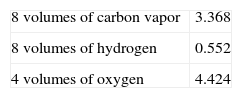To Auguste André Thomas Cahours (1813-1891) we owe the discovery and synthesis of a large number of chemicals, among them, amyl and allyl alcohol, toluene, xylene, cuminic and anisic acids, piperidine, organometallic compounds and radicals, and derivatives of phosphine and arsine, as well as important contributions to the theories of valence, isomerization in the aromatic series, density of vapors, particularly the abnormal ones, the use of PCl5 as a chlorination agent, and the mechanism of respiration of fruits and flowers.
A Auguste André Thomas Cahours (1813-1891) le debemos el descubrimiento y síntesis de un gran número de compuestos, entre ellos, los alcoholes amílico y alílico, tolueno, xileno, los ácidos anísico y cumínico, piperidina, compuestos y radicales organometálicos, y derivados de la fosfina y la arsina, así como contribuciones importantes a la teoría de la valencia, isomerización en la serie aromática, densidad de vapores, en especial los casos anormales, el uso del PCl5 como agente de cloración, así como el mecanismo de respiración de frutas y fores.
Auguste André Thomas Cahours was born on October 2, 1831 in Paris (Seine), the elder of the two sons of Rose Adelaïde Cartront and André Cahours, originally employed at the Ministry of Finances, and afterwards owner of a modest tailor shop on the rue de Provence.1 After finishing his first education at the local lycée he entered the
École Polytechnique in 1833 and after graduation (1835) he joined the corps of the Army Chief of Staff but promptly abandoned the military career to follow his interests in chemical research. Michel Eugène Chevreul (1786-1889), his former teacher, appointed him préparateur at his laboratory at the Muséum d’Histoire Naturelle, a position Cahours occupied for four years.
In 1839 he transferred to the private laboratory that Jean-Baptiste André Dumas (1800-1884) had on rue Cuvier. In the same year (1839) Dumas appointed Cahours as répétiteur at the École Centrale des Arts et Manufactures, and afterwards manager of the students’ laboratories (in those years it was customary for scientists to work at several places simultaneously, in order to improve their financial situation). In 1840 Cahours joined the École Polytechnic as répétiteur adjoint without salary, and remained in this position until 1851 when he was appointed examinateur de sortie2 and member of the Conseil de Perfectionnement, replacing Chevreul who had resigned. In 1870 he replaced Victor Regnault (1810-1878) in the chemistry chair at the École. Cahours became docteur-ès-sciences at the Faculty of Sciences of Paris in 1845 and in the same year he replaced Dumas at the chair of the course on general chemistry that Dumas was giving at the École Centrale and stayed in this position until 1870. In 1850 Cahours was appointed professor of chemistry at the École d’Application de la Manufacture des Tabacs; in 1851 Dumas appointed Cahours as suppléant (substitute) to the course he was giving at the Sorbonne and in 1868 Cahours substituted him at the chair after Dumas was appointed Secrétaire Perpétuel (permanent secretary) of the Académie des Sciences. In 1888 Cahours resigned his chair at the École Polytechnique.
Cahours’s family life was accompanied by tragedy; within a short period of time (1868-1871) he lost his only brother, his wife, and his two sons, Georges and André, younger than 23 years. His younger son André, died in 1871 during the Franco-German war. Some years later he married again. Cahours passed away in Paris on March 17, 1891. After his death Henri Moissan replaced him at the chemistry section of the Académie des Sciences.
Cahours received many awards and honors for his contributions to science and technology. In 1853 he was appointed assayer (analyst) at the Paris Mint, replacing Auguste Laurent (1807-1853) who had recently passed away. He was elected member of the Société Philomatique (1840); of the Académie des Sciences et Belles-Lettres of Rouen (1846); of the Académie des Sciences (chemistry section), replacing Dumas who had been elected Secrétaire Perpétuel (Permanent Secretary) of the same (1868); of the Académie de Cherbourg; of the Académie de Dijon; and of the Chemical Society of London (1856), replacing Gerhardt who had recently passed away. He was also corresponding member of the Berlin Academy, replacing Pelouze (1867); of the German Chemical Society (1870); and of the Académie des Sciences of Saint-Pétersbourg (1873). The Académie des Sciences awarded him twice (1867 and 1870) the Jecker prize for his work on amyl alcohol and on organometallic compounds, a prize established in 1851 to accelerate the progress in organic chemistry. He was also nominated commander of the Légion d’Honneur.
His research activities encompassed a wide range of subjects. While working for Chevreul at the Muséum, Cahours discovered by accident a bottle containing oil of potatoes, left from the time his mentor was investigating fatty materials. After painful work he proved that this oil was an alcohol, which he named amyl alcohol. Together with spirit of wine (ethanol), spirit of wood (ethanol), and éthal (spermaceti → cetyl alcohol, hexadecanol), the new substance was the fourth of this new natural family and a discovery that hinted the homologue series of ordinary alcohols (Cahours, 1839, 1840).
