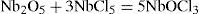Louis Joseph Troost (1825-1911) carried on a large number of researches, alone or with Deville, Hautefeuille, and other colleagues, on the isolation of lithium, determination of its atomic mass, and preparation of many of its salts, on allotropic phenomena, on the use of electromotive force of a cell to determine the heat effects of a reaction, determination of the density of vapors at high temperatures, dissociation of gases, niobium and its compounds, the hydrides of palladium, sodium, and potassium, and the influence of manganese and silicon on the properties of the different varieties of iron.
Louis Joseph Troost (1825-1911) llevó a cabo un gran número de investigaciones, solo o junto con Deville, Hautefeuille, y otros, acerca del aislamiento del litio, determinación de su masa atómica, y la preparación de muchas de sus sales, acerca de la alotropía, el uso de la fuerza electromotriz de una pila para determinar los efectos térmicos de reacciones químicas, la determinación de la densidad de gases a alta temperatura, disociación de gases, el niobio y sus compuestos, los hidruros de paladio, sodio y potasio, y la influencia del silicio y el manganeso en las propiedades de diversas variedades de fierro.
Louis Joseph Troost was born in Paris in October 17, 1825. He received his first education at the Lycée Charlemagne and in 1848 entered the École Normale Supérieure in Paris (founded by Napoleon) where he received his agrégé (license to teach) of physical sciences in 1851. In 1857 he was awarded his doctorate of science at the Faculty of Sciences of Paris (Sorbonne), under the direction of Henry Sainte-Claire Deville (1818-1881). After graduation he taught physics for four years at the Lycée d’Angouléme in southwestern France, and then returned to Paris to teach chemistry at the Lycée Bonaparte and do research at Deville’s thermochemical laboratory. In 1868 he was appointed Maitre de Conferences at the Faculty of Sciences of Paris (Sorbonne), following the signifcant papers he had published with Deville about the density of vapors. In 1874 he was promoted to the Chair of Mineral Chemistry, replacing Louis Pasteur (1822-1895), and in 1881 he was appointed to the Chair of General Chemistry, replacing Henri Debray (1827-1888). He kept this position until his retirement in 1900. Henry Moissan (1852-1907) replaced him in at the Chair. En 1884 he was elected to the Académie des Sciences, replacing Charles-Adolph Würtz (1817-1884) who had just passed away (Anonymous, 1911; Gautier, 1911; Jaubert, 1912).
Troost was a commander of the Légion de Honneur, a member of the French section of International Committee on the Meter, of the Consulting Committee of Arts and Manufactures, of the National Bureau of Weights and Measures, of the permanent Commission of Monetary Circulation, of the Council of Hygiene and Salubrity of the Seine, of the Commission of Inventions at the Ministry of War, and for some time, President of the Administration Council of the Parisian Gas Company (Anonymous, 1911; Gautier, 1911; Jaubert, 1912).
Troost’s first researches were about lithium and its salts. After discovering a practical method for preparing highly pure lithium chloride, he isolated the metal, determined its atomic mass, and showed that it constituted the bridge between the alkali metals (Na, K) and the alkali earth ones. He also prepared and determined the properties of many of its salts (Troost, 1856, 1857, 1862). Troost studied magnesium (Troost, 1865), chloral and its hydrate (Troost, 1877bc, 1878abc, 1879b), and the reaction of HCl with ammonia (Troost, 1879a). With Edme Hippolyte Marié-Davy (1820-1893) he studied the use of the electric cell to measure heat effects in chemical reactions (Marié-Davy and Troost, 1858abc); with Deville he studied the density of vapors at high temperatures (Deville and Troost, 1858, 1859, 1860, 1863ab, 1867a), metallic sulfides (Deville and Troost, 1861), the permeability of iron and cast iron (Deville and Troost, 1863c, 1868), niobium and its compounds (Deville and Troost, 1865, 1867b), the determination of high temperatures (Deville and Troost, 1880a), the density of selenium and tellurium vapors (Deville and Troost, 1880b), etc. etc. Together with Paul Gabriel Hautefeuille (1836-1902) he published a series of studies about boron and silicon (Troost and Hautefeuille, 1870ab, 1872, 1873), the formation of paracyanogen and its reversible dissociation to cyanogen, the transformation of cyanic acids into its isomers (Troost and Hautefeuille, 1868abcd, 1869ab), the allotropic conversions of white phosphorus into red phosphorus (Troost and Hautefeuille, 1873b), the hydrides of palladium, sodium, and potassium (Troost and Hautefeuille, 1874abcd, 1875a), the influence of silicon and manganese on the physical properties of commercial iron (Troost and Hautefeuille, 1873c, 1875b), etc. etc. (Anonymous, 1911; Gautier, 1911; Jaubert, 1912).
