This paper focuses on findings relating to chemical change that were part of a wider study exploring students’ understanding of the concept of a substance. Including pilot work, around 6000 students from 45 schools were involved in the project. An instrument using fixed-response items was developed with distracter options based on likely misconceptions reported in the research literature. The possibility of a learning progression was explored using Rasch modelling. Overall, the data show a good ft to the Rasch model and a learning progression towards understanding chemical change emerged. The progression is presented and significant implications for the chemistry curriculum are discussed. There is reason to suppose a curriculum better matched to students’ needs as learners could bring improvement in their progress.
Esta contribución se centra en resultados relacionados con el concepto de cambio químico obtenidos como parte de un estudio más amplio dedicado al estudio de las ideas de los estudiantes sobre el concepto de sustancia. Tomando en cuenta el trabajo de pilotaje, cerca de 6000 estudiantes provenientes de 45 escuelas distintas participaron en el proyecto. Los resultados fueron obtenidos utilizando un cuestionario con preguntas cerradas con opciones de respuesta basadas en resultados de investigaciones educativas sobre concepciones alternativas. La posibilidad de una progresión de aprendizaje fue explorada utilizando modelaje de Rasch. En general, los datos muestran buen alineamiento con el modelo de Rasch y describen una progresión de aprendizaje. Dicha progresión se describe en este artículo, junto con la discusión de sus implicaciones para la enseñanza de la química. Hay razones para suponer que un currículum basado en esta progresión tendría un efecto positivo en el aprendizaje de los estudiantes.
Like any experts within a field, chemists view the world in a specific way and have developed conceptual entities and an associated specialised language. For chemistry education, the key question is how to initiate students into what will be a new way of seeing and thinking. A curriculum capable of achieving such ends must be informed by the students’ perspectives. In planning any journey, one needs to know the where one starts as well as where one is going. We need to understand the demands being faced. For our students, looking forward into what is the unknown for them is very different from looking back over what is known for experts. This paper presents a route into chemistry — a learning progression — which may describe the journey most students must make towards a basic understanding of chemical change. By a basic understanding, I mean understanding the phenomenon as a change where old substances (reactants) cease to exist and new substances (products) are created in their place.
The proposed learning progression is informed by the literature on studies into students’ understanding in chemistry. For a detailed account of this literature the reader is referred to existing reviews (e.g. Andersson, 1990; Driver, Guesne & Tiberghien, 1985; Garnett, Garnett & Hackling, 1995; Harrison & Treagust, 2002; Krnel, Watson & Glazar, 1998; Liu, 2001; Talanquer, 2006; Wiser & Smith, 2008). This article draws more specifically on two studies conducted by the author. The first was a longitudinal study, in one secondary school in England, where a sample of students (n=33) was interviewed periodically over the first three years (Grades 7 to 9, ages 11 to 14). The study was based on four chemistry units taught across the whole year cohort (two units in Grade 7 and one in each of Grades 8 and 9, each unit around 14 one hour lessons). The interview sample, representing a full range of achievement, was drawn from all teaching groups in each year (six), taught by different teachers. Extensive interviews (lasting around 40 to 60 minutes) were interleaved with the teaching units to monitor individual student’s thinking before and after each unit. Johnson (2005) gives an overview of the study. The findings suggested a common pathway for the development of understanding, albeit with students progressing at different rates. Of course, this could simply have been a function of the common teaching units and have no wider significance.
