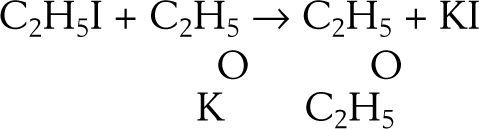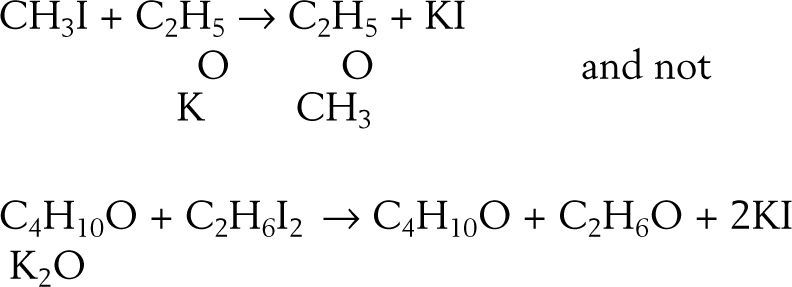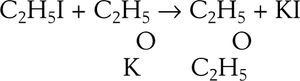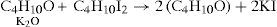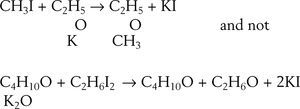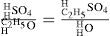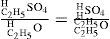Alexander William Williamson (1824-1904) discovered accidentally the synthesis of ethers by reacting an alcohol with alkyl iodide in the presence of sulfuric acid (Williamson's synthesis), a decisive fact in overthrowing Berzelius' dual theory. He promoted the use of water type as the single type for deducing the constitution of inorganic compounds and most of the organic ones. In spite of serious physical disabilities, he became one of the few students who were awarded a doctoral degree by Liebig.
Alexander William Williamson (1824-1904) descubrió en forma accidental la síntesis de éteres mediante la reacción entre un alcohol y un yoduro de alquilo en presencia de ácido sulfúrico (síntesis de Williamson), hecho que contribuyó en forma decisiva a destronar la teoría dual de Berzelius. Promovió el uso del tipo agua como tipo único para deducir la constitución de los compuestos inorgánicos y la mayoría de los orgánicos. A pesar ser físicamente minusválido, Williamson fue uno de los pocos estudiantes a los cuales Liebig les otorgó el doctorado.
Alexander William Williamson was born at Wandsworth, London, on May 1st, 1824, the second son of Alexander Williamson, a clerk at East India House, and Antonia McAndrew, a merchant's daughter. He had a sister, Antonia Helen, born in 1822, and a brother, James, who died in childhood (Forest, 1905).
As a child, and throughout his life, Williamson suffered from severe physical limitations: a semi paralyzed left arm, a blind right eye, and a myopic left one. In 1857, he was for a month almost deprived of sight by an explosion in his laboratory. These deficiencies undoubtedly promoted his later dissatisfaction for delicate laboratory work and encouraged his theoretical and speculative powers, which had been stimulated by his philosophical education by a father extremely interested in scientific matters (Divers, 1907; Forest, 1905).
When Alexander William was six years old his family moved to Kensington, and after his father's retirement from the East India Company's service, the family went abroad and resided for many years, first in Paris and then near Dijon. He attended day schools in Kensington, in Paris, and at the College at Dijon. Later on he spent three years (1841-1844) at Heidelberg, where he went to study medicine at the wish of his father. There he attended the lectures of Leopold Gmelin's (1788-1853) on chemistry and those of Friedrich Tiedemann's (1781-1861) on anatomy. Very soon, however, Williamson told his father that he wanted to become a chemist, not a physician, an announcement, which was not favourably received. Initially Gmelin also tried to discourage him because of his physical limitations but eventually he became so impressed by the zeal and intelligence of his pupil that he told his mother that her son “would be chemist” (Divers, 1907; Forest, 1905).
In 1844 Williamson transferred to Giessen to study under Justus von Liebig (1803-1883). At that time Giessen was the main European centre for chemical teaching and research; the Giessen Laboratory was the first ever built specially for the reception of students, and Liebig's research and discoveries were establishing a new horizons in chemical training and investigation. Williamson remained in Giessen for two years and during his first semester besides working at chemistry he attended Theodor Ludwig Wilhelm Bischoff's (1807-1882) lectures on physiology, then his favourite science next to chemistry (Forest, 1905; Divers, 1907).
