The quality of vascular access (VA) is a determining factor in clinical results of patients receiving periodic haemodialysis treatment (HD). The complications arising from VA dysfunction constitute one of the main causes of morbidity and mortality amongst patients and substantially increase health care costs.
The need for vascular access is as old as HD itself, given that access to the bloodstream is necessary to eliminate toxic substances.
Ideal VA should bring together at least three requirements: i) a safe, continuous approach to the vascular system; ii) provision of sufficient flow to supply an adequate HD dosage, and iii) present no complications.
Risk factorsIntravascular catheters are plastic devices that provide access to the intravascular compartment at a central level, they vary in design and structure according to if they are temporary (days) or permanent (weeks, months) as well as in the material they are made of and the reason why they are implanted.
These devices have been of great clinical use as they enable fast, safe access to the bloodstream to administer medications, treatments fluids and parenteral nutrition. However, they are not exempt from risks such as infectious or mechanical complications arising from their use.
- 1.
Infection from use of central vascular catheters (CVC) constitutes one of the main complications and the main cause of primary nosocomial bloodstream infection. The incidence of infection attributable to catheter use varies amongst hospitals and is about 4-5 events per 1000 days of catheterisation, and an average mortality of 3% is linked to this sizeable prevalence.1,2
More is starting to be known about the mechanism through which CVC contamination is produced thanks to the development of experimental models with animals. Studies using electron microscopes show that the immense majority of catheters, even those where the quantitative culture gives negative results, are colonised by microorganisms. The germs are usually immersed in a biofilm sticking to the interior and exterior of the catheter formed by the interaction of the catheter wall with host proteins.
Appearance of the film is very early, even in the first 24hours after insertion, but is not necessarily a determining factor in the appearance of infection. When the density of microorganisms reaches a certain level, the probability of catheter related sepsis increases considerably.1,2
- 2.
Vascular access dysfunction causes the highest consumption of resources in the population with chronic kidney disease (CKD). Experts consider that current standards for pump flow (Qb), venous pressure (Pv), molecular clearance and dialysis time could be improved and reductions in clinical complications can be reduced by enhancements in the process, thereby increasing the quality of life for patients and reducing the health care costs caused by this problem.
Reed et al, has described all the variables that play a part in CVC complications in a microbiological and ultrastructural study.2,3
- a)
Characteristics of patient
- •
Underlying disease
- •
Immune system compromise
- •
- b)
Personnel training
- •
Aseptic measures
- •
Choice of catheter
- •
- c)
Insertion of catheter
- •
Preparation of implant site
- •
Choice of site
- •
Insertion technique
- •
Tunnelling
- •
- d)
Handling
- •
Management
- •
Rescue
- •
- e)
Care of insertion site
- •
Skin antiseptic
- •
Type of dressing
- •
Local anti-bacterial application
- •
- f)
Clinical monitoring
- g)
Connections and perfusions
- •
Periodic washing and lumen sealing
- •
Impregnation of catheter with antiseptics
- •
There are factors that depend on the patient such as the existence of an underlying illness, immune system compromise, serious illness, comorbidity, etc. However, most of the other aspects can be regarded as institutional (place, insertion method, catheter material, care in handling, etc), and it is precisely these factors that have an important bearing on reducing infection.3,4
If there is septic shock, bacteraemia with hemodynamic decompensation or tunnelitis with fever, the catheter should be immediately removed. (Fig. 1)
If a patient with CVC presents fever, blood cultures of peripheral blood from both branches of the catheter should be extracted. Extractions should be simultaneous and cultivated using quantitative techniques whenever possible.
In cases of severe infection or when the catheter is not withdrawn, empiric antibiotic therapy should be set in motion prior to reception of microbiological test results. Conservative treatment without withdrawing the catheter is acceptable for tunnelled catheters infected by the customary microorganisms: i) associated systemic antibiotic therapy should be administered; ii) intraluminal sealing of the catheter with suitable antibiotics: iii) intraluminal sealing with antibiotics not associated with systemic therapy is ineffective. 3–5
Personnel trainingAsepsisKeep adequate hygiene of the hands by washing them with soap and water or with alcohol-based gel, maintaining aseptic techniques for any handling of the CVC. Use the maximum universal aseptic measures in the implant by maintaining all possible protective and safety barriers for the environment and handling. The skin area where insertion is to take place constitutes the most important source of CVC infection. Prior to implanting a central catheter, the following procedures are important.4–6
- 1.