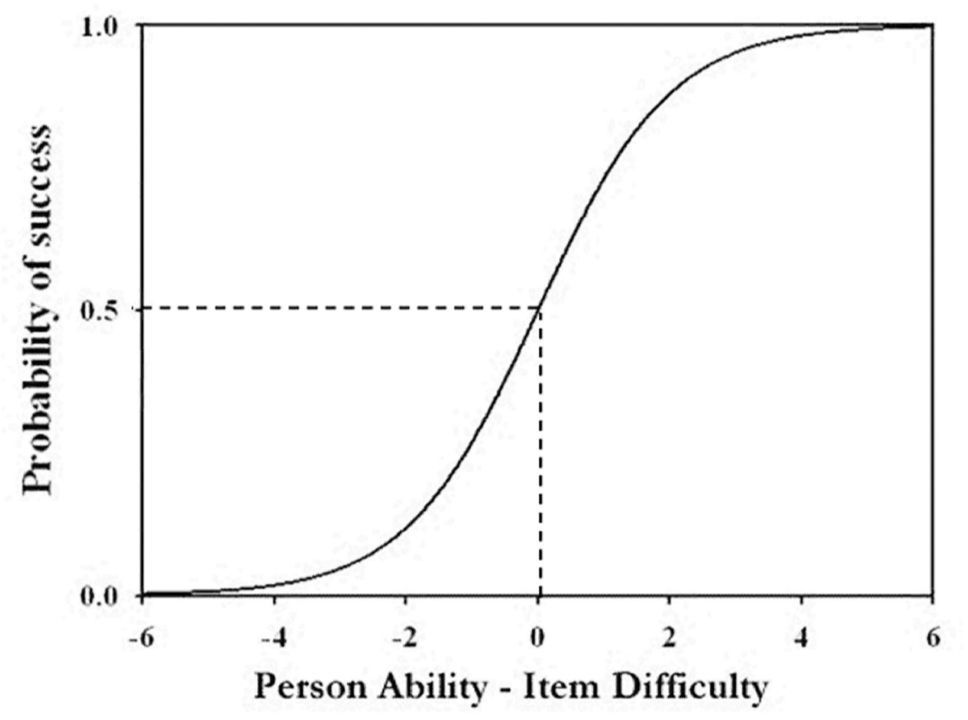
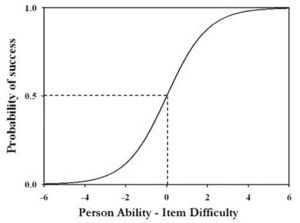
The second study, including pilot work, involved a sample of over 6,000 students (mainly ages 11 to 14) drawn from around 45 secondary schools of all types across England, covering a range of socio-economic backgrounds. A computer-based, fixed response instrument was developed to assess students’ understanding, where distracter options were informed by findings from the first study and those reported in the research literature. One hundred and seventy six items were developed and administered in three tests (of around 80 items). A kernel of 33 items common to all three tests, and some further items common to pairs of tests allowed for equating between tests. The student responses were scored dichotomously (1 or 0) and the whole data set was analysed using Rasch modelling (Bond and Fox, 2007). Rasch modelling is well suited to exploring the notion of a learning progression. The model presumes the existence of a continuous variable with an interval scale. For our purposes, the variable is ‘understanding chemistry’. Each student is said to have an ‘ability’ and each item a ‘diffculty’, both measured on the interval scale. The probability of a student answering an item correctly depends on the difference between student ability and item difficulty as described by the ogive shown in Figure 1.
When student ability equals item difficulty (i.e. the difference is zero) the probability of success is 50%. The likelihood of correctly answering an item increases the more positive the difference (ability is greater than difficulty) and decreases the more negative the difference (ability is less than difficulty). Through a series of iterations, Rasch analysis arrives at values for student ability and item difficulty which give the best overall match for the data set to the model. Indices show how well each individual student (in their responses to all of the items) and each item (in the responses from all of the students to that item) ft the model. For a data set with a good ft to the Rasch model, if success on an item refects the understanding of a particular idea, the relative difficulties of ideas can be established. Assuming students learn easier ideas before harder ideas, the ideas placed in order of difficulty can be taken to represent the order in which students are likely to acquire the ideas — i.e. an empirically established learning progression.
Overall, the data collected in the second study showed a good ft to the Rasch model. Taking success on an item to represent understanding an idea, rather than the consequence of some other factor(s), is a significant assumption. Using more than one item to target an idea helps to isolate what might be the difficulty of the idea and in different contexts. Within the item bank, some ideas were addressed by more than one item to give a zone of difficulty, but due to space constraints some were addressed by just one item (where context was judged to be less important). Notwithstanding the limitations of the items, the order of difficulty of ideas that emerged from the Rasch analysis produced a coherent progression in understanding that broadly matched the findings of the previous longitudinal study. This article presents that progression (Figure 2) and discusses its implications in relation to current practice. For details of the methodology, the reader is referred to Johnson and Tymms (2011).