His work at Giessen resulted in his first papers on bleaching salts, ozone, and Prussian blue (Williamson, 1845, 1846, 1847b). The material for a note regarding castor oil (Williamson, 1847a), published after he left Giessen, was also obtained there (Forest, 1905). During his first year at Giessen he became quite interested on the theory of galvanism. As a result, he wrote an essay on “a system that seems to me much more simple and natural”. In his letters to his parents he repeatedly referred to electrical experiments with which he was engaged, and in April, 1845, he spoke of a paper on electricity which he had submitted to Liebig, whose reception of it was much more favourable than he had expected, “for it was the theory of Humphry Davy which I had ventured to attack”. With Williamson's agreement, Liebig submitted the paper to Heinrich Buff (1805-1879), professor of physics. Although the comments of the latter were favourable, the paper was not published. In the course of their interview about the electricity paper Liebig proposed to Williamson that he should take the degree of Doctor of Philosophy. The degree was conferred in August 1845, without a thesis (Forest, 1905; Harris and Brock, 1974). Eventually Williamson did publish a paper on galvanism (Williamson, 1863).
After the summer of 1845, Williamson interrupted for a time his chemical studies in order to devote himself to mathematics and physics. He worked at the former under Friedrich G. K. Zamminer (1817-1858), Extraordinary Professor of Physics at Giessen, and at the latter with Buff, who accorded him special facilities and gave him access to the Physical Cabinet. In the summer of 1846 he went to Paris, and stayed there for three years, studying advanced mathematics under August Comte's (1798-1857) (Forest, 1905). During his stay in Paris he established his own research laboratory and became friend with Auguste Laurent (1807-1853), Charles-Frédéric Gerhardt (1816-1856), Charles-Adolph Würtz (1817-1884), and Jean-Baptiste André Dumas (1800-1884), all of who would strongly influence his approach to chemistry.
In 1849, on the death of George Fownes (1815-1849), Williamson was unanimously elected to the Professorship of Practical Chemistry in University College, London, and thus came into direct communication with Thomas Graham (1805-1869), who at that period held the Chair of General Chemistry (Divers, 1907). Williamson's first few years at University College constituted a period of outstanding activity and fruitfulness. The first session produced his ground breaking paper on etherification (Williamson, 1850, 1851), which was soon followed by important publications from his students; for example, Patrick Duffy on the composition and properties of stearin from different sources, using sodium methoxide as a methylating agent (Duffy, 1852), and A. Winkler Wills on heptanol from plant essences (Wills, 1854; Forest, 1905).
In 1855 Graham resigned the Chair of Chemistry in University College to become Master of the Mint, and Williamson was unanimously chosen to replace him. His researches on bleaching salts and on Prussian blue had gained him his first academic position; now, his break-through discoveries on etherification were instrumental in getting his promotion (Williamson, 1850, 1851, 1851-1854, 1852ab, 1854cd). Shortly thereafter he married Emma Catherine Key, daughter of his colleague, Thomas Hewitt Key (1799-1875), Professor of Comparative Grammar, to whom he had been engaged for more than a year. They had a son, Oliver Key (1866-1941), and a daughter, Alice Maude (1861-1946) (Forest, 1905).
In 1886 he moved to High Pitfold, Haslemere, and there began to devote most of his time to farming on scientific principles. Two years later he resigned his active appointments at University College where he had been a professor for 39 years, and was elected Emeritus Professor of Chemistry. Williamson passed away on May 6, 1904, at the age of eighty, and was buried at Brookwood, in Surrey (Divers, 1907).
From 1854 on, he became interested on the possibility of generating steam for locomotive purposes in a more efficient way. By 1860 he and his friend Loftus Perkins (1834-1891) had patented a water-tube boiler, a steam condenser, and a steam engine (Williamson and Perkins, 1859, 1860ab). The boiler was built such that “a number of slanting or vertical tubes, each acting as a separate steam generator, are firmly connected to a foundation at their lower ends, and are there made to intercommunicate by having connecting pipes attached to them for supplying the feed water, whilst the upper ends have no rigid connections, and are free to expand in the direction of their length, the steam being conducted, by small curved pipes attached to their upper ends, into main steam-pipes”. The sidewalls of the combustion chamber formed by stacks of this especially connected tubes and allowed the hot gases to circulate around the pipes, so as to raise steam and superheat it. In another of their patents they wrote “our chief object is to employ steam of very high pressure, as for example of 500 lb/in2, or more or less, and to expand this steam several times, and then condense it so as to obtain a great amount of power from a small quantity of steam” (500 lb/in2 is about 33 atmospheres; modern boilers are able to reach more than 10 times this pressure).
Although he published a small number of papers, Williamson was the most influential chemist in Great Britain during the period 1850-1870, which saw the downfall of Jöns Jacob Berzelius' (1779-1848) electrochemical dualism, the establishment of the theory of types by Laurent, Gerhardt, and Jean-Baptiste André Dumas (1800-1884), the creation of a rational system of atomic weights, the development of the concepts of valence and structure, and the realization of the first international meeting of chemists at Karlsruhe (1850), where Avogadro's hypothesis became accepted.