At Dumas laboratory Cahours carried on researches about several essential oils of plant origin. Together with his colleague and friend, Charles-Frédéric Gerhardt (1816-1856), they discovered that the essence of caraway (Carum carvi) contained two principles, a hydrocarbon (p-cymene, 4-isopropyltoluene) and an oxygenated body (cuminol), boiling at about 220°C and vapor density 5. Cuminol had a remarkable tendency to acidify; treated with oxygen, nitric acid, or chromic acid converted it into cuminic acid. This acid crystallized as prismatic tablets melting at 92°C. Heated in the presence of potassium hydroxide yielded potassium cuminate and hydrogen gas, treatment with nitric acid produced nitrocuminic acid, and treatment with calcium hydroxide reduced it to cumene (isopropylbenzene) a new hydrocarbon, and it could be oxidized to cuminic acid (4-iso-propylbenzoic acid). Afterwards, Cahours and Gerhardt showed that cuminic aldehyde, contrary to benzoic aldehyde, pre-existed in the plant (from which it could be separated by distillation) and did not originate from fermentation (enzymatic action). Encouraged by their discovery of cumene, they distilled cinnamic acid and isolated cinnamene (styrene) and prepared its crystalline bromide. The Académie judged their memoir valuable enough to be published in the Recueil des Savants Étrangers (Cahours and Gerhardt, 1841).
Other essential oils studied were those of anis, fennel, and wintergreen. Cahours determined the chemical identity of the essences of anis, star anis, and fennel, their centesimal composition, their elemental formula, described the preparation and properties of their nitro, chlorine, and bromine substitution derivatives, and while looking the action of nitric acid of different concentrations on the essence of anis, synthesized anisic acid (4-methoxybenzoic acid), and anisol (methyl phenyl ether). He also proved that the essence of Gaultheria procumbens (essence of wintergreen) was actually methyl salicylate, a compound that was also simultaneously an acid and a phenol. This was the first example of an acid playing a mixed function. Additional work was related to salicylic acid and anisic acids, their methyl and ethyl esters, and the products of their reactions with chlorine, bromine, nitric acid and KOH (Cahours, 1841, 1843ab, 1844a, 1849, 1857a).3
Another import work done at Dumas’ laboratories was related to the abnormal density of acetic acid. At Cahours’s time it was accepted that one mole of any substance occupied in the vapor state the same volume as one mole of an ideal gas. According to Avogadro’s law, relative molecular formulas corresponded to four volumes of vapor. Certain molecules, like acetic acid, behaved abnormally and presented an equivalent of three volumes. Cahours proved that these abnormal densities resulted from having been measured at temperatures a little above the boiling point of the compound. The density of a gas approached the theoretical value as the temperature was increased, and they became identical at about 120°C above the boiling point. His results gave a clear explanation to the apparent contradiction that existed between the equivalent of a body inferred from its density and from chemical considerations, which he then extended to the density of the vapors of phosphorus pentachloride, the alkyl chlorides, ammonia salts, etc. Cahours concluded that the change in density with increased temperature signaled the dissociation of molecular groupings and their transformation into simpler structures. A significant outcome of this research was finding and proving that at sufficiently high temperatures phosphorus pentachloride dissociated into phosphorus pentachloride and chlorine.
This finding suggested the possibility of using of phosphorus pentachloride as a chlorinating agent of organic compounds. He proved this point by carrying on the transformation of benzoic acid into benzoyl chloride, and thus opened the way for an easy procedure for preparing acyl chloride. Eventually Gerhardt would utilize it to discover acid anhydrides and thus provide a powerful argument in favor of the theory of types (Gerhardt and Chiozza, 1853; Wisniak, 2006). Cahours also studied the action of phosphorus pentachloride on aldehydes; from benzoic aldehyde he isolated benzylidene chloride, which transformed into benzoic aldehyde by the action of water at 150°C (Cahours, 1848b).
In 1846 he reported the preparation of di and trisulfides of dimethyl and triethyl (Me-S-S-Me), and the pertinent methyl and ethyl sulfocyanides (R-SCN), and that these compounds corresponded to the analog combinations formed by alkali metals (Cahours, 1846a, 1846b). In 1849 he discovered the superiority of the mixture of nitric acid and sulfuric acid over nitric acid alone for synthesizing nitro derivatives of aromatic compounds, illustrating it by the synthesis of dinitrobenzoic and dinitrocuminic acids (Cahours, 1847a, 1848a). In 1852-1853 he discovered in collaboration with Riche, organotin compounds, and in 1860 he extended the subject to the synthesis of organometallic compounds of magnesium aluminum, and tin (Cahours and Riche, 1852, 1854).
Collaborative work with August Wilhelm Hofmann (1818-1892) led to the synthesis of a large number of phosphorated bases, based on alkyl phosphine, by reacting phosphorus trichloride with alkyl derivatives of zinc (Cahours and Hofmann, 1855, 1856b). They also isolated allyl alcohol by reacting silver oxalate with propylene iodide and decomposing the resulting allyl oxalate with ammonia (Cahours and Hofmann 1856a).
Scientific contributionCahours did research in a wide range of subjects, among them density of vapors, essential oils, amyl alcohol, salicylic acid and derivatives, allyl alcohol, phosphorus pentachloride and phosphorated bases, sulfides and polysulfides, American petroleum, and organometallic compounds. The results were published in about 95 papers and several books (Cahours, 1845d, 1846c, 1856c, 1860ab, 1861b, 1875; Cahours and Pelouze, 1864; Cahours and Riche, 1868; Dumas and Cahours, 1842). As customary for all candidates to the Académie des Sciences, Cahours published a booklet describing his researches and achievements (Cahours, 1857b).
Here will describe some of his most important works.