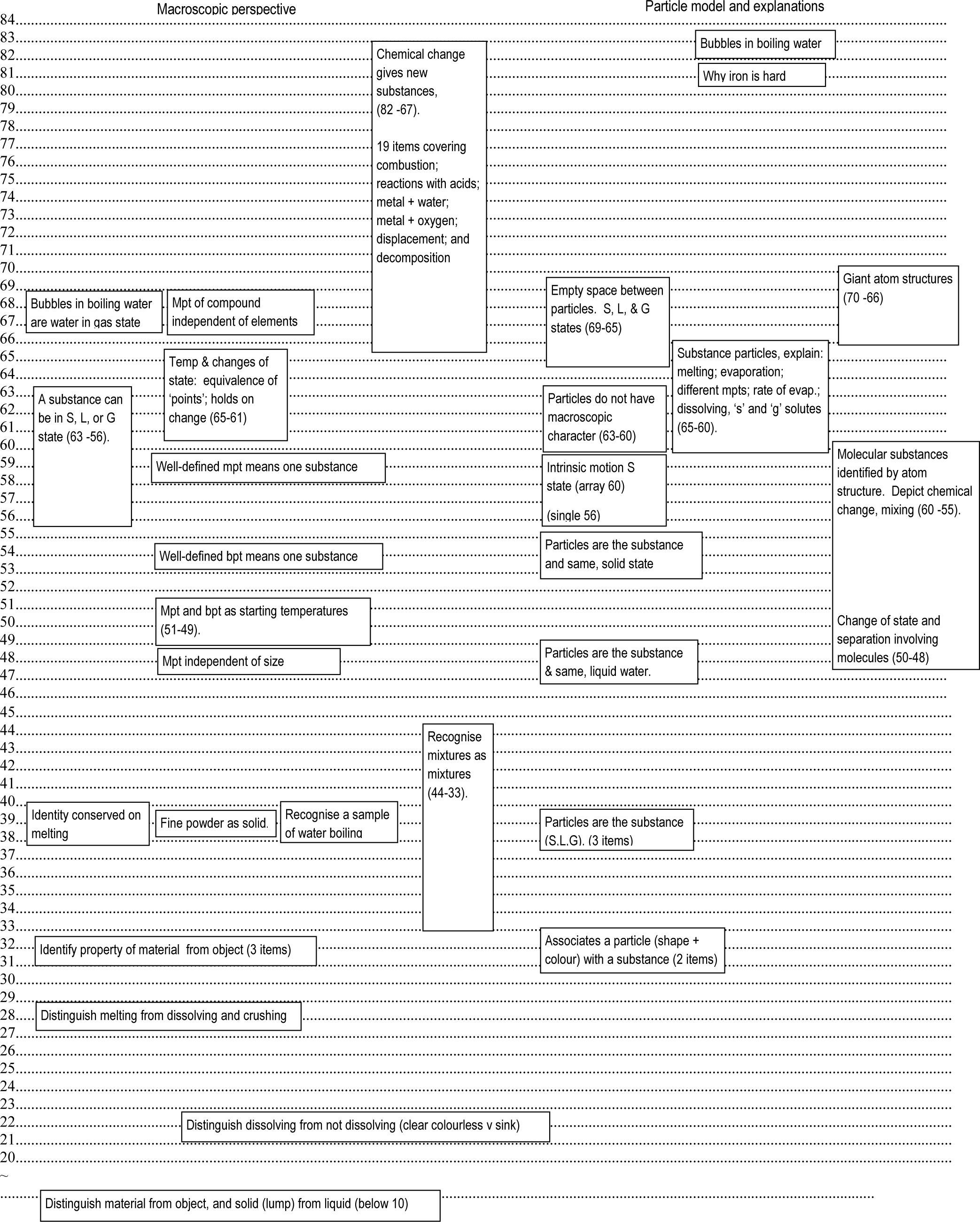
A learning progression towards understanding chemical changeFigure 2 picks out ideas leading to an understanding of chemical change. For presentational purposes it does not include all of the ideas addressed by the items in the study. Some of these ideas are incorporated into the discussion below, but those not directly related to chemical change (e.g. evaporation and separation of mixtures) have been excluded (Johnson and Tymms (2011) gives the full picture). On the left margin is the interval scale that forms the basis of the Rasch modelling. It is a relative scale and the numbers are quite arbitrary. With no zero as such, the positions show the difference in difficulty from one idea to another but not how many times one idea is difficult than another (like the celsius scale for temperature). On the left side are ideas in relation to macroscopic observations. On the right hand side are ideas relating to particle theory and particle explanations. Boxes giving a range of values arise from more than one item. The macroscopic side will be considered first.
