-10, and tumor necrosis factor (TNF)19. Findings that have been confirmed by a prospective randomized trial in severe sepsis without renal failure20. On the basis of the current evidence, use of standard CRRT in the absence of AKI cannot be recommended routinely.
Several options have been developed to improve clearence of inflammatory mediators and to use immunomodulation to reduce their levels. Small pilot studies using CVVH utilizing adsorptive capacities of filter membranes in patients with sepsis have shown reduction of IL-8 and IL-10 levels and vasopressor requirements using AN69 filters over a 9 hour period with filter changes every 3 hours21. High volume hemofiltration (HVH) with filtration volumes ranging from 45 to 125 ml/Kg/hour have been used to increase convective transport as well as adsorption22. Only a few observational studies in humans support this concept using volumes of 5-9 l/hour for 4-12 hours23. One of the largest involved 306 patients (30% of them with sepsis) and showed a significant lower mortality than expected by severity of illness scores24. Two small randomized control trials (RCT) investigating the effect of HVH in septic shock have demonstrated reduction in complement levels and vasopressor requirements as well as reduction of cytokines (IL-6) but they were not powered to show differences in mortality25,26.The European multicentre hIgh VOlume in intensive carE (IVORIE) will hopefully clarify the question of benefit of HVH in patients with AKI and septic shock27. With respect to immunomodulation the use of High Cut-Off (HCO) hemofiltration membranes (cut-off of 60-150 Kd) must be considered and alternative approach to HVH. Pilot trials in septic patients with AKI demonstrated immunomodulation by altering neutrophil phagocytosis as well as mononuclear cell function ex vivo28 and a Phase II trial has been conducted in 30 patients with septic shock using HCO in CVVH at an ultrafiltration rate of 2.5 l/ hour. Besides reductions in IL-6 and IL-1ra levels, more rapid reductions of norepinephrine (NE) requirements and significant reductions in Simplified Acute Physiology II scores were seen in patients treated with HCO-CVVH as compared with conventional CVVH at the same dose. Major adverse event was the finding of albumin loss observed during high ultrafiltraion rates29. Other techniques that have been developed to mimic the above mentioned results implied the use of plasma separation followed by adsorptive steps over activated charcoal sorbent30 and the more promising renal assit device (RAD) which uses non-autologous human renal tubule cells grown along the inner surface of hollow fibers aligned in a catridge that are later incorporated in an extracorporeal perfusion circuit where the ultrafiltrate is pumped though the RDA, allowing the renal cells to reabsorb and eliminate substances from the blood circuit and thereby emulating the transport, metabolic, and endocrinologic activites of the kidney. A recent multicenter, open label, randomized control (RCT) phase II trial including 50 critically ill patients was published. A relative reduction of both 28 and 180 days mortality of more than 50% could be achieved in a population with roughly 70% of the patients with sepsis and most of them with at least three organ failures31.
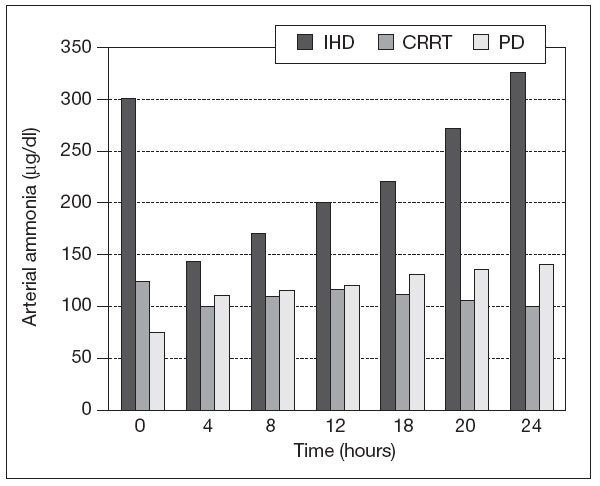
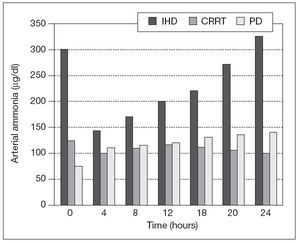
CRRT in patients with liver diseaseMortality is always increased in AKI requiring RRT in patients with liver disease. The importance of renal injury has been recently recognized, as evidence by the introduction of the model for end stage liver diseasse (MELD) scoring system for organ transplantation allocation. AKI may be due to hepatorenal failure32 both in the clinical setting of fulminant hepatic failure and chronic liver disease, but also immediately post-liver transplantation with primary graft nonfuncion. Other causes like preload responsive AKI, tubulointerstitial disease, glomerular involvement due to viral hepatitis, amyloid and alcohol, diabetes, hypertension and acute tubular necrosis (ATN) should also be considered. In Regards of RRT there is no prospective RCT showing improvement in survival in patients with acute or chronic liver failure33. In terms of possible toxins which accumulate in liver failure, RRT will remove both free plasma amino acids and ammonia in proportion to their plasma concentration, however, these losses are typically modest and generally continous therapies do not significantly impact on plasma concentrations, unless underlying hepatic synthetic function is improving. Although plasma ammonia and free amino acid concentration falls with intermittent hemodialysis (IHD), they typically rebound once therapy is discontinue34 (fig. 1).