Amyl alcoholCahours’s first scientific publication was related to the volatile oil of potatoes (Cahours, 1837, 1838), a substance that Carl Wilhelm Scheele (1742-1786) had noticed for the first time in 1780 during the distillation of fermented potatoes, and Dumas had reported its elemental composition and density (Dumas, 1834). Dumas and Eugène Melchior Peligot (1811-1890) had studied the volatile compound that Philipps Taylor had isolated in 1812 from the liquid product obtained by distilling wood and named wood spirit (methanol today) (Dumas and Peligot, 1834, 1836). According to Dumas and Péligot although wood spirit differed from common alcohol (spirit de vin) in having less carbon and hydrogen, it had very similar physical and chemical properties: oxidation of common alcohol yielded acetic acid, oxidation of wood spirit also gave an acid with less carbon and hydrogen (formic acid), etc. Dumas and Péligot had concluded that wood spirit was an alcohol, which they named methylic alcohol. Tus the first two terms of the series, an alcohol with one carbon atom and another with two carbon atoms had become known. Although the notion of homology was still to be established, was it possible to suppose the existence of other substances playing the same role? Dumas and Peligot tried to identify them among compounds previously studied and thus discovered cetyl alcohol (hexadecanol) in éthal, a neutral substance that Chevreul had prepared by during the saponifcation of spermaceti (Chevreul, 1823; Dumas and Péligot, 1836).
Cahours had accidentally discovered in Chevreul’s laboratory a bottle containing one liter of impure oil of potatoes. The close analogy between the compositions of methanol, ethanol, and oil of potatoes led him to think that the latter might behave in an isomeric way with the first two compounds. He went on to prepare a large number of derivatives of oil of potatoes and found them to behave in an analogous manner to the corresponding combinations of alcohol series. He recognized that oil of potatoes was an alcohol, which he named amyl alcohol, and went on to describe many of its derivatives, for example, amyl sulfuric acid, amyl chloride, bromide, iodide and acetate, and valeric acid. Hence, his finding of pentanol suggested there was a high probability that the C3, C4, C6, C7, etc. alcohols existed. In his second memoir about types Dumas referred to this discovery in the following words: “To discover or characterize a substance as an alcohol is enriching organic chemistry with a series of analog substances the same as what represents in mineral chemistry the discovery if a new metal” (Dumas and Stas, 1840).
France was well known for the wines it produced and several manufacturers had approached Antoine-Jerôme Balard (1802-1876) with the request to find the reason why certain types of marc brandy had a bad taste. The oil of marc had been studied by several scientists and was supposed to be composed entirely by enanthic ether (methyl heptanoate), but the samples that were provided to Balard were of more complex nature; they had been described by Dumas as potato oil (because it originated by the fermentation of the potato starch), and later as amyl alcohol by Cahours. Balard confrmed Cahours’ findings and went on to study the properties of the alcohol and prepare a large variety of derivatives. The alcohol was insoluble in water as were many of its derivatives. Balard reported the composition and method of preparation of fourteen derivatives of amyl alcohol, for example, amyl ether, amyl mercaptan, potassium xanthamylate, amylene (pentyne), amyl valeric ether, and amyl oxalate (Balard, 1844).
Toluene and xyleneHenry Sainte-Claire Deville (1818-1881) carried on extensive work on essential oils and resins, and their properties and chemical reactions. From the balsam of Tolu he obtained a hydrocarbon, which, because of its resemblance to benzene, he named benzoene (Deville, 1841). He added that this compound had the same composition and properties as the hydrocarbon that Pierre-Joseph (1788-1842) Pelletier and Philippe Walter (1810-1847) had isolated by distilling pine resin (rétinnaphthe), and that it should be considered an isomer of the same (Pelletier and Walter, 1837). In 1850 Cahours distilled the liquid that separated when adding water to wood spirit and found that boiled in a wide range of temperatures. In order to obtain a better separation he first treated the raw oil with concentrated sulfuric acid and found that it yielded a red brown viscous mass on top of which floated a clear liquid having aromatic odor. He washed the liquid with alkaline water, dried it then with calcium chloride, and distilled in the presence of phosphorous pentoxide. In this way he was able to separate several fractions; the one boiling between 108 and 110°C had the same properties than Deville’s benzoene. He named this product toluene. The fraction passing in the range 128°-130°C was found to be a homolog of toluene, which Cahours named xylene; the fraction boiling 145°-148°C proved to be cumene (Cahours, 1850a).
Allyl alcoholMany scientists had reported the synthesis of many compounds containing the allyl group (e.g. the alkyl halides, sulfide, sulfocyanide, and the essences of garlic and mustard), but none had been able to isolate the root alcohol. Cahours, John W. Reynolds, and Hofmann had previously discovered propylene chloride and propylene bromide when studying the action of chlorine and bromine on the gases that were obtained when heating amyl alcohol or valeric acid to the action of heat (Cahours, 1850b, Hofmann, 1851; Reynolds, 1851). A comparison with the corresponding series of ethyl derivatives suggested the presence of a same radical in the propylene series. This fact led Cahours and August Wilhelm Hofmann (1818-1892) to try to prepare an alcohol from this substance, which would have a relation similar to the one existing between methane and methanol and ethane and ethanol. In order to arrive at this result, Cahours and Hofmann reacted many silver salts (cyanate, acetate, butyrate, benzoate, oxalate, etc.) with propylene (allyl) iodide to obtain by double decomposition silver iodide and an ester, from which it would possible afterwards to separate the alcohol. They named the resulting series acrylic, a name that afterwards they changed to allylic (Cahours and Hofmann, 1856a). The best results were obtained reacting silver oxalate with propylene iodide to yield silver iodide and acrylic (allyl) oxalate. The filtrated liquid, after washing with water, drying with calcium chloride, and distillation, produced a colorless clear liquid, heavier than water, and boiling at 207°C. Treatment with an excess of dry ammonia yielded oxamide and regenerated allyl alcohol. The remaining water saturated with ammonia was eliminated by distillation over ferrous sulfate. Allyl alcohol was a colorless, very mobile liquid having a piquant smell, recalling at the same time that of ethanol and mustard. It boiled at 103°C and mixed with water in all proportions; treated with potassium it released hydrogen and become a gelatinous matter corresponding to the alkoxide.