Macroscopic phenomenaSince chemical change is a change of substance, items focussed on ideas contributing to the development of the concept of a substance. Science identifies a substance by its properties and of these melting and boiling behaviour might be thought to be the most accessible. How does such an understanding build up? In what follows, the term ‘material’ refers to any kind of recognisable stuff which may be a substance (e.g. copper, ethanol, carbon dioxide) or a mixture of substances (e.g. wood, orange juice, air).
A very basic starting point, below 10 on the scale, is the distinction between the material and object with regard to constitution; i.e. the same material can make different objects and similar objects can be made from different materials. Also at this early stage is the ability to distinguish between liquid and solid for reasonably large undivided samples — i.e ‘lumps’ and ‘pools’. At 22 is recognising the difference between not dissolving and dissolving for non-dissolving pieces that sink and those that disappear to give a colourless solution. Next (28) is being able to distinguish melting from dissolving (and also from crushing). Identifying properties depending on the material only (such as melting behaviour) from those of the object (shape, size and use) is a little higher up at 32. (Also at this level is recognising dissolving for coloured solutions.) Conserving the identity of a material on melting (e.g. the liquid from melting wax is still wax) comes at 39. Alongside are recognising the onset of boiling (large bubbles) and appreciating that fine powders are the solid state. Depending on the context, between 33 and 44, students are also able to identify materials that are mixtures as mixtures (e.g chocolate, orange juice) but they will also think of compounds as mixtures. At this stage, the groundwork for the concept of a substance is in place. The next phase develops the understanding of melting behaviour.
Appreciating that the onset of melting does not depend on the sample size (a small lump of wax does not melt at a lower temperature than a large lump) comes at 48. Otherwise, the idea of a melting point for a substance makes no sense. The region 49-51 covers the idea of melting and boiling points as the temperature at which melting and boiling, respectively, start to happen (rather than somewhat imprecisely, a few degrees beforehand). The significance of change of state behaviour in distinguishing between a pure sample of a substance and a mixture of substances follows. A steady boiling temperature indicating a pure sample of a substance is at 54. For melting, without monitoring the temperature, the observation of a sharp change from lump to runny liquid as opposed to going gooey (e.g. chocolate) signifies a pure sample of a substance. A full understanding of temperature and change of state comes later in the region 61 – 65. This covers two important ideas: that the temperature does not change during melting and solidifying and that a change of state reverses at a particular temperature (i.e. melting point = solidifying point and boiling point = condensing point). Moving into the territory of chemical change, the idea that the melting point of a compound (FeS) is independent from the melting points of the separate elementary substances (e.g not the mean, sum or one followed by the other) is at 68.
Alongside the developing understanding of change of state from 56 to 63 is the appreciation that any substance can exist in any of the three states given the appropriate conditions (avoiding the issue of thermal decomposition). Up to this point, students are likely to hold the misconception where the room temperature state is taken to define a substance as either ‘a solid’, ‘a liquid’ or a ‘gas’. ‘Solids’, ‘liquids’ and ‘gases’ are regarded as three different kinds of substance. That some substances can just change to a state adjacent to the room temperature state students find less difficult. A notable exception is the case of a beaker of boiling water. Here, appreciating that the large bubbles are water in the gas state is at a much higher level of difficulty (67). Confusions with what are known to be ‘gases’ through knowledge of dissolved air or water being made of ‘hydrogen’ and ‘oxygen’ seem to add to the difficulty. The bubbles in boiling ethanol are less difficult (59).