Figure 1. Changes in arterial ammonia concentration intermittent during haemodialysis (IHD), and during continous renal replacement therapy (CRRT) and peritoneal dialysis (PD).
Acute peritoneal dialysis for patients with AKI has been shown not to improve survival of patients with hepatorenal syndrome due to poor solute clearance and risk of infection35. IHD and or hemodiafiltration have not shown to improve survival either. The majority of patients with hepatorenal syndrome present with refractory hypotension despite therapy with vasopressors such as terlipressin or noradrenalin. Thus CRRT has a key advantage over intermittent techniques in terms of cardiovascular stability36. Slow extended daily dialysis or other hybrid systems may potentially offer similar benefits. Also, since patients with hepatorenal syndrome are usually hyponatremic, CRRT can increase the serum sodium concentration in a timely controlled fashion37.
Patients with acute liver failure are at risk of cerebral edema. In cases with fulminant hepatitis the therapeutic options are elective ventilation in combination with sedation and muscle paralysis. Studies in this type of patients have shown that changes in intracranial pressure occur during RRT, either due to fall in cerebral perfusion pressure from episodes of intradialytic hypotension or also secondary to a rapid fall in serum osmolality38. The blood brain barrier (BBB) is usually intact in liver failure, and if urea is rapidly cleared from the plasma faster than urea can move from the cerebral tissues, this will lead to an osmotic gradient, with the movement of water back into the brain, exacerbating underlying cerebral edema39. Patients with acute liver failure are more susceptible to hypotension, during RRT sessions, due to high circulating levels of nitric oxide40. This can cause cerebral ischemia with rebound local cerebral vasodilation and generation of idiogenic osmoles increasing intracranial pressure41. IHD is well recognized to cause increased intracranial pressure in this group of patients, with early reports of patients suffering brain stem coning whilst dialyzed. Even days after a toxic doses of acetaminophen, when liver function tests are improving with the patient conscious and alert, sudden brain stem edema can develop do to osmotic shifts42. CRRT causes the least cardiovascular and cerebral instability of any RRT. It also leads to thermal losses and patient cooling. Hypothermia has been reported to reduce intracranial pressure in patients with acute liver failure, although cooling trials have not shown benefit in trauma patients43.
Liver transplantation is offered to patients at a much earlier stage of liver failure, and most centers have dispensed intra-operative CRRT. AKI post-liver transplantation is managed by CRRT on the ICU allowing for correction of acid-base and fluid disturbances with greater cardiovascular stability. Studies have also shown that CRRT removes unidentified compounds that accumulate in acute liver failure44. This lead to the use of high volume CRRT to treat patients with this conditions. Some centers have reported impressive 28 day survival of patients treated by high volume CRRT in combination with plasma exchange45.There is a European trial evaluating this protocol. Due to the nature of the different types of acid-base abnormalities that a patient with liver disease may encounter, this population may not adequately metabolize lactate and/or citrate quickly enough to bicarbonate. Replacement fluids/dialysates without lactate, have been reported to be superior by allowing the dose of bicarbonate to be titrated to patient’s requirements46. Anticoagulation is always an issue especially in liver failure patients with increased tissue factor release from disseminated coagulopathy. Despite prolonged prothrombin ratios, many patients do not actively hemorrhage, but have an increased risk of CRRT circuit clotting, and post-liver transplant patients may need to be heparinized to prevent portal vein thrombosis. In view of the potential risk of hemorrhage, many centers use anticoagulation free CRRT circuits. Others use regional anticoagulation, such as citrate, nafamostat or prostanoids. Citrate can usually be used with dialysis based CRRT in all but severe cases of liver failure47. Patients with liver failure may show a degree of resistance to heparin due to reduced antithrombin. Patients with acute and/or chronic liver failure who develop renal failure have an increased mortality. RRT is an integral part of their management. Typically this patients are hypotensive and prone to hemodynamic instability during IHD and/or hemodiafiltration. For that reason CRRT represents a good therapeutic option.