Scientific contributionTroost was a very prolific researcher and writer; he published well over 100 memoirs, books, and booklets. His chemistry textbook (Troost, 1861), the result of his classes at the Sorbonne, became very popular and went through a large number of editions. As was customary for a candidate for membership in the Académie des Sciences, Troost also published two booklets describing his main fields of research and findings (Troost, 1878, 1884).
In what follows we describe some of his important results. His publications with Deville about the density of gases at high temperatures, dissociation, measurement of high temperatures, and related subjects have already been discussed in another paper (Wisniak, 2004).
Lithium and its saltsAs told by Troost, Johann August Arfvedson (1790-1841), a student of Jöns Jacob Berzelius (1779-1848), discovered lithium in 1817 while examining the Swedish mineral petalite (Arfvedson, 1818). He reported that petalite was composed of silica, alumina, and an alkali. At the recommendation of Berzelius, the new alkali was named lithion (stone) to symbolize the fact that it had been discovered in the mineral kingdom, while the two other known alkalis (sodium and potassium) had been found in the vegetable kingdom. The element was later found to be present in other minerals, such as triphane or spodumen, lepidolite, triphylline, amblygonite, minette, etc. etc., as well as in some mineral waters. In 1807 Humphry Davy (1778-1819) reported to the Royal Society the isolation of potassium (in very small amount) by decomposing a fragment of slightly moistened potassium hydroxide by means of a voltaic cell (Davy, 1808). Afterwards, Robert Bunsen (1811-1899) and his student Augustus Matthiessen (Bunsen, 1855) developed an alternative process for producing large amounts of lithium.
Many chemists believed that lithine (LiOH) was isomorphic with the other two alkalis and that it should be able to replace NaOH in any compound, in the same proportion it was present in it. As proof of the isomorphism, chemists had tried unsuccessfully to prepare the double sulfate of lithium and aluminum. Similarly, no one had prepared lithium bisulfate as the analog of potassium or sodium bisulfates. This lack of information was the motive that led Troost to make a detailed investigation of the position that lithium had to occupy in the metal series (Troost, 1857).
Not much was known about the properties of lithium because of the difficulties involved in its isolation; from lepidolite, the most common mineral, it was possible to separate perhaps 4 to 5 parts per hundred parts of raw mineral. Davy, Bunsen, and Matthiessen had reported many of the properties of lithium: it was a solid at room temperature, it was not affected by dry air, but in contact with humid air it slowly became tarnished. It was the lightest known body, with a density of 0.59; it floated in naphtha, it melted at 180 °C, and had an equivalent of 6.5 and an atomic volume of 11. It caught flame at elevated temperatures and burned quietly with a white flame. It decomposed water at room temperature without melting, and formed light alloys with sodium and potassium.
Troost mentioned that while attending the Universal Exposition held in Paris in 1855, he had been very fortunate to obtain a large quantity of lepidolite. Troost went on to prepare lithium in large amounts, using Bunsen and Matthiessen’s method. He also tried unsuccessfully to adopt Deville’s method for the preparation of sodium (Deville, 1855) and Joseph-Louis Gay-Lussac (1778-1850) and Louis-Jacques Thenard’s (1777-1857) method for extraction of potassium with the help of iron (Gay-Lussac and Thenard, 1808).