For the items on chemical change, the main focus was on how students’ viewed the products of a reaction in relation to the reactants. Were the products understood to be new substances with their own identity? Recall of reactions and the names of substances were not required. In most cases, the item included a video showing the reaction taking place. Otherwise, photographs were used. For example, students were shown a video of the reaction between calcium and a little water in a test-tube. To start with, the tube is at an angle with a few lumps of calcium resting above the water. On moving to a vertical orientation, the calcium slides into the water and the reaction causes a plastic bag attached to the mouth of the tube to inflate and the creation of white powder in the tube. Two questions followed, one about the white powder and one about what was in the bag. The options for the white powder were:
- A.
Calcium in a different form
- B.
A mixture of calcium and water
- C.
What is left after a gas escapes from calcium
- D.
A new substance that isn’t calcium or water
The most popular option was B, followed by A, then C with fewest choosing the correct answer, D, with a difficulty of 70.6. Rather than thinking in terms new substances being created students tend to conserve identities and think in terms of making mixtures (Option B), separating mixtures (Option C) or modification in form (Option A). The options for what was in the bag were:
- A.
A gas released from the calcium.
- B.
Water in the gas state.
- C.
A new substance that isn’t calcium or water.
- D.
Carbon dioxide.
- E.
A gas that was in the water.
The idea that the ‘gas’ is a new substance is more difficult at 73.4. Option A was by far the most popular with a relatively even split amongst the other options. Some students will always select ‘carbon dioxide’ for any question involving ‘gases’.
Nineteen items showing various examples of chemical change group together in the region between 67 and 82. At 67 is the reaction between copper oxide and warm sulphuric acid to give a clear blue solution. More pupils chose that a new substance had been formed in this case compared to the other items on chemical change. Termal decomposition seems to pose a particular challenge. For the decomposition of sucrose (at 76) most choices were divided equally between ‘sugar in a different form’ and ‘a mixture of sugar and oxygen’ as descriptions for the black product. The most difficult item relates to the idea that the condensation observed on placing a jar over a candle fame is new water made in the fame. That the substance water comes out of a hydrogen fame is also demanding at 76 (carbon dioxide is by far the most popular choice). Overall, a lighted candle is a very challenging event for students — even the role of the wax, explored in other items. A large majority think the wick burns to give the fame and the wax controls the rate of burning by melting down gradually. Wax is used because it does not burn! As might be expected, at 54, students are more able to agree that new carbon dioxide comes out of a candle fame (as opposed to carbon dioxide already in the air). Given students’ attachment to carbon dioxide, by itself, this doesn’t signify any meaningful understanding of chemical change.
The particle modelThe particle model side of Figure 2 deals with two degrees of resolution: a ‘basic’ model which talks of substance particles and then one at the level of atoms. The ‘basic model is good enough to explain the states, changes of state and mixing phenomena. Atoms are needed to explain chemical change. The ‘basic’ model will be considered first. To interpret particle representations, the first requirement is to associate a drawn particle with a substance. Items used different shapes and colour to represent different substances and identifying the number of substances involved is at a difficulty of 32. Next (39) is the idea that the particles are the substance as opposed to being something else extra embedded ‘in’ the continuous substance. This is for substances in each of the three states at room temperature. However, students are likely to think of the particles as being little pieces which have the macroscopic properties of the observed sample (i.e. little bits of ‘solid’, ‘liquid’ and ‘gas’). As such, the pieces can be of different sizes as can happen when something is broken up. That the particles for any one substance are all the same size comes at 48 for a substance in the liquid state and 54 for the solid state. In the region 60-63, attributing macroscopic properties to the particles is relinquished and students appreciate that the properties of a state depend on the arrangement and movement of particles as a collection and not their individual natures (whatever that might be). Nevertheless, students may still be unsure about what is between the particles — they may still want there to be ‘something’ and the idea of ‘nothing’ (empty space) is the most difficult aspect of the ‘basic’ model with difficulty in the region of 69–65 for the three states. Here, the gas state presents the greatest challenge.
