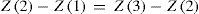Nature is clever in that no single and simple periodic chart can reveal all of the important relationships among the chemical elements. For some uses, however, we can maximize these revelations by giving up some simplicity, and we wish herewith to present what we may hope is an appealing way of doing precisely that. The purposes are both to promote teaching by calling attention to a novel periodic scheme, and to facilitate the discovery and use of similarities that may otherwise escape notice in research, writing and the development of materials. We begin with a very brief look at the history of such charts, as developed for example in The New Encyclopædia Britannica (1991). We do not attempt to review or even list all of the vast recent literature on periodicity, but Mazurs (a) (1974) gives a very useful earlier review.
La naturaleza es inteligente en cuanto a que ninguna tabla periódica simple o sencilla puede revelar todas las relaciones importantes entre los elementos químicos. No obstante, en algunos usos podemos maximizar esas revelaciones dándole algo de simplicidad y eso es lo que aquí pretendemos, presentar lo que esperamos sea una manera apropiada de hacer precisamente eso. El propósito es promover que la enseñanza ponga atención en un nuevo esquema periódico, así como facilitar el descubrimiento y uso de similitudes que, de otra forma, pueden escapar a la consciencia, la memoria escrita y la investigación del desarrollo de materiales. Empezamos por dar una ojeada a la historia de tales tablas, como la desarrolla la Encyclopædia Britannica (1991). No pretendemos dar una lista o una revisión a la vasta literatura que existe sobre periodicidad, lo cual puede lograrse al consultar Mazurs (1974).
Chemists know of the Russian Dmitri Mendeleev's very useful Периодическая система элементов, or Periodicheskaya Sistema Elementov, or Periodic System of the Elements, of 1869. Before this, there were numerous attempts to systematize the similarities and differences among the elements.
In 1817 J. W. Döbereiner noted that the atomic weight of Sr is about halfway between those for Ca and Ba. He later found other such “triads”, including Cl, Br and I; and Li, Na and K. We will comment further on triads below.
In 1862 A. E. B. de Chancourtois placed the elements with their atomic weights in a helix on a cylinder with a circumference of 16 units, so that some elements occurred above or below similar ones.
In 1864 J. A. R. Newlands proposed a “law of octaves” because of the repeated recurrence of similar properties in the eighth element from various starting points in the list according to rising atomic weights; the noble gasses were not yet known. This analogy to octaves in music led unfortunately to scornful dismissal by unimaginative colleagues.
Mendeleev, however, also proposed a form with eight columns, after his earlier table was rotated 90 degrees. In this, the eighth column was headed by Fe, Co and Ni all together in one space, with blanks in this column VIII in alternate rows. Such a chart was perhaps not attractive esthetically, but it led later to the now prominent 18-column charts that unite two of the previous rows of seven and “eight” in rows of 18. Lothar Meyer proposed a similar table.
Mendeleev in 1871 predicted the existence and some properties of Sc, Ga, Ge, Tc, Re, Po, Fr, Ra, Ac and Pa, as they are now symbolized. The noble gasses were discovered and isolated in 1894-1898 by Lord Rayleigh, W. Ramsey and M. W. Travers, thus providing the true eighth or 18th column of the most common charts. In 1944 G. T. Seaborg proposed that an “actinide” (actinoid) series exists parallel to the “lanthanide” (lanthanoid) series.
In 1913 H. G. J. Moseley used X-ray spectra to identify the atomic numbers, rather than atomic weights, as the proper basis for the sequence of elements. This eliminated the previous distress from the fact that in some pairs of elements, notably Co and Ni, together with Te and I, their chemical properties seemed to require placing the lighter element after the heavier one in the order of atomic weights. Most regrettably, Moseley was then killed in World War I. In retrospect, it seems surprising that perhaps no one had previously considered that a few of the increases of weight in a proper sequence might be negative, because those increases were so irregular and often non-integral anyway.
Many more recent additions and changes have been made or offered. Mother Nature is clever in denying all the advantages to any single form. However, let us show just one that has an unusual appearance but with all of the following advantages over the common forms in modern chemistry classrooms and books: The elements are in a continuous sequence with no interruptions or gaps. No groups are relegated separately to the bottom of the chart as if they were belated afterthoughts in creation. The d-block (“transitional”) metals, i.e. Sc through Zn and their heavier congeners, and the f-block (“inner transitional”) metals (lanthanoids and actinoids), i.e. La through Lu and their heavier congeners, each appear as coherent series of their own in the fuller series. Figure 1 is from Mazurs (b), (1974), Janet (1928 and 1929), and Quintana Mari (1931).