Troost’s separation procedure was based on the fact that if a mixture lepidolite, barium carbonate, and barium sulfate were heated in a crucible, the materials fused and underwent a kind of liquefaction and separation into two phases. The lower one was a perfectly fused but viscous glass and the upper one, an extremely fluid liquid, easily removed from the crucible while hot either with an iron spoon or by decantation. This liquid, on cooling, produced a crystalline mass, white or slightly colored rose by manganese. If the whole mass was left to solidify in the crucible, it formed two solid non-adherent masses. The white crystallized portion was a combination of barium sulfate, potassium sulfate, and lithium sulfate. Simple washing with boiling water separated the barium sulfate from the alkaline sulfates. This simple procedure only succeeded with petalite by adding to it an amount of sodium or potassium sulfates such that the total mass of alkali was more or less the same as that present in lepidolite. These results suggested that adding these alkalis to lepidolite would increase the yield of lithium oxide. This allowed Troost to obtain by a simple fusion, a yield of 3% of lithium oxide (Troost, 1857).
Troost described the preparation and properties of a large number of lithium inorganic and organic derivatives, among them, the oxide and the hydroxide, lithium salts such as the chloride, bromide, iodide, sulfide, fluoride, carbonate, sulfate, and phosphate; the double sulfates of lithium and potassium, and lithium and ammonia; and organic salts such as acetate, the oxalates (acid and neutral) and double oxalates, tatrates and double tartrates, etc. (Troost, 1857).
Troost concluded that although the results of his experiments indicated that lithium should be classified the same as the two known alkalis (sodium and potassium); it should be clearly separated from them and approach more those of magnesium. Thus, lithium could not be prepared by the procedures that yielded easily potassium and sodium; lithium chloride when dissolved and heated, experimented a partial decomposition as magnesium chloride; solutions of lithium salts were not precipitated by ammonium carbonate, it formed with them double salts, the same as magnesium did. Lithium carbonate was soluble in a very large amount of water; if this solution was treated with a stream of CO2, the solubility of the carbonate increased as it did for magnesium carbonate, while the solubility of the other alkaline carbonate decreased. Lithium phosphate was insoluble in water, the same as magnesium phosphate; the equivalent value of lithium hydroxide was small with relation to sodium hydroxide, the same like that of magnesium hydroxide with respect to calcium hydroxide. Lithium chloride heated to its fusion temperature lost part of its chlorine, like magnesium chloride, etc. etc. In summary, lithium seemed to have the same role in the series of alkaline metals as magnesium did in the series of alkaline earth elements (Troost, 1857).
Several scientists had reported highly different values for the equivalent of lithium. Arfvenson had used an impure salt and calculated the value to be 10.224 while and Bunsen and Matthiessen and others had obtained values about 6.5. In his first paper about lithium Troost had also found the value 6.5 (Troost, 1857). It was clear that the real value depended on the nature and amount of the impurities present. Troost decided to repeat the determination of this property after spectral analyses showed that the lithium salts used also contained, in addition of sodium and potassium, notable amounts of caesium and rubidium (Troost, 1862). His findings indicated that highly purified lithium carbonate did not contain these foreign elements. His procedure was as follows: Lithium chloride was treated with ammonium carbonate; the precipitated lithium carbonate was washed, dried, suspended in water, and the milky liquid treated with a stream of CO2, which dissolved the carbonate rapidly. Heating the solution to boiling precipitated the crystalline carbonate. After repeating the process one more time, spectral analysis showed that the purified carbonate did not contain sodium, potassium, caesium, or rubidium. Their absence was easily explained on the basis of the high (relative) solubility of their carbonate.