Some items targeted the idea of intrinsic motion, using animations involving single and arrays of particles. For the solid state the ‘single’ and ‘array’ items were relatively close together at 56 and 60 respectively. For the liquid and gas states there was a big difference between the two formats. However, on average, these were much less difficult with the gas state easier than the liquid state as might be expected. Positions for these states cannot be given with any confidence but it seems safe to assume that the idea of intrinsic motion (for any state) has been accepted by 60 on the scale.
During interviews for the development of items, a preoccupation with the closeness of particles emerged. For many students this was the key issue and for items exploring other aspects it was important to have the same spacing in each of the option diagrams and put this in writing too. Otherwise, many students would ignore all else and choose on the basis of perceived differences in the spacing, alone. One item specifically targeted the spacing in the liquid state, but the responses to this item did not fit the Rasch model (the item was underfitting) and so a difficulty value on the ‘understanding chemistry variable’ of Figure 2 does not apply. A student’s ability as measured by the other items is not a good predictor of success on this item — for the best fitting level of difficulty that could be assigned to the item, not enough of the middling ability students were getting it right. Closeness of particles ought to belong to the variable and it could be that there was a fault with the item. Or the item could be hitting a pocket of confusion that persists despite progress elsewhere. The most popular choice showed particles spaced too far apart — mid-way between the solid and gas states. Unfortunately, it is not uncommon for text books to portray this misconception (Harrison and Treagust 2002), which may well be the cause of the confusion — perhaps reinforced by some teaching.
Seven items explaining a range of physical phenomena using a ‘basic’ particle model cluster together between 60 and 65. For evaporation of water, students needed to choose the option ‘simply’ showing water particles leaving to mix into and then remaining in the air unchanged. This was as opposed to changing to a ‘gas’ either directly or after entering the air, or going straight up to the clouds. For dissolving in water as a solvent, items used solutes in the solid and gas states at room temperature when on their own (pure samples). Students needed to choose the option representing the solution which ‘simply’ showed a mixture of water and solutes particles close together, the particles unchanged. With sugar as solute, alternative choices were evenly distributed between options showing sugar particles disappeared in a continuous solution — i.e. no particles showing, sugar particles in a continuous water background, ‘sugar’ and ‘water’ particles in a continuous sugar solution background and sugar particles inside water particles. With carbon dioxide as solute the main alternative choice showed tiny bubbles (labelled as such) dispersed amongst water particles. Also, a significant minority chose water particles with a continuous carbon dioxide background.
For melting, students were asked to choose between the following options to best explain the change from solid to liquid.
- A.
The particles move apart
- B.
The wax around the particles melts
- C.
Solid particles (hard) change to liquid particles (runny)
- D.
The particles start to move about from place to place, keeping close together.
Options A and D juxtapose ‘spaced apart’ and ‘moving around’ as the key criterion for the liquid state. In keeping with the misconception of spacing for particles in the liquid state, Option A was the most popular choice. Significant minorities also chose C and B. Using the idea of the strength of attraction between particles to explain different melting points and rates of evaporation is also in this 60-65 region of the scale. Different strengths in the ability of particles to attract each other for different substances is a crucial idea since it explains why different substances appear in different states at room temperature.
Two further items explaining physical phenomena were much higher up the scale. One (at 81) asked why iron was hard where most opted for ‘the particles are close together’ with only the most able choosing ‘the particles don’t easily change neighbours’. Again, the preoccupation with spacing is evident. The other (at 83), concerned the representation of a bubble in boiling water. In addition to the idea of empty space between particles as opposed to air (already at 69 for water in the liquid state), knowledge of hydrogen and oxygen as gases seemed to add to the difficulty. Few chose an image of water particles spaced apart with nothing in between. For a bubble of carbon dioxide in water represented in a similar way, the difficulty level was 63.