Vigorously and thoroughly wash your hands
- 2.
Wear sterile clothing
- 3.
Careful and full skin disinfection
- 4.
Avoid shaving the skin whenever possible
- 5.
Widely cover the implant area, ensuring sterility
- 6.
Carry out the implant in a clean environment free of air currents
- 7.
Do not inject anaesthetic into the tunnel track
- 8.
Adequate nursing personnel for the care activity ratio
Ensure that professionals responsible for managing catheters are present to provide them with the necessary knowledge and training with regard to suitable recommendations, indications and procedures for management and avoidance of incorrect management or prevent CVC-related infections with the appropriate measures.
Personnel training programs significantly reduce infectious complications. Likewise, deviations from recommendations for care of accesses have been directly related to an increase in catheter-related infection.6
Antiseptic (aqueous chlorhexidine at 2%) for preparing the skin prior to insertion reduces infection and the risk of catheter-related infection. Chlorhexidine has been shown to be more effective than povidone iodine (betadine) for cleaning and disinfecting the skin and the catheter for its bactericidal capacities in reducing CVC-related infections. 7–10
Handling the connection systems of long-term CVCs increases the risk of catheter-related infection. Impregnating the connections with an antiseptic or antibiotic reduces the probability of risks. This method effectively prevents germs that migrate via the lumen to the internal or external surface of the catheter.11
Recommendations cannot be made for the use of chlorhexidine sponges to reduce the incidence of infection. This issue is unresolved.
Observe and frequently touch the tunnel track, the catheter exit hole and the extenders, changing the location of the forceps. The ease with which contamination from the skin and hands is caused by these devices implies poor practice on the professional's part, as well as unnecessary replacements or handling.12
PCT is a new highly specific and sensitive bacterial infection marker. It differentiates between severe bacterial and viral infections or any other non-bacterial pathology that triggers a systemic inflammatory response. It is currently the best test for early diagnosis of early or late sepsis. It is an easy, low-cost method.
The pacemaker or implantable cardioverter defibrillator (ICD) should not be the cause of a clinical expression amongst patients with CVC.