Troost used this highly purified sample to determine the value of the equivalent by two different procedures. In the first one, he heated lithium chloride in a porcelain boat, and treated it first with a stream of dry HCl, followed by another of dry oxygen. The cooled material was then treated with silver nitrate to precipitate the chlorine. The results of two different runs gave the values 7.030 and 6.99 for the equivalent, average 7.01. For the second procedure Troost reacted one sample of the highly purified lithium carbonate with sulfuric acid, to titrate the lithium, and titrated the carbonate by heating another sample, intimately mixed with an excess of pure quartz. The results of two runs gave 7.00 and 7.02 (average 7.01) for the titration with sulfuric acid, and 7.06 for the carbonate. All these results indicated that the value of the equivalent was close to 7. This number was confirmed by the value 7.026 (the atomic mass of lithium is 6.941) reported by Karl Diehl (Diehl, 1862) (Troost, 1862).
Niobium and tantalumIn 1801 Charles Hatchett (1765–1847) communicated to the Royal Society his discovery of a new metal, which he named columbium (Hatchett, 1802). Shortly thereafter, Anders Gustaf Ekeberg (1767-1813) announced the discovery of a new element, which he named tantalum (Ekeberg, 1802). Some years later, Wollaston became suspicious that columbium and tantalum were the same metal (Wollaston, 1809) and after much experimental work, in which he compared the physical and chemical properties of the materials used by Ekeberg and by Hatchett, Wollaston concluded that except for a large difference in the specific gravities of the two minerals (5.918 for columbite and 7.953 for tantalite), which he assumed to be due to a different state of aggregation or of oxidation, there was no reason to consider columbium and tantalum as different elements. Wollaston opinion was generally accepted until 1844 when Heinrich Rose (1795-1864) showed that the American mineral contained a metallic acid different from than present in tantalite. Rose named his acid niobic and its metallic component niobium (after Niobe, one of the children of Tantalus) (Rose, 1844).
According to Jean-Charles Galissard de Marignac (1817-1894), although Rose had done an exhaustive study of the combinations of niobium, some of his conclusions seemed to be not based on factual data. For example, Rose assumed that hyponiobic acid and niobic acid were two oxygenated compounds of the same metal, completely irreducible one into the other: It was impossible to convert hyponiobic chloride into niobic chloride using chlorine in excess. In addition, both acids belonged to the sesquioxide and dioxide groups, respectively, although Rose’s results seemed to indicate that they were able to combine with bases in different proportions, the same as silicic and stannic acids did. Marignac’s experiences proved that niobium hypochloride and hypofluoniobates salts contained oxygen, contrary to Rose’s statement that the constitution of the hypochlorite was Nb2Cl3 instead of Nb2O2Cl3. Not only that, Marignac showed that the value of the equivalent of hyponiobic acid was about 266 instead of Rose’s 243.2, and that hyponiobic chloride was actually niobic oxychloride (NbOCl3) and hyponiobic acid was Nb2O3 (Marignac, 1865).
In a paper published in 1865, Deville and Troost reported their results on the constitution of niobium compounds (Deville and Troost, 1865). After a short review on the work done by Rose and Marignac, they indicated that the value 9.6 for the density of niobium chloride at the boiling temperature of mercury that they had reported previously (Deville and Troost, 1863a), corresponded more correctly to Marignac’s formula, Nb2Cl5, than to Rose’s one of 8.6 for his formula NbCl2. Deville and Troost also determined that the density of niobium oxychloride was 7.87, at the temperature of boiling sulfur, a value corresponding to that Marignac’s formula (Nb2O2Cl3) for this compound, and concluded that their results proved that Rose’s hypoychloride was actually an oxychloride and that all the properties of niobium, claimed to be extraordinary, were actually common properties (Deville and Troost, 1865).
In a following paper, Deville and Troost used a synthetic method to prove the presence of oxygen in niobium oxychloride (Deville and Troost, 1867b). Their procedure was as follows: Niobic acid was heated to redness in a platinum boat in a glass tube through which a current of dry CO2 was passed; niobium chloride NbCl5 (fusing at 194 ° and boiling at 240 °C) was then repeatedly volatilized over the acid. The niobic acid disappeared almost completely to form a white silky body volatilizing at 400 °C, which possessed all the properties of niobium oxychloride, according to the reaction
The same experiment, repeated using instead tantalic acid and tantalum chloride (Ta2Cl5), produced no tantalum oxychloride. Deville and Troost determined that tantalum chloride was a pale yellow, solid crystallizable compound, melting at 211.3 °C and boiling at 241.6 °C at 753 mmHg. It decomposed rapidly in contact with air producing fumes of HCl, and reconverting into tantalic acid. Its vapor density was 12.8 at 360 ° and 13.0 at 440 °C, confirming Marignac’s formula Ta2Cl5 and the equivalent value of 182. The density of tantalic acid, precipitated from the chloride by ammonia and ignited to low redness, was 7.35 (Deville and Troost, 1867b).