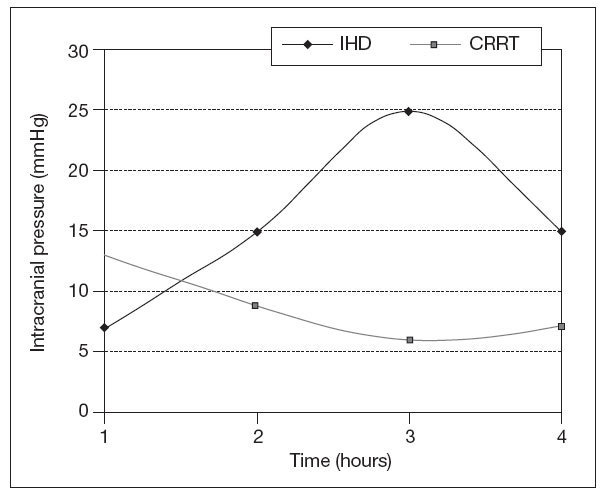
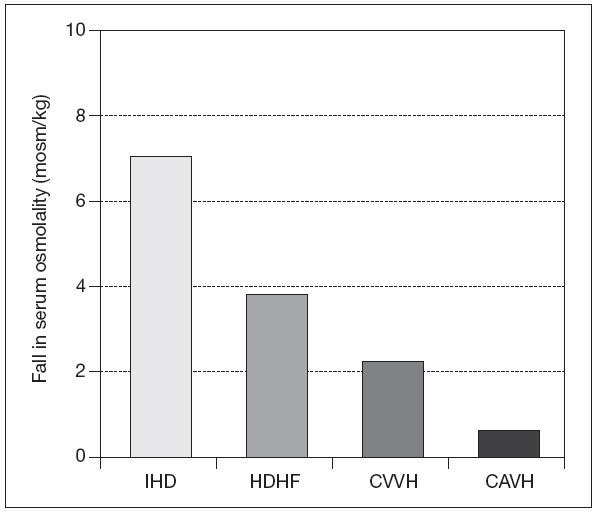
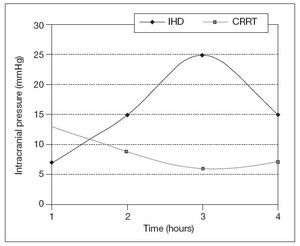
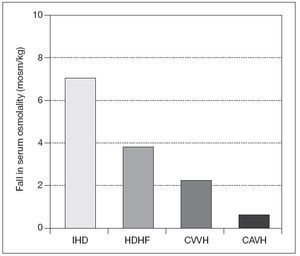
CRRT and neurological injuryAKI may develop due to renal ischemia following hypoperfusion as a complication of traumatic brain injury, sepsis use of nephrotoxic drugs or exposure to different types of nephrotoxins. Patients with baseline chronic kidney disease (CKD) on dialysis have an increased risk of stroke and also both subdural and /or intracranial hemorrhage especially on anticoagulants48. Acute neurological injury following trauma, acute intracerebral hemorrhage (AIH), or ischemic stroke typically takes 10-14 days to stabilize. To maximize recovery, RRT need to be tailored during this period to prevent therapy-induced cerebral ischemia. Urea and other solutes increase as serum osmolality increases and so does intracerebral osmolality. The BBB is usually intact, except in cases of traumatic brain injury, AIH, small vessel vascular disease, including vascular occlusion, hypertensive encephalopathy, and infection. Urea and water pass the BBB through active urea transporters and aquaporin channels, respectively. This results in an osmotic gradient developing between intravascular and cerebral extracellular compartments due to the fast moving ability of water molecules through aquaporin channels. IHD can lead to cerebral edema not only on experimental animal models but also in the outpatient setting49. The rapid infusion of bicarbonate in supra-physiological doses during hemodialysis can cause a paradoxical intracellular acidosis. After forming H2CO3, it dissociates into water and carbon dioxide which crosses the BBB. This leads to a compensatory production of intracellular osmoles and water movement into the brain along a concentration gradient50. Paradoxically tradialytic hypotension, specially during IHD, causes reduction in intracranial pressure (ICP) due to cerebral vasodilatation51. As previously mentioned before, IHD causes brain swelling by increases in ICP, not only due to changes in osmotic gradients and rapid increase in arterial PH, but also due to sudden falls in mean arterial pressure51 (fig. 2). For that reason intermittent RRT should only be consider in patients without mid line shift on cerebral imaging. If IHD is the only RRT available, the prescription should be altered to diminish changes in efective blood volume and reduce cardiovascular instability by using high sodium dialysate concentration and cooled dialysate. The changes in serum osmolality should be minimized by utilizing slower blood pump and dialysate flows, smaller surface area dialyzer membranes, and lower bicarbonate concentration. There are no randomized clinical trials which have investigated the optimum pre-dialysis urea to lessen changes in ICP during dialysis. Perhaps a pre-dialysis urea of less than 30 mg/dl reduces the risk of raising ICP during therapy. When continous renal replacement therapy is use in critically ill patients with AKI and increased ICP, filtration is preferable to dialysis, as this leads to a slower rate of change in serum urea and other small solutes and also greater cardiovascular stability due to cooling with pre-dilutional fluid replacement. A replacement fluid with a serum sodium concentration than greater than 140 mmol/l should be initially used. Initially small exchange volumes are also used follow by small increases based on patients condition. The reason behind this measures has been that with the introduction of continous forms of pumped veno-venous hemofiltration, dialysis, and hemodialfiltration, and in particular high volume exchange, greater changes in osmolality are possible (fig. 3). Also RRT can be used to clear several