Documenting patient data2,13,14- •
Demographic data
- •
Presence or not of pathologies
- •
Previous accesses and associated failures
- •
Monitoring sheet and physical examination of access
- •
Blood flow and venous pressure.
- •
Anomalies
- •
Orifices and deterioration of the skin
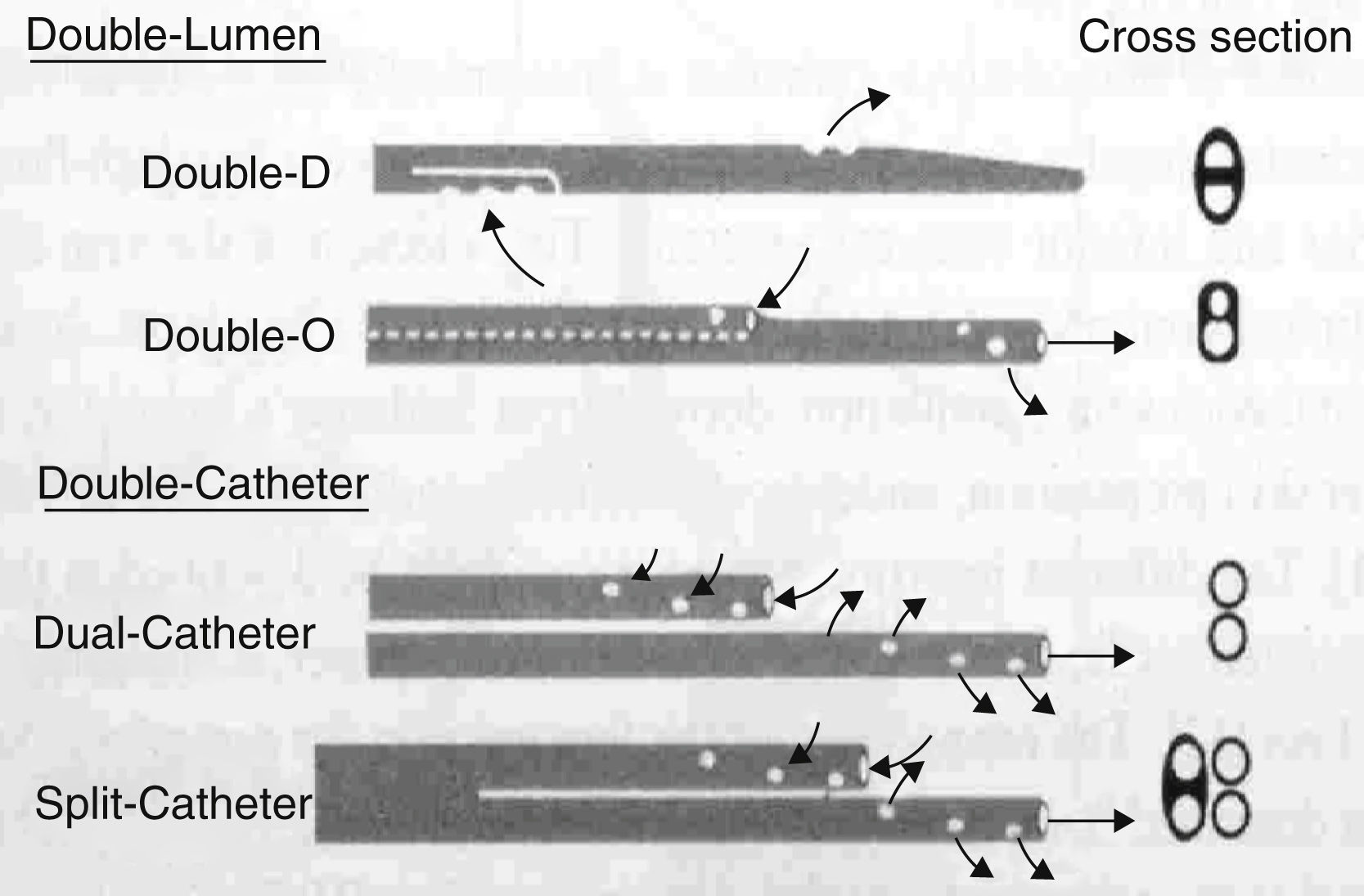
Single and double or multi-lumen catheter (Fig. 2)
Catheters are divided into:
- a)
Single lumen catheters; used in continuous renal replacement therapy (CRRT) where two vascular accesses are inserted (artery-vein or vein-vein)
- b)
Double-lumen catheters; where only one vascular access is punctured. The proximal lumen acts as an arterial line and the distal lumen as the venous line
- c)
Double split catheters are two catheters that make it possible to select the length and location of their points.
This is the main determining factor of flow. There are different measurement units that are mutually related. The most commonly used ones are:
- 1.
French(F, FG)
- 2.
Gauge (Gg)
- 3.
In adults, the calibre of the catheter basically depends on the desired flows. Recommended calibres for adults start from 12-14 Fr to achieve the right flows without exceeding the pressure limits for the inlet and return. In paediatrics, vascular access depends on the age and weight of the child.16,17
Catheters are classified according to their cross-section (Fig. 3):
- 1.
single catheter with different types of section
- 2.
double catheter with independent distal ends (the dual has two lumens throughout its length and the split catheter divides into two at the distal third). This design minimises support of the catheter on the vessel thus allowing it to function better
- 3.
double split catheter, enabling length to be chosen
According to the lumen section, the catheter can (Fig. 4)
- •
improve flow characteristics
- •
improve system pressure
- •
reduce complications, including coagulation of the system from high transmembrane pressure
The length should be as short as possible depending on the vessel to be treated; the length has a negative effect on flow from increase in resistance.1,15,17
In adults, the routes depending on the superior vena cava system should be:
- 1.
The arterial and venous line in the right atrium
- 2.
The routes that depend on the inferior vena cava system (femoral veins) should reach 25-30cm in length. The artery is below the vein
- 3.