Heat and work effects in cellsIn 1858 Troost joined forces with Edme Hippolyte Marié-Davy (1820-1893) to study the possibility of using the electromotive force of a cell to determine the heat and work effects of some chemical reactions. Marié-Davy had done fundamental work on batteries and the electromagnetic motor and had found, among other things, that the resistance encountered by an ondulatory or continuous electric movement, at any point in an electric circuit, was proportional to its intensity at that point; that the work done by the resistance was proportional to the square of the intensity of the movement; and that in an active cell, the total work of the resistances of the circuit was proportional to the electromotive force of the cell and to its useful expenditure of zinc (Marié-Davy, 1855).
James Prescott Joule (1818-1889) had shown that the heat developed by a chemical reaction was due to the resistance that the media opposed to the establishment of an electric movement, and that the electromotive force of a cell was proportional to the algebraic sum of the heat effects of all the chemical reactions that took place in the cell. Using different arguments, Rudolf Julius Emanuel Clausius (1822-1888) had shown that the electromotive force of a cell could be used to measure the amounts of heat released during the chemical reactions taking place in the cell.
Marié Davy and Troost carried on their experiments along two lines: (1) whenever possible, they built a cell with the substances they wanted to combine and measured the electromotive force generated. Although this seemed to be to most direct approach, it was applicable only to a limited number of cases; (2) passing the current of a cell through the substances they wanted to combine or decompose, and thus force reactions that otherwise would not take place. The electromotive force of the cell was measured before and after the current flowed through the pertinent substances, and the difference between both values gave the result desired (Marie-Davy and Troost, 1858ab). In their calculations they used the fact that the solution of one gram of zinc in dilute sulfuric acid released 567.9 calories, or that one equivalent of zinc (32.5 g) released 18,457 calories (Favre, 1843).
In a paper published in 1858, Marie-Davy and Troost reported the calculation of the molecular work produced by the reaction of 10 inorganic acids (among them, sulfuric, nitric, HCl, HBr, boric acids, and zinc oxide) and five organic acids (oxalic, tartaric, acetic, formic, and citric), with KOH, NaOH, and ammonia (Marie-Davy and Troost, 1858ab). The results were expressed as the amount of heat released during the combination of one part of alkali in dilute solution with one part of the acid in dilute solution. Their results agreed completely with those obtained by Pierre Antoine Favre (1813-1880) and J. T. Silbermann (1806-1865) using standard calorimetric procedures (Favre, 1843).
In a following work, Marié-Davy and Troost used their electrochemical procedure to determine the heat of reaction on one equivalent of chlorine with one equivalent of 33 different metals (Marié-Davy and Troost, 1858c).
Cyanogen and derivativesJoseph-Louis Gay-Lussac (1778-1850) discovered cyanogen and paracyanogen during his studies about hydrocyanic acid (prussic acid). In order to obtain pure cyanogen it was necessary to distill mercury cyanide, neutral and well dried, in a small glass retort. It soon began to blacken, seemed to melt like animal matter, and then cyanogen was abundantly released in a very pure state, as long as the temperature was not too high. In contact with heat intense enough to melt the glass it released a little nitrogen. The residue was a charcoal matter of the color of soot, and as light as lamp black (paracyanogen) (Gay-Lussac, 1815).