The alcohol was easily esterified using the same procedures as those with ethanol. Thus, each hydrocarbon of the ethylene series could be related to two alcohols, one obtained by hydration (reaction with sulfuric acid and alkali, to give propanol), the other by substitution (to give allyl alcohol).
Cahours and Hofmann reported the reaction of allyl alcohol and its alkoxide with several reagents, for example, acrylic iodide, ethyl iodide, phenol, phosphorus pentachloride, sulfuric acid, phosphorus pentoxide, carbon disulfide, silver salts, etc. etc., to produce about 20 different derivatives, among them allyl sulfide, mercaptan, acetate, cyanide, and benzoate, allyl ureas, allyl amines, etc. They also gave a short description of each product (Cahours and Hofmann, 1856a).
Density of vaporsAt Cahours’s time it was accepted that one mole of any substance occupied in the vapor state the same volume as one mole of an ideal gas. The equivalent of volatile organic compounds corresponded to four volumes of vapor, where the unit of volume was represented by a quantity of oxygen equal to 8 grams. What characterized the true grouping of the atoms was the invariability of the density over a long temperature range. Nevertheless, Dumas (Dumas, 1828) had reported that the vapor of acetic acid presented an anomalous density and Amand Bineau (1812-1861) had shown that the vapors of formic acid and sulfuric acid presented the same anomaly (Bineau, 1844). Cahours repeated Dumas’s experiments using glacial acetic acid and obtained the value of 2.72 (between 150° to 155°C) against 2.781 reported by Dumas, and 2.75 at 145°C (Cahours, 1844b). The theoretical calculation indicated that one volume of the acid corresponded to
for a total of 8.344 per equivalent, a figure that division by 3 (8.344/3 = 2.781) matched the experimental results.
Cahours raised the question if the anomaly observed by Dumas was due to the fact that the measurements had been made at a temperature very close to the normal boiling point of the acid. Indeed, measurement of the density of the vapors of acetic acid 100° to 110°C above its boiling point yielded a value of 2.17, which indicated that the state of the acid was the same as that of other volatile acids. The same results were obtained when using acetic acid prepared from potassium acetate. Hence, the anomaly presented by acetic acid disappeared completely when the density of the vapor was measured at a conveniently high temperature.4
In another publication (Cahours, 1845b) Cahours reported that the value of the density of different alcohols (methanol, ethanol and amyl alcohol), compound esters, water, and many hydrocarbons, at 30° to 35°C above their normal boiling point was very near the theoretical value, but measured at only 10° to 12°C above their normal boiling point, the deviation could be considerable. Against this behavior, the density of the vapors of acetic, butyric and valeric acids at temperatures 30 to 40° above their normal boiling points, showed significant deviations from the ideal value. For example, the density of acetic acid vapors was 3.20 at 125°C and decreased to 2.08 at 338°C. Similarly, the density of butyric acid vapors was 3.68 at 177°C and 3.07 at 330°C. He also reported that the density of more complex molecules, such the essence of anis, was also anomalous, 5.98 at 245°C and 5.19 at 338°C, against the theoretical value (ideal gas) of 5.18.
In another paper Cahours (Cahours, 1845c) mentioned that Eilard Mitscherlich (1794-1863) had reported that the density of phosphorus perchloride (pentachloride) vapors was 4.85 at 185°C, a value corresponding to the exceptional value of 6 volumes of vapor when the formula was assumed to be P2Cl10, and 3 volumes for the formula PCl5. Again, Cahours thought that this result was another example of anomalous density, suggesting that the density of the vapors of the perchloride should decrease if the temperature was increased. His experimental results confirmed this assumption: the density of the vapors was 4.99 at 190°C and decreased to a constant value of 3.656 beyond 300°C. In order to obtain a result invariant with temperature it was necessary to determine the property at a sufficiently high value beyond the normal boiling point. Cahours ended his paper with the crucial comment that it was possible to match the theoretical value with the experimental one if above 300°C phosphorus perchloride dissociated into phosphorus trichloride and chlorine.
Phosphorus pentachlorideIn a following publication Cahours pursued the idea that fact that the density of the vapors of an anomalous substance became constant at sufficiently high temperatures could be explained by its dissociation into simpler (and hence more stable) bodies (Cahours, 1847b). For the case of phosphorus perchloride the theoretical volume of the equivalent was
for a total of 28.840, which only divided by eight (28.840/8 = 3.61) yielded essentially the same figure as the experimental value (3.65). Hence, to assume that phosphorus perchloride was formed by the union of one volume of phosphorus vapor and 10 volumes of chlorine, condensing into 8 volumes, was an extremely unusual molecular combination. Considering known facts about the behavior of phosphorus perchloride with certain agents, wouldn’t it be more rational to consider it as formed by the union of equal volumes (equal number of moles) of chlorine and phosphorus protochloride (trichloride)? This assumption was totally in accordance with Gay-Lussac law of volumes for gaseous combinations. It was known that gases such as HCl, HBr, HI, and HCN resulted from the combination of equal volumes of hydrogen and chlorine (bromine, iodine, or cyanogen), ammonium chloride resulted from the combination of equal volumes of the acid and ammonia, etc., without contraction. On the contrary, when formation took place between different volumes of gases (e.g formation of water, hydrogen sulfide, ammonia, etc.) the phenomenon occurred with a considerable contraction of volume (Cahours, 1847b).