In paediatrics there are different lengths depending on the vascular access and and age of the child (10-15cm for femoral route)
Catheters were initially made of polyvinyl chloride (PVC) or polyethylene, and were later rejected after showing a tendency to cause vascular traumas due to the rigid material, making them easier to break along with a tendency to thrombogenicity and infections from an increase in adhesion of microorganisms.
The material used in manufacture is important as there are certain antiseptic and antibiotic solutions that are habitually used when handling them which can be incompatible. Alcohol, the polyethylene glycol used in mupirocin ointment and povidone-iodine interfere with polyurethane and crack it, leading to possible breakage of the catheter. Povidone-iodine also interferes with silicon leading to degradation and breakage.
Bacterial adhesion in polyvinyl chloride and polyethylene > teflon > silicon > polyurethane e.g.: Staphylococcus aureus adheres more easily to polyvinyl chloride and to a lesser extent to polyurethane. A prospective study in which polyvinyl catheters were compared to ones made of silicon showed a lower proportion of catheter related sepsis of the latter type when compared to the former (19-0.83 per 1000 cat/day). Experimental studies on pigs have shown that the use of catheters impregnated with antiseptics (chlorhexidine and sulfadiazine) before insertion significantly reduce both bacterial adhesion of Syaphylococcus epidermidis and the formation of biofilms.1,5,9,18
Host proteinsCertain host proteins are deposited in the irregularities of the catheter surface, favouring the formation of a biofilm. There is a degree of protein selectivity in this process, thus Syaphylococcus epidermidis is especially adherent to fibronectin. Flexible polyurethane tunnelled catheters are used nowadays (silicon catheters are no longer used). They are very flexible and present lower risks of lesion of perforation of the vascular wall. Studies have shown a lower frequency of infections from these catheters than others made of PVC and polyethylene. In vitro, polyurethane catheters impede to a greater extent the adhesion of some bacterial species.1,2
Insertion of catheterPreparation and choice of location. Insertion and tunnelling techniqueThe choice of location for inserting the catheter is determined by many factors, but the most common points for implanting the central vascular accesses are: femoral, jugular, subclavian and occasionally the axillary. The most commonly used approach in all these cases is a direct one via percutaneous access. The larger the number of punctures for the CVC implant, the more difficult the CVC implant procedure is likely to be, which may increase mechanical complications.1,15,18,19
c.1.1) The femoral route should reach the inferior vena cava. The arterial point should be lower than the venous point to minimise blood recirculation during dialysis, the distance between the arterial and venous orifices should be more than 2.5cm. The femoral route approach is linked to greater frequencies of colonisation due to greater constant humidity and rubbing, and so tunnelling the catheter is advisable. Urgent insertion should be avoided for the femoral route, and extreme safety and precautionary measures should be taken.
c.1.2) The subclavian route presents greater risks of severe complications and must be channelled by experienced members of staff. It is not recommended for patients with chronic renal failure because of the risk of stenosis and thrombosis that could lead to the later development of arteriovenous fistulas.
c.1.3) The internal jugular route is the first choice for tunnelled CVC. The right jugular is better than the left jugular in this case as the latter takes a longer and more convoluted route. The arterial and venous routes should be located in the atrium to avoid recirculation.
c.1.4) In paediatrics the accesses are similar although in neonatal treatment there is also the possibility of channelling of the umbilical vein/artery.
Once the catheter is in place, check that all the air in the lumen inside the catheter and the extender to avoid air embolism. The blood should leave and enter the vascular circuit correctly, both during aspiration and when returning. Confirm using X-ray imaging that the post-implant catheter is suitably placed; for superior vena cava implants the arterial and venous points should be located in the right atrium.12,13
TunnellingTunnelling uses a subcutaneous path (adipose tissue) before inserting the CVC in the vessel lumen. This path should not be shaved or anaesthetised to avoid the possible entry of germs through the skin.19,20
The catheter has a Dacron (polyester) cuff in the extravascular section. The purpose of this catheter cuff or Dacron is to adhere to the muscle and cause a fibrosis to impede the entry of infectious agents and act as an anchor, thereby preventing any accidental escape of the catheter and retarding extraluminal migration of pathological germs towards the distal end of the catheter and the interior of the circulatory flow, reducing the level of risk of catheter related sepsis.