According to Troost and Hautefeuille, paracyanogen was an “isomer” (allotrope) of cyanogen; it was a substance that had all the properties of a simple body, and hence it was of interest to find out if its two forms were comparable to red and white phosphorus (Troost and Hautefeuille, 1868a). In their first series of experiments they investigated how the temperature of decomposition of mercury and silver cyanide and the pressure of the cyanogen generated, affected the amount of paracyanogen formed. To keep the temperature constant they heated the salt, enclosed in a sealed tube, in a bath of a liquid boiling (mercury, sulfur), at the desired level. At the boiling temperature of mercury, 350 °C, mercury cyanide was completely decomposed after seven hours; the conversion to paracyanogen was 35% at 14 atm and 40% at 34 atm. At the boiling temperature of sulfur, 440 °C, the decomposition was almost instantaneous and the conversion to paracyanogen was 12% at 1 atm, 15% at 30 atm, and 40% at 65 atm. Silver cyanide started decomposing at a temperature slightly above 350 °C; heated slowly to 440 °C and then kept at that temperature resulted in total decomposition without fusion or ignition. The conversion to paracyanogen was about 64% at 60 atm.
To purify the remaining paracyanogen, the tube was opened, heated back to 440 °C, and then a stream of cyanogen gas passed through to eliminate the residual metal (Troost and Hautefeuille, 1868a).
In the second part of their research, Troost and Hautefeuille studied the laws that regulated the inter conversion of cyanogen and paracyanogen (Troost and Hautefeuille, 1868b). They had already found that that paracyanogen gradually decomposed into cyanogen as the temperature was increased and that the pressure generated could be used as a measure of the degree of decomposition. Their experiments consisted in putting a certain amount of paracyanogen inside a glass tube connected with a manometer and to a sampling tube (to test the purity of the resulting gas), and heating the contents to the desired temperature with combustion gases. In this manner they determined the values of this pressure at nine different temperatures in the range 502 ° to 640 °C. The corresponding values of the pressure varied between 54 to 1310 mmHg. Troost and Hautefeuille assumed that these values also represented the inverse reaction, that is, the conversion of cyanogen into paracyanogen. To confirm their assumption, they heated small amounts of pure liquid cyanogen and measured the resulting pressure. Their results indicated that, as expected, the equilibrium was achieved at the same levels of the pressure and temperature, but the reaction was extremely slow. This result pointed out the great analogy between this reaction and that of the inter conversion between red and white phosphorus.
The conversion of red phosphorus to white phosphorus was very rapid while the opposite one was very slow (Troost and Hautefeuille, 1868b).
In another publication, Troost and Hautefeuille extended their previous analysis to the equilibrium between cyanuric acid (the cyclic trimer of cyanic acid) and its “isomer” cyamelide, or insoluble cyanuric acid. The latter was known to transform into gaseous cyanic acid upon heating, hence the pressure of the gas could be used as a measure of the degree of dissociation. The conversion of cyamelide and cyanuric acid into cyanic acid occurred very slowly at temperatures below 150 °C and became very fast about 228.9 °C (the boiling temperature of sulfur). The reverse reaction (the transformation of cyanic acid into cyamelide) not only occurred at a much lower rate but the result depended on the temperature: under 150 °C the acid appeared as ordinary transparent crystal, soluble in water, below, the product was cyamelide insoluble and amorphous. Troost and Hautefeuille determined the values of the dissociation pressure at nine different temperatures in the range 160 ° to 350 °C. The corresponding values of the pressure varied between 56 to 1200 mmHg (Troost and Hautefeuille, 1868d).
In three additional publications Troost and Hautefeuille reported their measurements of the density of cyanic acid in the vapor and in the liquid state, as well as the coefficient of expansion of the latter (Troost and Hautefeuille, 1868c); the heats of transformation of cyanic acid into cyamelide, of cyamelide into cyanuric acid, of vitreous arsenic acid (arsenic pentoxide) into opaque arsenic acid, and of amorphous sulfur (Troost and Hautefeuille, 1869a); and the heats of combustion of cyanic acid and its “isomers” (Troost and Hautefeuille, 1869b).