To substantiate his claims, Cahours mentioned that Georges Simon Sérullas (1774-1832) had found that the reaction of phosphorus pentachloride with hydrogen sulfide took place according to PCl5 + 2SH = 2HCl + PCl3S2, while Würtz had shown that the reaction of phosphorus pentachloride with water (the homolog of hydrogen sulfide) yielded a chloroxide representable by the formula PCl3O2, PCl5 + 2HO = 2HCl + PCl3O2.
As a consequence of the above statements, although the phosphorus chlorosulfide and oxysulfide corresponded quite well with the perchloride by their composition in equivalents, they had to differ completely by the way they were grouped. Indeed, although the latter gave 8 volumes of vapor, the chlorosulfide and the oxysulfide yielded only 4 and the density of their vapors did not offer the anomalies of the pentachloride; a conclusion easily verified by experiment.
Assuming now, that phosphorus perchloride decomposed into equal volumes of phosphorus protochloride and chlorine yielded
for a total of 3.62, which divided by 1, yielded the value 3.62 for the theoretical, a figure quite similar to the experimental value measured at a sufficiently highly temperature (Cahours, 1847b).
For this research the Académie des Sciences awarded Cahours the 1860 Jecker prize.
In a later paper, Cahours repeated that in the doctoral thesis he had presented to the Faculté des Sciences de Paris, he had shown that the vapors of certain liquids behaved as ideal gases only at temperatures quite above their normal boiling points (Cahours, 1863). Tus, when determining the density of one of these substances from the normal boiling point up to the temperature where it stopped changing, it was easy to prove that only until this limit was achieved, there existed a well-defined equilibrium state. This anomaly, observed in quite different substances (simple, acids, essential oils, alkyl chlorides, etc.) seemed to be independent of the material that constituted the walls of the vessels used or the experience. In this new publication he showed that substitution of the acid hydrogen atom in the molecule of acetic acid to form a volatile compound, such as methyl, ethyl and amyl acetate, caused disappearance of the anomaly, but the anomaly persisted if the hydrogen substituted was one located in the acid radical, for example, monochloracetic acid, thioacetic acid, etc.
Phosphorus basesIn 1845 Paul Thenard (1819-1884) had reported that the reaction between methyl chloride and calcium phosphide resulted in the formation of a series of bodies in which the hydrogen of phosphorus hydrides had been replaced by an equivalent quantity of methyl group (Thenard, 1845ab). One of these bodies was phosphine, a compound, which appeared to occupy in the phosphorus series the same position that cacodyle had among the arsenic compounds. In addition to phosphine, two other solid substances were formed; one of them was the hydrochloride of a very volatile phosphorated base. Its analysis suggested that it could be viewed as an ammonia derivative in which the nitrogen had been substituted by phosphorus and the methyl group by hydrogen. At the time of these discoveries, the ammonia base had not been discovered and Thenard’s work had not been continued. Discovery of methylamine, dimethylamine, and trimethylamine, of the corresponding terms in the ethyl and amyl series, and the corresponding bases of arsenic and antimony (Cahours and Riche, 1852, 1854; Hofmann, 1854-1858), had shown that groups such as methyl ethyl, and phenyl, were able replace the hydrogen in ammonia, partially or totally, generating products that retained the basic character of the original ammonia molecule. Cahours and Hofmann believed that phosphorus, which by its chemical character stood between nitrogen and arsenic, could exhibit a similar behavior (Cahours and Hofmann, 1855, 1856b).
As a first step, Cahours and Hofmann repeated Thenard’s procedure to prepare the phosphorated compounds corresponding to ammonia and ammonium and found that a much better synthesis method was to use methyl iodide (a liquid) instead of methyl chloride (a gas), and sodium phosphide instead of calcium phosphide. The reaction was very energetic at room temperature and its products were flammable and detonating. The results of these experiences indicated that the product of the reaction was a complex mixture, hard to separate, of dimethyl phosphine (a liquid, corresponding to cacodyl), trimethyl phosphine (a liquid, corresponding to trimethylstibine and trimethylarsine) and tetramethylphosphonium iodide (a solid, corresponding to tetramethylammonium iodide). Afterwards, Cahours and Hofmann improved their procedure by reacting phosphorus trichloride with alkyl derivatives of zinc. The products of this reaction were alkylphosphines united with zinc chloride. The alkyl phosphines were then liberated by simple distillation in the presence of alkali.
Cahours and Hofmann paper gave a detailed description of the physical and chemical properties of 24 derivatives prepared according to their procedure: triethylphosphine and its hydrohalide derivatives, tetraethylphosphonium iodide, tetraethylphosphonium chloride, triethylphosphine disulfide and diselenide, methyltriethylphosphonium iodide, trietehylamylphosphonum iodide, and the corresponding compounds of the methyl series.
Their results gave clear proof that the phosphorus compounds hold a position intermediate between the corresponding series of nitrogen compounds and the arsenic and antimony ones (Cahours and Hofmann, 1855, 1856b).