Honors and awardsWilliamson received many honors for his contributions to science and public life. He was honorary, correspondent, or associate member of the most important European scientific societies, among them, the Literary and Philosophical Society of Manchester; the German Chemical Society, the American Chemical Society; Académie des Sciences; Royal Turin Academy of Sciences; Accademia dei Lincei, Royal Berlin Academy or Sciences, and the Athenaeum Club. In 1862 the Royal Society awarded Williamson one of its medals in recognition of his work on etherification; he also received honorary doctorates from the universities of Dublin, Edinburgh, and Durham, and was Fellow of the University of London.
Williamson was elected a Fellow of the Royal Society in 1855 and served three times on its Council, and as Foreign Secretary from 1873 to 1889. He joined the Chemical Society in 1848 and was elected its President in four opportunities. In 1872 he started publishing in the journal of the Society monthly reports in the form of abstracts of all papers of a chemical nature. In several opportunities he was President of the Chemical Section of the British Association for the Promotion of Science meetings, and served as its General Treasurer from 1874 to 1891. In 1876 he was appointed by Sir Charles Adderley (1814-1905) to succeed Dr. Henry Letheby (-1876) as Chief Gas Examiner under the Board of Trade (Divers, 1907; Forest, 1905).
Scientific contributionWilliamson did work on a wide range of subjects, among them, the action chlorine on oxides and salts (Williamson, 1845), Prussian blue and other compounds of cyanogen and iron (Williamson, 1846), ozone (Williamson, 1847b), organic chemistry (Williamson, 1852c, 1853ab, 1854ab, 1855d, 1854-1855ab) and the measurement of gases (Williamson and Russell, 1859, 1864). His most important contributions, which brought him the most fame, was the discovery of a new method of synthesizing ethers, the mechanism of the reaction, and its implications regarding the type theory, kinetics, atomicity, and chemical nomenclature and notation (Williamson, 1864ab, 1865bc, 1869). He suggested that water type should be sufficient for deducing the constitution of inorganic compounds and most of the organic ones. He published under his name less than forty papers and one book (Williamson, 1865d); some papers were published only under the name of his students.
Here we describe some of his most important contributions.
Bleaching SaltsWhile at Giessen, Williamson, at the age of 20, published in London the results of his first research (Williamson, 1845). More as an exercise than for purposes of investigation, Liebig set him to work upon the subject of the bleaching salts, which had recently been receiving attention in France, and even in Germany in the Giessen laboratory itself. To Williamson's chagrin, Liebig at first discredited these results as too improbable, but he was eventually convinced (Divers, 1907).
As ordinarily carried out, the production of a chlorate is the result of the catalytic action of chlorine upon the hypochlorite present in the solution of (so-called) chloride of lime or similar bleaching salts; a concept that Joseph-Louis Gay-Lussac (1778-1850) had already advanced. But Williamson succeeded in showing, in the first place, that once chlorine in an aqueous solution has combined with an alkaline earth or an alkali to form chloride and hypochlorite of the metal, additional chlorine can be made to dissolve in the solution by converting the hypochlorite itself into chloride, along with hypochlorous acid, and a little of chlorate. In the next stage, Williamson found that upon heating, the solution of metallic chloride and free hypochlorous acid transforms into one containing an amount chlorate equivalent to one-half that of the chloride, with the simultaneous release of as much chlorine as had been absorbed by the bleaching solution. To prove this point more convincing he dissolved some potassium chloride in a concentrated solution of hypochlorous acid, and on heating it changed this chloride into chlorate and free chlorine, thus establishing what had seemed to Gay-Lussac (1842) to be most unlikely. Nearly 40 years later (1883), Georg Lunge (1839-1923) and Paul Naef, unaware of Williamson's results, succeeded in showing that, even in the cold, hypochlorous anhydride freely decomposes solid hydrated calcium chloride by converting it partly into hypochlorite (Divers, 1907).
Finally, Williamson proved, contrary to what Liebig had allowed his English student, M. Detmer, to publish (Detmer, 1841-1843), that hypochlorous acid, which so readily decomposed a chloride, was unable to decompose a dissolved alkali carbonate or bicarbonate. Williamson saw this result as proof that in the abundant production of a chlorate that may take place when passing chlorine through a solution of potassium or sodium bicarbonate, the production of the chlorate depends only upon the interaction of the first-formed alkali chloride and hypochlorous acid (Divers, 1907).