At the resolution of atoms, which atoms are bonded to which — the atom structure — defines a substance. For molecular structures this is the atoms making up a molecule. For giant structures this is the repeating unit of the array. Items targeting the link between atom arrangement and substance identity for molecular structures occupy the region of 48 to 60. The easiest required students to recognise a change of state and the separation of a mixture of substances; i.e. where the molecules remained intact. Items depicting a chemical change were at 55 and 60. As might be expected, two items showing a chemical change where one of the substances involved had a giant structure were more difficult (66 and 70).
Where were the students on the scale?Independent data for about 1000 students in each of Years 7, 8 and 9 were held by Durham University’s Curriculum Evaluation and Management (CEM) Centre (MidYIS, 2011). The CEM data base involves just over 2000 secondary schools in England and constitutes a good representation of the English school population. From a battery of standardized tests, the CEM data give a good indication of academic ability. The scores for the students were very similar for each of the year group samples, fairly normally distributed, but with a mean about one standard deviation above the national mean. For these CEM students, for each of Years 7, 8 and 9, the mean ability on the scale of Figure 2 is 50.1, 52.5 and 54.9 respectively. The corresponding standard deviations are 7.4, 7.7 and 8.6 (Johnson & Tymms, 2011). Since the sample is above the national average, adjusting the Year 9 mean by its standard deviation gives an estimated national mean of around 46. The data for these CEM students were collected at the end of the academic year. If the study is accepted as giving even just a rough picture of the situation in England, it seems that students make very modest year on year progress and that by the end of Year 9 the average student has barely begun to develop the concept of a substance.
Implications for the curriculumThe coherence of the progression emerging from the empirical data (Figure 2) is quite striking and supports the validity of the items and the appropriateness of applying the Rasch model. Relative difficulties of the various ideas make sense, and it seems plausible that this represents a path of learning that would be suitable for most students. Recognising chemical change is contingent on understanding what is meant by ‘a substance’. Without a means of attributing identity it is logically impossible to conceive of a change of substance. Chemists ascribe substance identity through physical and chemical properties. At an introductory level, chemical properties (e.g. iron goes rusty, carbon dioxide turns limewater milky) could be used, but of course these themselves are chemical changes and students could only learn these in a superficial way. Such happenings can only appear as little more than magic since students are not in a position to understand what is going on in terms of substances. With regard to physical properties, density has limited value since it does not give a means to recognise the distinction between a pure sample of a substance and a mixture of substances and it does not deal with the independence of identity from state. It is difficult to see any other route to developing the concept of a substance apart from through melting and boiling behaviour.
Traditionally, changes of state feature prominently in introductory chemistry (science) curricula. However, usually if not exclusively, this takes place within a ‘solids’, ‘liquids’ and ‘gases’ framework where the attention is on the generic properties of the states and not substance identity. The distinction between the behaviour of mixtures and pure samples is often ignored and there is a strong emphasis on linking identity to the room temperature state. Activities which ask students to classify materials as ‘solid’, ‘liquid’ or ‘gas’ carry the danger of teaching the misconception of three types of material as do descriptions such as ‘iron is a solid’ and ‘oxygen is a gas’. Elsewhere, we have argued that the ‘solids, liquids and gases’ framework impedes the development of students’ understanding of particle theory (Johnson and Papageorgiou, 2010).
With regard to melting, if not their first experiment, students often encounter the ‘cooling curve’ very early on. However, understanding the significance of the observations is in the region of 60-65 in Figure 2, which will be far above the likely ability levels of the students. It is anything but a ‘simple’ introductory event. Few have any chance of making sense of what is going on. From the perspective of the expert (i.e. looking down in Figure 2 from very high up off the scale) one does not necessarily appreciate all that is involved from the perspective of looking up from the bottom. In fact it is very easy for experts to assume that the concept of a substance is obvious. Introductory chemistry courses often start with chemical changes and usually in very difficult contexts involving substances in the gas state (such as combustion and reactions of acids). Superficially, this can be ‘fun’ for students, but without having developed a conceptual structure which allows them to make sense of the various events they are denied the deeper satisfaction of understanding.