Essence of plantsAccording to Cahours, it was known that most volatiles oils are composed of two fractions, one solid at room temperature, and the other liquid. The solid fraction could be made to crystallize and volatilize at a fixed temperature, hence assumed to be a pure immediate principle. The liquid fraction was known to be a mixture of many substances, hard to separate because of their similar properties and their being thermolabile. Many solid oils had been studied and shown to present some interesting chemical reactions. Cahours indicated that the purpose of his first publication was to report the results of his experiences with the solid fraction of the essences of anis, badiane (star anis), and bitter fennel (Foeniculum vulgare) (Cahours, 1841).
Cahours separated the solid fraction of the essence by pressing commercial essence between blotting paper, followed by solution of the retained material with alcohol, and 2 or 3 crystallizations from the solvent. The resulting product was a very pure white sparkling crystalline substance having specific weight similar to that of water. It was very friable, melted at about 18°C, and boiled at 222°C, completely volatilizing, and leaving little residue.
Cahours proceeded then to study its chemical properties. The solid fraction exposed for a long time to dry or humid air did not surfer alteration but when in the liquid state it slowly lost its ability to crystallize. It reacted vigorously with bromine and chlorine yielding substituted products. Alkalis, boiling and concentrated, did not affect it. Strong acids such as sulfuric and phosphoric, at room temperature, transformed it into an isomeric substance. Oxidizing acids, such as nitric and chromic acids, gave place to interesting results, explained below. Determination of the density of the vapor led to the elementary formula C40H24O2 (Cahours, 1841).
Addition of bromine in small portions resulted in a highly exothermic reaction; the brown color of bromine disappeared and abundant vapors of hydrogen bromide were released. Purification of the resulting material with ether yielded a colorless crystalline mass, insoluble in water, little soluble in alcohol, and a little more soluble in ether. Chemical analysis of the substance, which Cahours named bromanisal, led to the formula C40H18Br6O2. The reaction with chlorine was more complicated; it was very exothermic and the chlorine content and amount of hydrogen chloride released, increased with the reaction time. different products formed were semi liquid at room The temperature. Treatment with concentrated sulfuric acid yielded, after purification, a white odorless substance, melting at above 100°C, which Cahours named anisoïne. Anisoïne was found to be insoluble in water, slightly soluble in alcohol, ether, and volatile oils. It dissolved in concentrated sulfuric acid producing a red solution. Heated strongly in contact with air it caught fire and burned like a resin. Cahours reported that other strong acids, particularly phosphoric acid, also produced anisoïne. Elemental analysis corresponded to the formula C40H24O2, identical with the original solid fraction, indicating that treatment with strong acid had led to an isomerization reaction (Cahours, 1841).
The reaction with nitric acid of different concentration originated a variety of products of different nature, composition, and properties. For example, treatment with very concentrated acid produced a yellow resinous substance; treatment with a less concentrated acid produced a red oily liquid, destroyable by distillation, treatment more diluted nitric acid produced two products, a yellow resin (similar as the one described before) and a new acid exempt of nitrogen, capable of crystallization, volatile without decomposition, which Cahours named anisic acid and classified in the group formed by benzoic and cinnamic acids (Cahours, 1841).
Anisic acid crystallized as long brilliant needles, slightly soluble in cold water and more soluble in boiling water. It was very soluble in alcohol and ether and formed soluble salts with alkalis and alkaline earths. Chemical analysis led to a composition described by the formula C32H14O6. Cahours reacted the acid with ethanol to prepare anisic acetate and also with barium oxide to obtain a compound he named anisole. The latter was a colorless liquid, having a very agreeable aromatic odor, boiling about 150°C, insoluble in water and soluble in alcohol and ether. In contact with chlorine or bromine it generated crystalline derivatives, which distilled without decomposition. An elemental analysis corresponded to the formula C28H14O2 (Cahours, 1841).
As described above, treatment of the solid fraction of essence of anis with concentrated nitric acid produced a yellow resinous substance, which dissolved in excess acid. Addition of water precipitated yellow fakes of impure nitro-anisic acid. The purified acid was a white substance, little soluble in cold or hot water and soluble in hot alcohol. Treated with potassium, sodium, or ammonium hydroxide it formed very soluble salts; the salts formed with baryta, strontia, calcium hydroxide, and magnesium were slightly soluble. The silver and lead salts were insoluble. Elemental analysis corresponded to the formula C32H12N2O10 (Cahours, 1841).
The next experiments were done with the essence of bitter fennel, an essence composed of two oil fractions. One of them could be obtained very easily in a pure state and shown to be identical to the solid fraction of essence of anis, although they differed in some of their reactions. The second fraction was more difficult to purify and seemed to have the same composition as the essence of turpentine, although probably as an isomer. An interesting fact was that treated with NO2 it yielded a crystalline substance of formula C30H24O4N4 (Cahours, 1841).
In 1843 Cahours published his first paper about the essence of Gaultheria procumbens, which was just being commercialized under the name of essence of wintergreen (Cahours, 1843a). This oil was heavier than water and practically composed of only one substance, which boiled at 244°C without decomposition. Chemical analysis of the substance indicated that it contained, by weight, 63.13%, 5.45% hydrogen, and 31.41% oxygen, which according to Cahours corresponded exactly to methyl salicylate, as proven by the product of their reaction with fuming nitric acid (indigotic acid). According to Cahours, this was the first time that methanol was found to be present in a vegetable product. Although the ester had a neutral character, it behaved like a true acid. Treatment with potassium or sodium hydroxide converted it into crystallizable salts soluble in water and alcohol; addition of an acid restored the original oil. A similar behavior was observed with ammonium hydroxide. Addition of diluted hydrogen chloride liberated salicylic acid.