Chemists already were familiar with the bleaching properties of the compounds obtained by the direction action of chlorine on the alkalis and the alkaline earths hydroxides. They had considered these compounds to be the chlorides of oxides, that is, the direct combination of chlorine an oxide, but Berzelius had been the first to fight this idea, by affirming that the bleaching compounds were actually a mixture of chloride and a salt formed by the oxygenated acid of chlorine. Experiences done by Eugène Soubeirau (1797-1858) had supported this opinion: by evaporation of the aqueous solution of an apparent oxide chloride he obtained crystals of chloride while the mother liquor retained all its bleaching power. Afterwards, François Ernest Balard (1833-1894) prepared an oxygenated combination Cl2O by reacting mercuric dioxide and chlorine, where the bleaching powers are like the product of Soubeirau (Williamson, 1845).
Williamson studied the amount of chlorine that can be absorbed by a base, particularly barite and potash. Pure chlorine was bubbled at room temperature through a barite solution; saturation was assumed to be complete when the liquid acquired a yellow tint. The liquid, agitated with air, maintained its yellow color, and chlorine. At the end of the process, it presented a slight odor of hypochlorous acid, followed by a strong chlorine odor. A portion of the solution was saturated further with ammonia, and after being heated for some time, it was acidulated with nitric acid. Chlorine was determined using silver chloride, and the barite, in the filtered liquid, in the state of sulfate. Three different experiences gave essentially the same result, that is, there was one equivalent of barium for each two equivalents of chlorine. In order to determine the state of chlorine inside the liquid, Williamson added silver nitrate to a portion of the barite water saturated with chlorine, resulting in a white precipitate of silver chloride, which increased after the solution was left to rest. To another portion he added barite water until the odor and the bleaching properties of hypochlorous acid had disappeared completely. Addition of the silver salt resulted in a black precipitate that decomposed slowly with release of oxygen. If in the first case, the acid was not completely free then it should have formed in addition to the white precipitate, a certain amount of black precipitate (Williamson, 1845).
The following experience was more conclusive. The hypochlorous acid was neutralized with barite water; the resulting salt did not bleach litmus paper but the bleaching became considerable after addition of an acid. Treatment with carbon dioxide resulted in a precipitate of carbonate that was eliminated by filtration. The filtered liquid was boiled for some time; the freed hypochlorous acid escaped and the residue did not contain barite, showing that the salt had been completed decomposed by the carbon dioxide. Next, a solution of pure potash was saturated with chlorine, where it contained 3/2 equivalents of chlorine per equivalent of potassium. The resulting liquid had the smell of chlorine, which could not be eliminated by agitation with air. It had enough chlorate and hypochlorous acid was also present in the free state. The results indicated that if potash produced chloride and hypochlorous acid, then the latter decomposed fast into free chlorine and chlorate, while with barite the two phases of this metamorphosis were less complete (Williamson, 1845).
Other experiments showed that hypochlorous acid was unable to displace carbon dioxide from carbonates. There was no effervescence when a concentrated solution of hypochlorous acid was added to a solution of sodium carbonate. The liquid kept its odor of hypochlorous acid, like if it had only been diluted with water. But addition of a drop of caustic made to odor and the bleaching properties disappear immediately. Treatment with chlorine resulted in a solution of sodium carbonate, chloride and free hypochlorous acid, which after rest, became loaded with chlorate. The latter was formed by the action of hypochlorous acid on the chloride (Williamson, 1845)
In this publication Williamson also reported the preparation of hypochlorous acid, and the action of hypochlorous acid on potassium chloride, sodium phosphate, sodium pyrophosphate, sodium sulfate, potassium nitrate, lead acetate, potassium chromate, borax, cyanogen, etc. Hypochlorous acid was described as a weak acid with the chemical formula HOCl, formed when chlorine dissolved in water. It could not isolated in pure form due to rapid equilibration with its precursor (see below). The acid could be used as bleach, an oxidizer, a deodorant, and a disinfectant (Williamson, 1845).
OzoneIn 1840 Schönbein announced the discovery of ozone in a memoir presented to the Academy of Munich (Schönbein, 1840). He reported that during the electrolysis of water oxygen was released at the positive pole accompanied by an odorous substance, which could be preserved for a long time in well-closed vessels. The production of this material was influenced by the nature of the metal that served as the pole, by the chemical properties of the electrolytic fluid, and by the temperature of that fluid as well of the electrode. He also remarked that the odor was the same that accompanies a flash of lightning. In his memoir Schönbein speculated that the odorous body was a new electro-negative element, belonging to the same class as chlorine and bromine, and for which he proposed the name ozone (from the Greek, ozein: smell). Schönbein soon afterwards discovered that ozone is also formed when phosphorus oxidizes slowly in the presence of moist air or oxygen.