Of course, we would now replace at least Ku, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117 and 118 with Rf, Db, Sg, Bh, Hs, Mt, Ds, Rg, Cn, Uut, Uuq, Uup, Uuh, Uus and Uuo. The u, t, q, p, h, s and o are derived from the Latin or Greek names for 1, 3, 4, 5, 6, 7 and 8 in the atomic numbers, so that Uut, for example, stands for 113 before an official name is assigned. After 118 or even before, relativity disturbs the simple picture of atomic-energy levels too greatly to extend any such table in any simple way.
Now let us consider suggestions for further possibilities, with brief discussion, some elaborated elsewhere (Laing, 2005; Rich, 1965; Rich, 2005; Rich, 2007 pp. 3 and 49; also pp. 479-490 for special uses; regrettably, many minor linguistic and chemical errors, chiefly in splitting words and formulas at the ends of lines, were added to this book after the author returned the final proofs). Herewith we present additional innovations for evaluation and use by readers.
Part of Nature's cleverness is that many elements have notable chemical similarities to several others, which cannot all be placed adjacently in any simple chart, i.e. with only one position for each element. We can, however, show the most significant of these relationships by writing some elements in several places. Hydrogen, for example, can head both the column of alkali metals (even though it is not metallic at low pressures but does form a unipositive ion) and also the column of halogens (even though it does not occur as salts of metals in ores but does form a diatomic molecule and a uninegative ion). Thus it adds to the list of “triads”, groups of three elements such that
so that the atomic number of the middle element is the exact average of the other two; e.g. for 1H, 9F and 17Cl:emspScerri (2010) has discussed triads recently. There is vigorous disagreement, at times dismissing inconvenient facts on one side or the other, touched on by Rich (2007, p. 49), sometimes using triads at least implicitly, on whether 57La, 71Lu, neither or both should be included in Group 3 with the lanthanoids and likewise for the actinoids. See, e.g. Jensen (1982) and Lavelle (2008).
One can construct interesting sequences of four chemically similar elements, each containing one triad as defined here, such as:
to support or oppose each extreme argument. Fortunately, these disagreements are often less punitive inside than outside natural science!We see that 57La and 89Ac can each be taken as the first member of a series of 15, with 71Lu and 103Lr as the last members of the same series. Also, if we choose to take triads somewhat seriously, 57La, 64Gd and 71Lu make an especially nice one, with extremely similar chemistries, albeit not e.g. magnetism. The new group symbol, Rth, may be convenient for the so-called rare earths when one includes Sc and Y. (We note in passing that the elements themselves are not “earths” or oxides and that many of them are not rare.) Without Sc and Y, we have “lanthanoid”, Ln, and “actinoid”, An, as useful names and symbols.
Hydrogen even resembles carbon in electronegativity, in having its valence shell of electrons half full, and in bonding covalently with various d-block metals, thus possibly heading Group 14 in the format of the International Union of Pure and Applied Chemistry (Cronyn, 2003). We may object that H does not resemble Pb in Group 14, but neither does C resemble Pb in the overwhelming majority of carbon compounds.
Theoreticians may also object that the electronic structures of the LS-Coulomb-coupled and relativistically spinorbit split ground levels of the isolated atoms of some of these chemically similar elements are quite different, and that fact is important for some considerations, though rather irrelevant in descriptive chemistry, where the chemical resemblances are crucial at least for non-theoretical chemists. These questions are discussed thoroughly elsewhere (Schwarz and Rich, 2010, and Schwarz, 2010), albeit with considerable repetition, which teaching may require. Then let us examine Table 1.
Beside hydrogen, we consider another example to explain or rationalize the multiple groupings, expecting that readers somewhat acquainted with the properties of the elements will see similar reasons for the others, or that this chart will, on the other hand, lead to discovering new relationships.
We find that S and Cl, but not O and F, form the salts K2XO4 and KXO4, like K2CrO4 and KMnO4, where X represents S or Cl respectively, like Cr or Mn. Therefore S and Cl, but not O and F, may appear above Cr and Mn in such a chart.
This chart also suggests correctly that the actinoid U and its neighbors have important chemical resemblances to W and its neighbors just above them, while the corresponding lanthanoid Nd and its neighbors lack similar resemblances to Mo and its neighbors just above them, as shown by the different horizontal separating lines.
For example, W and U both form the common anions MO42– with some similar salts, albeit, of course, also with various differences in reactivities such as are found everywhere in the chart. Additionally we may cite, say, the waterinsoluble white solids HfF4 and ThF4.
On the other hand, Mo is well known in the stable MoO42– , while Nd does not occur in NdO42– in water.
Chemists should learn that different formats are possible, and they should use the arrangement that is most useful and which gives back the most information for the smallest input of printers' ink.
Finally, let readers design their own periodic charts of the elements to reveal relationships that are not otherwise elucidated in the many previously proposed tables (Mazurs (a), 1974); good luck!