The central venous routes should be tunnelled when treatment requires long periods of duration and permanence. Tunnelling for internal jugular approaches reduces colonisation by 39% and infection from the catheter by 44% in comparison to standard procedures. Tunnelling of the femoral significantly reduces the risk of catheter related infection in comparison to non-tunnelled catheters.1,17,21
ManagementVascular haemodialysis catheters should only be used for haemodialysis sessions. Do not use haemodialysis catheters for taking blood samples or other applications that are not for dialysis therapy except in emergency situations.4,5,15
Connection and disconnection should only be managed by specialist dialysis unit personnel following recommended protocols and procedures.1,5,13
Connection, disconnection and handling should all be managed using stringent aseptic measures.12,14,22,23
Inform, warn and remind the patient to report any signs or symptoms he/she has or notices. Take standardised notes of any observations on handling and maintenance of the CVC.4,5,24
Care of the catheter is essential and the use of alcohol-based antiseptics, ointments or non-breathable dressings is not recommended.8,10,19,25
Thrombosis of a central vessel is an important and common complication in CVCs. Incidence varies between 4 and 35% of patients.1,2,26
Several factors contribute to a growth in frequency:
- a)
Time period of catheter use
- b)
Endothelial injury during the insertion process
- c)
Situation and location of catheter
- d)
Polymer characteristics
- e)
Patient's clinical situation
This strategy uses anticoagulants such as heparin, unfractioned heparin, citrates or other anti-microbial compounds such as gentamicin to fill the dead space of the CVC lumens at the end of each dialysis session and after washing with 20ml of isotonic saline with positive outward pressure.4
d.1.1) Intralimunal heparin is retired before starting the next dialysis and before washing the lumens with isotonic saline.
A significant and important reduction has been shown in rates of overall infection, and a prolongation in the useful life of the catheter, in patients under haemodialysis with tunnelled catheters. However, plasmatic levels of gentamicin determined before every dialysis session fall within a toxic range for a fraction of patients that received this compound, showing associated symptoms.
As regards the concentrations of heparin to be used, there are some discrepancies in the scientific literature, where concentrations vary from 20 UI/ml to 5.000 UI/ml. The use of single doses of 20 UI/ml generates permeability of the catheter and reduces the risks of systemic involvement caused by a dose of 5.000UI/ml due to chronic repetition of treatments (risk of bleeding).21,26
d.1.2) The catheter and extender should be sealed when therapy ends by closing the access with the clamp and luer/lock caps. Check that the connections fit and that all the system components are compatible to minimise risks and system breakages.
The SEN and CDC do not recommend the use of systemic antimicrobial sealing.4,15
d.1.3) Catheter rescue
Fibrin in the catheter lumen
- a)
Intraluminal
- b)
At the tip of the catheter
- c)
Formation of pericatheter fibrin sheath
Assessment of tunnel orifices and tracks should be planned in accordance with accepted internal evidence. (Fig. 5)
Skin cures are carried out in accordance with set internal protocols, and a recommended antiseptic is an aqueous 2% chlohexidine gluconate solution of the biguanides group. The mechanism of action is quickly absorbed by passive diffusion via the membranes of the bacteria and the yeasts. The bactericidal effect of the chlorhexidine starts with it linking to the bacteria cellular wall (negatively loaded) because of its physiological Ph cationic molecule. Its time of action is 6h, due to its affinity to adhering to the skin and mucous membranes.4,5,7
DressingsThe dressings that cover the outlet orifices and the catheter itself should be products that keep skin health intact and prevent erosions and infection. There is no evidence-based nursing about which dressing to use.12,21
Types of protection:
- 1.
Gauze + tape
- 2.
Transparent polyurethane dressings have become popular recently as they allow the incision site to be seen. It was demonstrated that this type of transparent dressing could be linked to CVC related bacteriemia. This mechanism has been attributed to bacterial colonisation in the insertion site that may be increased by the humidity created below the transparent dressing from lack of permeability or less frequent changes.
Changes have been made to the design of transparent dressings to increase permeability.