Williamson did not agree with Schönbein's claim that ozone was also the substance formed during an electric discharge in air. It was true that during electrolysis the new substance appeared at the positive pole, but with static electricity generated by friction, it developed at both poles. Williamson believed that the particular properties attributed to ozone were actually determined by a mixture with a peroxide containing hydrogen more oxidized than in hydrogen peroxide. This peroxide was not hydrogen peroxide because it was volatile and smelled (Williamson, 1847b).
A second paper quickly followed that on chlorine and purports to have proved that ozone is a peroxide of hydrogen (Williamson, 1847c). Ten years afterwards, however, Thomas Andrews (1813-1885) and Peter Guthrie Tait (1831-1901) proved that ozone is not a compound at all (Andrews and Tat, 1856-1857, 1857-1859). Williamson had worked with a mixture of ozone and oxygen obtained by electrolysis, and these mixed gases, apparently, had not been kept quite free from electrolytic hydrogen (Divers, 1907).
Prussian blueVery soon after the appearance of his paper on ozone, Williamson published his important results on the composition of Prussian blue (Williams, 1846). This beautiful preparation was then of special interest as a salt because in it iron functioned partly as basic and partly as acid radical. It had been easy to ascertain that it contained both ferrous and ferric cyanides and potassium cyanide and that these cyanides were not present in a constant proportion. It had also been possible to anticipate, with some confidence, that among its component substances, the two complex iron cyanides Fe7C18N18 and Fe5C12N12 were present but it had hitherto not been possible to verify this conjecture because Prussian blue, being amorphous and highly colloidal was incapable of being separated into the several substances of formed it (Divers, 1907).
Williamson succeeded in preparing both iron cyanides in a very pure state, notwithstanding their colloidal nature. He also produced a new blue salt, a constituent likewise of Prussian blue, a double cyanide of iron and potassium, KFe2C6N6, by the limited oxidation of the insoluble, nearly white, potassium ferrous cyanide K2Fe2C6N6, known as Everitt's salt. The new salt, since obtained in various ways, proved to be of perhaps greater interest than either of the other constituents of Prussian blue, namely, Turnbull's blue, Fe5C12N12, and the substance, Fe7C18N18, (Williamson's blue). One interesting feature of the latter compound is its being the parent salt of Turnbull's and Williamson's blues; in 1875 Zdenko Hans von Skraup (1850-1910) showed (Skraup, 1875) that is it also the first substance to precipitate when a ferrocyanide is mixed with a ferric salt or a ferricyanide with a ferrous salt. In other words, well-washed and freshly prepared Prussian blue is always a mixture of these three blue salts and of these only. Another interesting feature about Williamson's new salt was the remarkable facility with which it could be converted into a form freely soluble in water. It appeared to be possible, indeed, to get all three blue constituents of ordinary Prussian blue into solution in water. Nevertheless, such dissolution of two of them takes place rarely, while that obtained by Williamson, is usually found to be so soluble in water as to have become known as soluble Prussian blue, and is only obtainable in a form insoluble in pure water when it is prepared by Williamson's method (Divers, 1907).
Another important result that came out from Williamson's researches on the subject, is that when potassium ferrocyanide in solution is digested with Prussian blue, the ferric cyanide present in each of the three blue salts will always take potassium cyanide from the ferrocyanide and transform into potassium ferricyanide in solution, leaving place an insoluble pale blue substance containing all the ferrous cyanide together with some of the potassium cyanide of the potassium ferrocyanide, apparently as Everitt's salt. Williamson proposed this way to manufacture potassium ferricyanide in a pure state (Divers, 1907).
The molecular structure of aluminum saltsUntil the middle of the nineteenth century, aluminum chloride had always been represented by the formula Al2Cl3, or as AlCl3 when selecting the high atomic weight of aluminum, as required by its specific heat. In their studies on the dissociation of compounds, Henry Sainte-Claire Deville (1818-1881) and Louis Joseph Troost (1825-1911) found that aluminum chloride decomposed at high temperatures and that at 500°C the degree of decomposition corresponded to the formula Al2Cl6, while at 100°C it was AlCl3 (Deville and Troost, 1860). This result led many chemists to adopt the formula Al2Cl6 and to double the previously accepted formulae for the entire series of aluminum compounds. According to George Boudler Buckton (1818-1905) and William Odling (1828-1921), the experimental data available was not enough to justify the definite formula (Buckton and Odling, 1865). For these reasons they decided to examine organo-aluminum compounds to throw light upon the question. Ethyl aluminum was prepared by reacting mercuric ethide with an excess of aluminum clippings in a heated sealed tube; elemental chemical analysis of the product accorded well with the formula AlEt3 or Al2Et6. At 234°C the vapor density, calculated using Guy-Lussac's method, was found to be 4.5 against the theoretical density of 3.9 calculated for the formula AlCl3. From these results Buckton and Odling concluded that ethyl aluminum would appear to have the simple molecular formula AlEt3, for the difference between the experimental and the theoretical results was an obvious consequence of the extreme oxidizability of the compound. Methyl aluminum was prepared by a similar procedure and its elemental chemical analysis and density measurements corresponded to the formulae AlMe3 or Al2Me6. Buckton and Odling observed that the density increased very rapidly every decrease of temperature, a peculiarity also noticed by Frankland for boric methide. Hence aluminum methide appeared to belong to that class of bodies presenting anomalous vapor densities under certain circumstances, either because they dissociate or deviate from ideality, particularly when heated well above their boiling points. These results led Buckton and Odling to question the procedure of determining the general formula of aluminum compounds through measurement of the density of their vapors (Buckton and Odling, 1865).