drugs and/or toxins in cases of coma due to drug toxicity and some metabolic encephalopathies. Due to increases in volumes of distribution and water solubility, this drugs are easily removed by IHD but a rebound in plasma concentration will follow. CRRT, slow extended hemodialysis and hybrid techniques would prevent this rebound. Recently isovolemic CRRT has been advocated for treating patients without AKI who have been resuscitated post-cardiac arrest and not regained consciousness. Up to eight hours of high volume hemofiltration can increase survival in this cases52.
Figure 2. Changes in mean intracranial pressure during intermittent haemodialysis (IHD) and continous veno-venous hemodiafiltration (CVVHDF).
Figure 3. Reduction in plasma osmolality after 1 hour of treatment with intermittent haemodialysis (IHD), high volume hemofiltration (HVHF),continous veno-venous hemofiltration (CVVH), and continous arteriovenous hemofiltration (CAVH).Values expressed as mean (SEM) *P<.05 vs IHD. CRRT in decompensated heart failure (DHF)
“Cardiorenal syndrome” is an entity characterized by the simultaneous dysfunction of two organs. Cardiac dysfunction leads to renal hypoperfusion and dysfunction, causing diuretic resistance and exacerbating heart failure. This creates a vicious circle nurtured by a bidirectional interaction resulting in the development of refractory volume overload and AKI. Intermittent isolated ultrafiltration (IUF), slow continous ultrafiltration(SCUF), and continous veno-venous hemofiltration (CVVH)/hemodialysis (CVVHD) represent potential extracorporeal options to unload the failing heart and restore cardiac and renal function. 30% of hospitalized patients with heart failure also have renal insuficiency53. Understanding of the hemodynamics and neurohumoral aberrations that occur in the setting of DHF and worsening renal function that supervenes with it, and recognition of the limits of conventional therapy have led clinicians to employ various forms of RRT. Fluid removal with extracorporeal therapies has clear-cut benefits in patients with volume overload and pulmonary edema, resulting in better gas exchange, lung compliance and symptomatic relief. Fluid removal in this fashion probably does not stimulate the adrenergic or renin angiotensin aldosterone (RAAS) systems through extracorporeal means and should support the failing heart to restore function. Ultrafiltration (UF) by extracorporeal means lowers filling pressures, norepinephrine, renin, and aldosterone levels while preserving blood pressure, cardiac index, increasing renal perfusion pressure and urinary sodium excretion as well54. Only patients with low cardiac output and stimulated adrenergic and RAAS system, without evidence of volume overload, did not benefit from UF. Echocardiography also demonstrates improved diastolic dysfunction after UF55.
IUF resembles conventional hemodialysis but the blood circuit is only a pressure gradient established between the blood and the UF compartment, allowing for the removal of isotonic fluid. IUF is performed in intermittent sessions of 1 to 4 hours where a goal volume removal is set. Unfortunately the large volume of fluid removed in a short period of time adds more hemodynamic stress56. The is minimal data regarding the use of this modality of RRT in the management of acute or chronic decompensated heart failure. As a tool for diuretic– refractory cases, it demonstrated improved New York Heart Association (NYHA) classification from functional class IV to III. Benefit that unfortunately was not sustained57. There was also an increased risk of hospitalization after IUF was initiated in this population. Further, there is no data on improvement or stabilization of renal function on patients treated with IUF.