The reaction with ammonium hydroxide and anhydrous bases was studied in more detail (Cahours, 1843b). Mixing methyl with an aqueous solution of ammonium hydroxide resulted in a slow reaction, during which the solution was complete. The resulting liquid had a yellow green color; evaporated at a moderate heat in it yielded a precipitate of long needles. Total evaporation produced a brown crystalline resin, which on distillation released initially ammonia vapors and then a liquid which crystallized on the cold walls of the retort as a crystalline mass colored yellow like sulfur. Purification of the latter gave a substance little soluble in cold water and more soluble in boiling water, from which it crystallized as long needles, very soluble in alcohol and ether, and strongly reddening litmus. Chemical analysis showed it to be salicyamide.
The reaction with dry calcium oxide and barium oxide also produced interesting results. Drop-to-drop addition of methyl salicylate over finally powdered barium oxide gave place to a highly exothermic reaction ending in a well-defined crystalline compound, which was found to be anisole. Cahours remarked that the two isomeric substances, anisic acid and methyl salicylate, having the same chemical equivalence, gave place to totally different chemical reaction when treated with hydrated bases (KOH and NaOH), but yielded the same product when treated with anhydrous bases (Cahours, 1843b).
In a following paper Cahours extended the information about his experiences with this essence. The essence was located in the flowers from where it could be extracted directly by maceration with ether. Wintergreen essence differed from other essences such as bitter almonds and meadowsweet, in that it not existed preformed in the seeds or flowers of the plant but was the result of the action of water and ferments (enzymes) on particular compounds existing in the these elements. Another interesting fact was that since it was essentially composed of methyl salicylate, it was possible to compare the properties of the natural essence with the compound prepared synthetically.
Some characteristic properties of the essence were being the denser (1.18) of the known oils; it begun boiling at about 200°C and distilled without alteration. The first fraction was a light oil mixed with a little of methyl salicylate. The essence was slightly soluble in water and completely soluble in alcohol, ether, and the essences of turpentine and lemon. The aqueous solution was neutral and assumed a violet color when treated with a few drops of a ferric salt. If the initial solution was slightly acid, then the color became a strong violet (Cahours, 1843b).
Treatment of the essence with a concentrated solution of KOH or NaOH produced a crystalline mass completely soluble in water. Addition of acid to the crystals recuperated the original oil with all its primitive properties. Heating the mixture of essence and alkali or left to rest for about 24 hours, followed by treatment with acid, did not separate the original oil as before but resulted in the precipitation of a solid substance crystallizing in prisms and having the composition and properties of salicylic acid. Distillation of the essence mixed with and excess of barium oxide transformed the latter into barium carbonate while distilling a neutral oil, which Cahours named anisol. This substance was also obtained by carrying on the distillation of crystalline anisic acid. Chlorine and bromine reacted vigorously with the oil yielding crystallizable substitution derivatives. Treatment of the later with mercuric cyanide resulted in substitution of the chlorine or bromine with a cyanide group. Iodine dissolved in the oil without reaction. Treatment of the oil with a small amount of fuming nitric acid generated methyl indigotate. Cahours went on to describe the reaction of the oil with many other reagents, for example, potassium, ammonium chloride, etc. (Cahours, 1843b).
Cahours determined the centesimal composition of the oil and found it to be identical with that of synthetic methyl salicylate, prepared by reacting salicylic acid with methanol in the presence of sulfuric acid. He found that methyl salicylate behaved like a true acid with respect to alkaline bases and metal oxides and for this reason he named it gaultheric acid. In his memoir Cahours described the preparation and properties of the gaultherates of potassium, sodium, barium, silver, etc., as well as description of the preparation and properties of several bromine and chlorine derivatives of methyl salicylate, and the products of the reaction with fuming nitric acid, ammonium hydroxide, anhydrous alkalis, for a total of about 20 different derivatives (Cahours, 1844a).
Another publication described the properties and reactions of anisic acid and its comparison with salicylic acid (Cahours, 1849).
Organometallic radicalsCahours conducted extensive research on the subject of organometallic compounds and published his results in two long papers (Cahours, 1860ac, 1861a). In the introduction of the first paper he recalled the accepted definition of radicals as more or less groups complex that behaved as simple bodies, and then went on to summarize the information available on the subject. In 1837 Robert Wilhelm Bunsen (1811-1899) had reported the preparation, composition, and properties of cacodyl (tetrametyldiarsine) (Bunsen, 1837) and 15 years later Edward Frankland (1825-1899) had done the same with ethyl zinc (Frankland, 1852). It was known that a simple body could not combine with another in any possible proportion; two of these bodies were able to form a very limited number of combinations. Even more, when this number exceeded 2 or 3, the resulting combinations were rather unstable (e.g, combinations of chlorine with oxygen). Within the possible combinations, some were more stable, for example for alkaline metals it was RX, for iron and analogs it was R2X3, and for titanium and tin it was RX2. It was known that of all the combinations that phosphorus could form with oxygen, the most stable was P2O5; under particular conditions other proportions were possible, but all these converged into P2O5 under the action of heat. Yet, there were certain substances that on joining together, they yielded products that had a much larger tendency to combine than the original simple ones (higher reactivity). Examples of these were carbon monoxide and sulfur dioxide; they not absorbed additional quantities of oxygen more easily than carbon or sulfur alone but could form with chlorine, iodine, etc. compounds corresponding with those of maximum oxidation. These new groups, which could be separated intact from their combinations and hence exhibited all the appearance of true simple bodies, had been named radicals (Cahours, 1861a).