Buckton and Odling's results elicited a prompt reply from Williamson (Williamson, 1865a). He believed that even if the density of aluminum chloride was unknown there was enough information for assigning to aluminum methide the molecular formula Al2Me6, and a vapor density corresponding to Al2 Me6 = 2 volumes. The close analogy of aluminum and ferric salts was perfectly notorious and the constitution Fe2O3 for ferric oxide clearly suggested Al2O3 as the formula for alumina. In the same manner, the most probable molecular formula for the chlorides of these metals should be FeCl3 and AlCl3.
A considerable number of other compounds have been found to occupy in the state of vapor nearly double the volume which corresponds to one molecular; but with very few exceptions, all of them have been proved to have undergone decomposition, so as to consist of two combined molecules. Thus ammonia chloride was admitted to have the molecular formula NH4Cl, although in the state of vapor this quantity occupied the volume of nearly two molecules, viz. four volumes. The arguments for admitting that the low vapor densities of the aluminum compounds are anomalous were even stronger than those which were admitted for nitric peroxide; very severe heating was required to get aluminum compounds to near four volumes, while very ingenious devices were needed to get nitric peroxide out of the four volume state. The decision on the atomic weight of aluminum was more difficulty than with most other metals because only one oxide of the metal (and the salts corresponding to it) was known, but the analogies connecting aluminum with other metals were so close and so numerous, that there were probably few metals of which its position the periodic table of the elements could be settled so easily. Aluminum is a metal singular for only appearing in the pseudo-triatomic character in which iron and chromium appear in their sesquisalts (Williamson, 1865a).
The question of the atomic weight also interested Charles Friedel (1832-1899) and James Mason Crafts (1839-1917) in their intents to explain the mechanism of their reaction. Friedel and Crafts' original explanation assumed the formula of aluminum chloride to be Al2O6, but Lars Frederik Nilson (1840-1899) and Sven Otto Pettersson (1848-1941) (Nilson and Pettersson, 1887) had recently reported that the molecular weight of the salt, calculated according to Viktor Meyer's (1848-1897) method, pointed to the formula AlCl3, that is, aluminum was tervalent. Given the theoretical importance of the subject, Friedel and Craft decided to perform their own measurement claiming that the Meyer method was unsuitable because it required operation at a temperature (440°C) well above that of the normal boiling point of the compound (183°C). Friedel and Crafts determined the vapor pressure and the density of aluminum chloride at different temperatures and found it to correspond with that required by the molecular formula Al2Cl6. They speculated that at higher temperature aluminum chloride decomposed into two gases, which would explain the results of Nilson and Pettersson (Friedel and Crafts, 1888). Today we know that aluminum is a tri-covalent compound in which the outer shell of the aluminum is incomplete. The combination of the aluminum chloride with the alkyl chloride completes the octet atom and gives an addition compound in which the alkyl radical has a positive charge.
In a later paper on the classification of the elements in relation to their atomicities (Williamson, 1864a), Williamson proposed doubling the atomic weights of all the metals in Gerhardt's system of atomic weights, except for the alkali metals, silver, gold, boron, and the metals of the nitrogen series.
EtherificationAccording to Williamson (Williamson, 1850) when sulfuric acid reacts with alcohol a new arrangement takes place in which the constitutive elements of the alcohol molecules divide into two groups to form ether and water. This phenomenon may be explained in two ways, according to the molecular weight assigned to the alcohol. Assuming the molecular weight to be 23 (corresponding to the formula C2H6O) then two molecules of alcohol are required to form the ether, one molecule taking C2H4 from the other and setting water free, If the molecular weight is assumed 46 (corresponding to the formula C4H12O2) then alcohol contains ether and water. The purpose of Williamson's initial experiments was not to discriminate between the two explanations but to obtain new alcohols by substituting a carburetted hydrogen for hydrogen in a given alcohol. For this purpose he first prepared a potassium alcoholate and then reacted it with an alkyd iodide. To his “astonishment” the reaction between potassium ethylate and ethyl iodide produced diethyl ether, instead of an ethylated alcohol (this reaction is known today as Williamson's synthesis). Accidentally, a totally new way of preparing ether under alkaline conditions was discovered, as opposed to the centuries old reaction of sulfuric acid with ethanol.