SCUF has become a better-studied modality of extracorporeal fluid removal. Fluid removal occurs over an extended period of time at a slower rate providing better hemodynamic stability. Treatment of high-risk populations admitted with DHF with early UF demonstrated sustained improvement in volume status. Patients with NYHA class IV showed 30 and 90 days symptomatic improvement after treatment with SCUF58. The Relief for Acutely Fluid-Overloaded Patients with Decompensated-CHF (RAPID-CHF) trial offered more insight into the benefit of SCUF. Patients assigned to SCUF in addition to diuretics had significantly more volume removal and symptomatic improvement than those receiving conventional therapy with diuretics59. Unfortunately the evidence for the superiority of SCUF over conventional therapy is lacking. The UNLOAD (Ultrafiltration versus Intravenous Diuretics for Patients Hospitalized with Acute Decompensated CHF) trial provided a randomized comparative therapy for DHF. In this case SCUF versus vasodilators and diuretics. Patients were refractory to diuretics therapy. As expected the group assigned to UF had significantly more weight loss and decreased rate of re-hospitalization within 90 days, but the dyspnea score was similar in both randomized groups. There were also no major differences in the rates of creatinine and serum urea nitrogen increases. Patients were excluded with serum creatinines above 3.0 mg/dl60. A recent study of SCUF versus furosemide showed similar outcomes regardings changes in GFR61. This type of therapy has demonstrated effectiveness as a therapeutic option for DHF at least regarding hemodynamics and neurohumoral effects. Unfortunately the original renal benefits have not been able to be mimic again in further trials, probably due to the heterogenous population of the trials. There is lack of consensus regarding patient selection for UF in DHF and how to employ this therapy still remains controversial.
CVVH provides a means to correct metabolic derangments while removing volume through UF. For example hypocalcemia, which is common in patients with acute on chronic renal failure, is a reversible cause of heart failure due to its effect in cardiac myocytes. Correction of hypocalcemia by CVVH may improve cardiac function in DHF, even though data on this potential benefit is lacking62. For that reason and others, CVVH may be a better alternative to SCUF for the treatment of DHF in the intensive care unit. This technique can not also accomplish fluid removal but can also utilize solute clearance for the removal of “middle molecules” via convection during hemofiltration in DHF63. “Myocardial depressant cytokines” have been isolated from the ultrafiltrate of patients with renal dysfunction and DHF64. Two specific cytokines, interleukin-8 and antimonocyte chemoattractant protein-1, were effectively removed along with improvement in volume status via hemofiltration. Serum concentrations were greater in patients with DHF than controls. Diuretic (furosemide) use, in the other hand, accomplish volume removal without decrement in cytokine serum concentration. Unfortunately there was no significant clinical benefit. In another study, a significant improvement in ejection fraction occurred in the hemofiltration group, with a 30 days decline in diuretic dose in the same group65. Robust data demonstrating better outcomes is needed to recommend its use as first-line therapy in the clinical arena. Little is known about the best parameters to follow in the monitoring of fluid removal and indications to discontinue therapy. Some clinical trials have established goals of fluid removal through several clinical indicators which have not prevented rehospitalization for heart failure in these population. Several surrogate markers have been also evaluated specially brain natriuretic peptide and continous serum hematocrit concentration monitoring with inconclusive results based on patient variability. Until a reliable method is device in the future, the combination of non-invasive and invasive methods of assessing cardiac output, filling pressures, and tissue perfusion must be utilized to determine when to stop extracorporeal removal and support.
ConclusionThere is clinical benefits in the horizon for the use of different extracorporeal techniques in the management of volume, electrolyte and acid-base abnormalities that will help in the armamentarium against different clinical scenarios specially sepsis, liver disease, acute neurologic injury, “cardiorenal syndrome” and DHF. There are also risks involved with the use of this techniques, like catheter placement complications, possible blood loss related to the procedure, and hemodynamic instability with volume management. This will need adjustments to the therapy. Identifying which patients benefit the most from these extracorporeal therapies is paramount in order to maximize fluid removal, cardiac and renal function and maybe survival outcomes in a population not only subject to cardiovascular burden, refractory to conventional diuretic therapy, but also with MOF and a particular metabolic milieu. Future studies that will help us evaluate hard outcomes will soon be available for scrutiny.
INFORMACIÓN DEL ARTÍCULOHistoria del artículo:
Recibido el 28 de agosto de 2009
Aceptado el 31 de agosto de 2009
* Autor para correspondencia.
Correo electrónico: lortega2@med.miami.edu (L. M. Ortega).