Figure 2 makes no specific mention of elements and compounds as two types of substance. When ideas of atoms are introduced it becomes relatively straightforward to distinguish between substances where there is only one type of atom and those with two or more making up the structure. The idea of elementary and compound substances is a further differentiation of the concept of a substance. However, if ‘elements and compounds’ are introduced before the concept of a substance has been established (not uncommon in beginner chemistry courses) students do not have the grounds to distinguish between compounds and mixtures — a mix of atoms is no different from a mix of substances. Recognising the bonded atom structure which defines the substance is crucial here too. The formal distinction between molecular and giant structures is usually made when different types of bonding (covalent, ionic and metallic) are introduced in later secondary school (after atomic structure). There is a case for dealing with structures in relation to the substance when atoms are first introduced.
Teaching about the elemental composition of water too early seems to be seriously detrimental to students’ understanding of physical changes between the liquid and gas states. Without establishing water as a substance which can exist in either the solid, liquid or gas states (as any other substance), many students will think it must change to hydrogen and oxygen on boiling — since these are ‘gases’. The bubbles themselves, forming as they do within the body of the rest of the water remaining in the liquid state, seem to add to the demand. Choosing the option ‘water as a gas’ for an item showing a drop of water changing to a clear body of gas within a hot glass syringe was at 59 on the scale, compared to 68 for a bubble. The students’ predicament is not helped by text books which label the mist above boiling water as water in the gas state (not uncommon in England). As with the spacing for the liquid state, an item on this was underfitting — suggesting another pocket of confusion caused by instructional materials.
There often seems to be an implicit assumption that the role of the particle model is to explain macroscopic phenomena; i.e. the event is first understood at the macroscopic level and then explained at the particle level. However, in the longitudinal study there was evidence to suggest that in two key places particle ideas helped students to think about previously inconceivable macroscopic interpretations of events. Basic particle ideas open up the possibility that a substance could exist in the gas state: i.e. that ‘gases’ are substances. Ideas of atoms opened up the possibility that substances could change into other substances: i.e. that chemical change could happen. The progression in Figure 2 is compatible with these directions of operation but, of course, do not prove causality. Appreciating that particles do not carry macroscopic properties coincides with the idea of a substance existing in any of the three states. Interpreting molecular atom structure representations of chemical changes comes before the macroscopic understanding of chemical change as a change of substance. It seems plausible that particle ideas do help students see events in these new ways and perhaps these learning mechanisms should be built into curriculum design. Interestingly, interpreting molecular atom structure diagrams comes before the appreciation that the particles are not literally little pieces of what is observed. If particles are thought to carry the macroscopic properties it is difficult to see where atoms ft in. Perhaps the incompatibility (cognitive conflict) helps to move students on from macroscopic thinking.
ConclusionRasch modelling uses the difference between student ability and item difficulty on a supposed variable to predict the probability of success. In theory, the relative difficulties of items that emerge from the analysis do not depend on the sample of students taking the items (and the relative student abilities do not depend on which items they responded to). That such a large sample of students drawn from many different schools has produced data giving a good overall ft to the model with coherence in terms of ideas suggests the notion of a common learning pathway has traction. One prominent feature of the research literature on students’ problems in understanding chemistry is the commonality of findings across countries. This gives reason to suppose Figure 2 has wider application beyond the borders of England. The study has taken place within the context of the current Science National Curriculum in England, which is similar to that in other countries for introductory chemistry (Martin et al., 2004). The progression in Figure 2 shows the pathway along which understanding is developing, but the measure of student abilities shows most students are making slow progress. Given the mismatch between the curriculum and students’ needs, discussed above, this slow progress is perhaps not surprising. With better alignment between the curriculum and the learning progression there is every chance that students will make better progress. Johnson and Roberts (2006) and Johnson (2011) show how a curriculum based on developing the concept of a substance might look.