Every compound could be considered as a molecular system in equilibrium in which the atoms were attracted to each other by more or less strong affinities. Replacing one or more of the atoms of one of the elements by an equal number of atoms of another substance yielded new compounds having the same mechanical grouping as the primitive body, but with a equilibrium state varying largely, according to the reciprocal attractions present in the new substance. For example, its was possible to replace all or part of the hydrogen present in ammonia by chlorine; ammonia resisted very well the action of dark red heat but nitrogen chloride was destroyed at temperatures well below that of boiling water. It was easy to explain the difference: the mutual affinity between nitrogen and chlorine was substantially weaker than that between nitrogen and hydrogen (Cahours, 1861a).
When an alkyl group such as methyl, ethyl and amyl, coupled with a simple body it generated products in which the affinity for oxygen, chlorine, etc. was substantially larger than that of the simple body for these elements. Tus, zinc absorbed oxygen very slowly; atmospheric oxygen, which did not act on pure water at room temperature, carried on with extreme violence the decomposition of this liquid when it was combined with a methyl or ethyl group. The molecule split into zinc that combined with oxygen and the alkyl group combined with hydrogen to form the hydrocarbon. The same behavior was observed with magnesium and aluminum. Metals more electronegative than zinc, such as tin, lead, and mercury, combined with alcoholic radicals to form compounds that have a large affinity for oxygen, chlorine, etc. Nevertheless, these affinities were less energetic, and when saturation was achieved (in today’s terms, when the central element is at its maximum valence), the combination now behaved like an inert body with respect to these elements. The composites of methyl or ethyl with the metal may in these circumstances separate in an intact from their new combinations (Cahours, 1861a).
When a simple body formed with other simple bodies compounds where ABx represented the saturation state, experience showed that this simple body was able to form with diverse alcoholic radicals compounds in different saturation states. As long as the number of alcoholic molecules that become combined was less than x, the new compound ass able to combine with oxygen, chlorine, etc. and behave like a true radical.
Metals formed with and oxygen, chlorine, sulfur, etc., compounds of definite composition. It was perfectly logical to assume that these metals could form similar compounds with groups such as methyl and ethyl. Frankland had already proven the existence of methyl and ethyl zinc, having the group ZnX. James Alfred Wanklyn (1834-1906) (Wanklyn, 1857-1859) had shown that alkaline metals were also capable of forming analog combinations, although these were highly unstable. In contact with water, all these compounds decomposed immediately into oxide and hydrocarbon (Cahours, 1861a).
According to Cahours, the results of his experiments proved that a large number of metals were able to react with methyl iodide and ethyl iodide, if heated in a closed vessel at temperatures between 120° and 200°C. Depending on the metal, the product of the reaction was the pure ethyl derivative pure, while with others it was a mixture of the ethyl derivative and derivatives containing iodine (Cahours, 1861a).
In his first memoir (Cahours, 1860c) Cahours went on to describe the results of the reaction of methyl or ethyl iodide with magnesium, aluminum, tin, and tin-sodium alloys, and to give the composition and main physical and chemical properties of the different derivatives obtained. He also described the physical and chemical properties of additional derivatives, such as the chloride, bromide, fluoride, sulfate, nitrate, oxalate, and formiate of ethyltin, and the chloride, bromide, sulfate, acetate, and oxalate of sesquiethyltin. He ended this paper stating that the methyl and ethyl combinations of tin fully demonstrated his hypothesis that radicals were unsaturated compounds. The limit was achieved in a product that presented absolute neutrality. Hence, for a body to play the role of a radical, it had to be, before anything else, below its saturation limit and present such an stability that the equilibrium of its grouping could not be broken by the affinity of simple bodies with which it came into contact, or by the influence of forces applied to separate the combinations in which it formed part (Cahours, 1860c).
Cahours second memoir (Cahours, 1861a) was devoted to describe a large number of additional organometallic compounds that he had prepared from tin, titanium, lead, and arsenic, as well the action of alkyl (methyl and ethyl) halides (chloride, bromide, and iodide) on cacodyl, and the action of methyl and ethyl iodide on metallic phosphides (potassium, sodium, zinc, and cadmium).
Cahours’s work on organometallic radical won him (for the second time) the Jecker Prize of the Académie des Sciences.
The archives of the École Polytechnique contain a statement by auguste’s father by which he guaranties payment of his son fees in the case he will be admitted to the École: «Je soussigné André Cahours, vérificateur à la comptabilité générale des receveurs de l’enregistrement et des Domaines, au ministère des Finances, demeurant à paris rue Buffault nº7, je promets et m’engage envers l’École royale polytechnique, dans le cas que mon fils, Auguste André Thomas Cahours, soit admis à la dite École, comme élève, de payer annuellement la pension de mille francs» (courtesy of olivier Azzola, in charge of the archives at the library of the École).
The examinateur de sortie is the officer that follows the students’ progress during their schooling. He sits on the jury, which classifies the pupils in order of merit, at the end of the schooling at the École polytechnique, and before their entrance in engineer and offcers’ training schools (École des Mines, École des ponts et Chaussées, School of the artillery and engineering) (courtesy of olivier azzola, in charge of the archives at the library of the École).
Salicylic acid continued to be extracted from wintergreen until 1859 when adolphe Wilhelm Hermann Kolbe (1818-1884) developed a synthetic procedure based on heating sodium phenolate with CO2 under pressure (Kolbe, 1860).
We know today that lower fatty acids associate through hydrogen bonds, resulting in very high values of the density of their vapors around the normal boiling temperature, which diminishes as the temperature is increased.