- 3.
Chlorhexidine-impregnated patches (Biopatch™) reduce the risk of colonisation of the catheter and have shown no adverse effects during use (Fig. 6)
Hoffman (1992) stated that transparent dressings are a determining factor for a higher risk of infection than gauze ones. However, Gilles (2003), in a systematic revision, states that there is uncertainty over which is more appropriate and recommends further rigorous research on this issue. More recent studies confirm that there is no difference in terms of the risk of infection when using a gauze or transparent polyurethane dressing.
It is considered that non-tunnelled catheter dressings should be changed every two days (48) if they are gauze, and every week if they are tunnelled and when the dressing is clean and intact.1,4,5,18
Advice on choosing a dressing- a)
Protection against microbial infection
- b)
Keep skin dry
- c)
Make sure catheter is securely fixed
- d)
Minimise growth of skin flora under dressing
- e)
Good tolerance and non-irritant
- f)
Comfortable and accepted by the patient
- g)
Easy to apply and remove
It should be highlighted that not all transparent polyurethane dressings are indicated to cover central venous catheters as not all of them breathe adequately or their water vapour transmission rate is low, with consequent increase in moisture below the dressing and skin maceration, leading to an increase in microbial activity. The increase in humidity below the dressing means that it also comes loose, which leads to a risk of contamination and increase in the number of dressing changes.4,14,25
Recommended- a)
Check manufacturer's indications
- b)
Only use transparent polyurethane dressings recommended for CVCs.
- c)
pocket dressings increase patient comfort
Different types of pocket dressing are currently available:
- a)
Plastic; non-breathable, rigid dressing that creates an increase in patient sweating. They are uncomfortable as they create heat and noise at night when the patient moves.
- b)
Padded dressings, more comfortable than the ones mentioned above, but the design means that a first dressing needs to be applied to cover the inlet orifice; they are also impermeable to water, which makes personal hygiene more difficult
- c)
The databases do not show research studies on these dressings. Although they are more comfortable for patients, some unsuitable features need to be modified.
Any accidental or voluntary breakage or disconnection of the catheter or the caps must be avoided to prevent gas embolisms. The clamps at the extensions do not always guarantee closure of the extenders and so luer/lock caps should be used (Fig. 7). The zone where the clamps are used should be changed to prevent wear and breakage of the lumen.5,18
Handling CVC connections with fluid systems leads to an increase in the risk of catheter-related sepsis (CRS), especially in long-term CVCs. The use of a new model impregnated with antiseptic (alcohol iodine) has reduced the probability of CRS by a factor of 4 (IC95% 0,1-0.7). This method, which effectively prevents germs that migrate through the lumen towards the internal wall of the catheter, is not obviously effective against migration via the external surface.26
Multiple studies (mostly cohort) show an increase in the frequency of CRS when traditional connection systems are profusely used. The ease of contamination of these devices suggests design problems and poor practice by professionals (especially the latter), insufficient replacement of contamination during handling when washing the lumen, since adequate practice and handling of the CVC prevents the risk of CRS. However, newly improved designs of connectors have been developed.3,24,26
e.3) Sutureless™ devices may be more advantageous than sutures in preventing CRS, these avoid external sutures that tend to scratch and erode the skin at the catheter outlet. (Fig. 8)
Clinical follow upClinical follow-up should be based on the search for signs and symptoms that lead to the suspicion of an infection or malfunction. Body temperature and signs of inflammation of the outlet or tunnel orifice should be investigated at each dialysis session. The presence of oedema in upper extremities or the face indicate the possibility of thrombosis in the central veins, any sudden change should be considered as a possible severe complication.
The aim of the functional follow-up is to detect alterations that impede effective dialysis, such as lack of flow, high pressures, recirculation or symptoms of malaise or pain
Assessment- a)
Skin intact
- b)
No signs or symptoms of inflammation or pain
- c)
Lines and extender intact
- d)
Lumen closure intact
- e)
Permeability of catheter lumens
Recirculation is effectively minimal in double and split catheters placed in the internal right jugular, if and when the arterial and venous points are distanced in the atrium, and so any recirculation above 5-10% indicates alteration in the catheter.13,24