Williamson understood immediately that his results were inconsistent with the assumption that he molecular weight of alcohol was 46 because if true, then the final product should have contained twice as many atoms of oxygen atoms as they are in ether. In other words, the reacting compounds were actually C2H5 and C2H5 and the reaction could be written:
which required accepting that both alcohol and ether belonged to the water type where, for alcohol, half the hydrogen had been replaced by carburetted hydrogen and for ether, both atoms of hydrogen had replaced by carburetted hydrogen.Williamson realized, however, that his experimental results could also be explained using a four-volume formulation for water, H4O2, if it were supposed that both potassium ethylate and ethyl iodide contained ether:
To test this option he reacted the potassium ethylate with methyl iodide reasoning that if the compound was ether and potash, then the result should be a mixture of ether and methyl oxide, if not then he should obtain a body of composition C3H8O, which he actually did:
Similar results were obtained when reacting amyl iodide with potassium methylate or potassium ethylate. In the latter case the product was an ether boiling at 111°C and having the composition C7H16O.
From these results Williamson concluded that “alcohol is therefore water in which half the hydrogen is replaced by carburetted hydrogen, and ether is water in which both atoms of hydrogen are replaced by carburetted hydrogen” (Williamson, 1851). Ethers could thus be built about the water type. Inherent in Williamson's proposal was that water is H2O and not OH, as had been maintained by many of his colleagues, and that alcohol was not hydrated ether from which water has been removed by the action of the acid.
Williamson then proceeded to explain the process of etherification by the action of sulfuric acid on alcohol by using the analogy of the simple and compound radicals in their compounds. He first showed how a substance analogue to ethyl iodide is initially formed (sulfovinic acid), followed by its double decomposition with alcohol to produce ether. Sulfovinic acid is strictly analogous to ethyl iodide plus hydrogen iodide, which is obtained by replacing SO4 in its formula by an equivalent of iodine. In other words, sulfuric acid and alcohol are first transformed into sulfovinic acid (ethyl sulfate) and water by an exchange between half the hydrogen in the acid and the carburetted hydrogen in the alcohol. Thus
In Williamson's words: “Now from this point it is clear that the process is the same as in the decompositions above described; for by this sulfovinic acid coming in contact with an atom of alcohol, it reacts exactly in the same manner as the iodide did, forming of course sulfuric acid and ether:
The sulfuric acid thus reproduced comes again in contact with alcohol, forming sulfovinic acid, which reacts as before; and so the process goes on continuously, as found in practice…We thus see that the formation of ether from alcohol is neither a process of simple separation not one of mere synthesis, but that it consists in the substitution of molecule for another, and is effected by double decomposition between the two compounds…I therefore admit the contact theory… as a necessary condition of the reaction of the molecules upon one another…On the other hand I attach equal importance to the essential facts of the chemical theory (catalysis)…for one-sixth of the hydrogen in the alcohol truly exhibits different reactions from the remaining five, and must therefore be contained in that compound in a different manner from them…” (Williamson, 1851).*
Williamson then added (Williamson, 1851-1854): “Having proved by a direct experiment that the formation of ether from alcohol is effected by substituting ethyl (C2H5) for 1/6 of the hydrogen of that body, the process of etherification by sulfuric acid was explained by a diagram in which half the hydrogen in sulfuric acid was shown to change places with its analogue ethyl in alcohol, and that the peculiarity of the process, i.e., its continuity, is owing to this change of place between hydrogen and ethyl, first taking place in one direction and then in the opposite, that is, that sulfuric acid becomes sulfovinic acid by taking an ethyl instead of an atom of hydrogen, and it is then reconverted into sulfuric acid by resuming hydrogen instead of this ethyl, the first change forming water, the second ether. By using successively two different alcohols…the two steps of this decomposition can be separated and their reality proved. The process of etherification is thus effected by a succession of double decompositions, each of which considered individually is perfectly conformable with the law of definite proportions, but the alternation and continuous succession so clearly proven in them, is a fact unexplained by the law. A complete analogy between this process and the more familiar cases of chemical action is therefore only to be established by finding in these latter a similar atomic motion. A little reflection is sufficient to show that such a motion actually exists. The fact of diffusion is in reality nothing but a change of place between atoms effected by the mere action of the particles on one another. We have in etherification an evidence of the tendency of atoms of analogous nature to change places continuously, and it is natural to suppose that the facility of this interchange must be greater in proportion to the analogy between the molecules, and greatest between like molecules” (Williamson, 1851-1854).
Williamson claimed that the above reactions were the best evidence of the nature of the action of sulfuric acid in forming ethyl ether (or in accelerating the formation of mixed ether) for acetic ether was formed from acetic acid just as ethyl ether from alcohol by the replacement of hydrogen by ethyl…”and if the circumstance of containing hydrogen, which is replaceable by other metals or radicals, be the definition of an acid, we must consider alcohol as acting the part of an acid in these reactions. Common ether is its ethyle salt, the 3-carbon ether is the methyle salt, and so on, just as potassium-alcohol or ethylate of potash is its potassium salt…Viewing…alcohol as water in which half the hydrogen is replaced by ethyle…we shall consider acetic acid as containing one equivalent of oxygen in the place of two atoms of hydrogen in that radical…acetic acid differs from alcohol by containing instead of ethyl, this other radical…and which may be called oxygen-ethyl or othyle” (Williamson, 1851-1854).
August Kekulé (1829-1896) went on to prove that adopting the idea that the series of organic compounds based on sulfuretted hydrogen (hydrogen sulfide) is a type corresponding in every aspect with the series of water type, then it would be possible to prepare new compounds in which the oxygen atom would be replaced by sulfur (Kekulé, 1855). Accordingly, by reacting phosphorus trisulfide with acetic acid he prepared thiacetic acid and by reacting phosphorus pentasulfide with acetic acetate he obtained ethyl thiacetate, compounds in which only half the oxygen was replaced by sulfur and which were very to the original acetic compounds. These results confirmed Williamson's action in formulating acetic acid with half its oxygen in the radical, acetyl or “othyle”.
Williamson's ideas were violently attacked by Kolbe (Kolbe, 1855) who complained of Williamson rejecting too lightly the old theory, which he considered to be “as a main pillars of organic chemistry…these facts carefully considered should convince Williamson that his othyle does not exist in the acetyl compounds in the form which he assigns to it”. Williamson rebutted Kolbe's arguments (Williamson, 1855) in the following terms: “I am not aware of having pretended, by my experiments…to refute either of the old theories of etherification, for they were refuted by facts known by chemists before ever I worked on the subject…The two theories were generally described…as the contact theory and the chemical theory. The latter of these attributed the formation of ether to the action of heat on sulfovinic acid (Liebig); the former (Berzelius) invented the name Contact-force, or Catalysis, and said that sulfuric acid acted by this force and not by its usual combining force. Now by thus inventing a name for the cause of an anomalous decomposition, the contact theory did not in any degree advance our knowledge of its nature, although some persons have imagined that the difficulty was solved by the invention of this name (!!)…It is easily perceived…that the real question at issue between Dr. Kolbe and ourselves has been overlooked by him…for, in accordance with the older habits of reasoning upon chemical reactions, he represents chemical combination as consisting simply in an addition of atoms, and his formulae for the representation of processes of chemical combination are essentially additive, although numerous reactions…are so clearly proved to consist of double decompositions..”.
Williamson's work on the water type inspired Gerhardt (Gerhardt, 1853) to explore the reactions of the salts of carboxylic acids with acid halides, thereby preparing the true anhydrides, which, in his view, were of the water type. These formative studies allowed Gerhardt to define the basic types: H2O, NH3, HCl, and H2.
Williamson's impressive study, together with his explanation of the process of continuous etherification by an exchange or double decomposition mechanism, led him to a number of important historical consequences. Firstly, he was led to reject completely the notion of a catalytic force and decide on chemical intermediates in catalyzed reactions. Secondly, he was led to picture atoms and molecules in motion, and not as the static particles of traditional Daltonism. The mechanism of etherification was inconceivable unless it was viewed as a process of continuous atomic exchange. Finally, and most important, the study suggested that analogies for the classification of both organic and inorganic chemistry should be based on the inorganic type water (Harris and Brock, 1974).
Gerhardt, Odling, Kekulé, and many other chemists immediately adopted the idea of the water-type set forth in Williamson's paper. The then recent discovery of ethyl amine by Würtz, and August Wilhelm Hofmann's (1818-1892) discovery of diethylamine and triethylamine and the corresponding derivatives of aniline made chemists familiar with the idea of the existence of a considerable number of compounds whose properties and constitution could be better understood by regarding them as being built up on the same type as ammonia (Forest, 1905).
Priesner has published a comprehensive review of the history of ether and the etherification reaction (Priesner, 1986).
Almost simultaneous with Williamsons' report, Gustave Charles Bonaventure Chancel (1822-1890) informed the Académie des Sciences that he had synthesized diethyl ether by reacting potassium ethylate with ethyl sulfate, and methyl ethyl ether from potassium methylate and ethyl sulfate (Chancel, 1850).