DissociationAccording to Troost, some chemists believed that there were no compounds having an equivalent of 8 volumes. They admitted, a priori, that the equivalent of organic compounds was always 4 volumes; whenever they found that the density of the vapor corresponded to 8 volumes it meant that the compound in question had decomposed (Troost, 1877a). Jean Baptiste Dumas (1800-1894) had found that the density of the vapors of chloral hydrate was 2.76 and concluded that that his compound was formed by 4 volumes of chloral vapor and 4 volumes of water vapor uncondensed (Dumas, 1834). Hence the equivalent of the hydrate corresponded to 8 volumes of vapor, having theoretical density 2.86. In 1876 Alexander Naumann (1837-1922) measured the density of chloral hydrate at 78 ° and 100 °C and found it to be 2.82. From this result he concluded that chloral hydrate could not vaporize at these temperatures without decomposing completely into 4 volumes of chloral and 4 volumes of water vapor (Naumann, 1876).
Due to this contradictory conclusions, Troost decided to find an experimental method that would allow establishing if the vapors of chloral hydrate at 78 ° and 100 °C were really a mixture of 4 volumes of chloral vapors and 4 volumes of water vapor, or if this vapor was a stable compound representing 8 volumes (dry vapor). In the first case, the vapor pressure F should be considered a mixture of two vapors, each exerting a partial pressure 0.5 F (assuming ideal gas behavior); in the second case (no decomposition), the vapor behaved as a dry gas having pressure F. The method proposed by Troost was very simple and elegant: addition to the vapors of chloral hydrate a hydrated salt having a dissociation pressure of f. The compound should be selected such that f < 0.5 F at the temperature of the experiment. Under these circumstances, if the vapor of chloral hydrate was decomposed, then the salt would not dissociate and the pressure would remain at the value F. If the chloral hydrate was not dissociated, the salt would decompose and the total pressure increase to F plus the dissociation pressure of the salt, that is, F + f (Troost, 1877a).
For the particular case in question, Troost selected the hydrate of potassium oxalate, having a dissociation of pressure of 53 and 182 mmHg at 78 ° and 100 °C, respectively. The apparatus was charged with an amount of chloral hydrate that completely vaporized at 78 °C, yielding a pressure of 117.5 mmHg. Afterwards, the potassium oxalate was introduced. If the salt came into contact with a mixture of chloral and water vapor, each exerting a pressure of 117.5/2 = 58.5 mmHg, then the total pressure would remain unchanged at 117.5 mmHg. It was found that the pressure increased little by little until it reached 164.5 mmHg, that is to say, it increased by 47 mmHg. If the mixture of chloral hydrate and water vapor was ideal and there was no dissociation of the former, then the increase should have been 53 mmHg. The experimental result indicated that the potassium salt dissociated into chloral hydrate behaving as a dry gas. The same result was obtained at 100 °C. In summary, at 78 ° and 100 °C chloral hydrate vaporized without decomposition, against what was claimed by Naumann (Troost, 1877a).
It is clear that Troost did not consider the possibility of a small dissociation of chloral hydrate as an alternative for the pressure increase.
A few months later Würtz reported that he had used Troost’s technique and found that chloral hydrate could not vaporize without decomposing completely into anhydrous chloral and water vapor (Würtz, 1877). According to Würtz, the apparatus used by Troost could only handle very small amounts of the oxalate (about 1 gram) so that it was enough to introduce one milligram of water as hygroscopic water to change completely the results. In addition, Würtz brought into attention another possible source of error: the fact that potassium oxalate acted upon chloral and decomposed it into chloroform and formiate, at the same time that it transformed into potassium mono-oxalate anhydrous (Würtz remarked that this reaction was probably insignificant under Troost’s conditions). In his response to this criticism, Troost wrote that at the beginning of his experimental work he had operated under the same conditions used by Würtz but found that they were improper because of the extremely low rate of dissociation of the potassium oxalate hydrate. After Würtz’s communication he had carried on more experiences and found that they gave the same result as his previous ones. Troost remarked that his experimental method required that the measurements be made at a pressure not larger than 50% that of chloral hydrate at the temperature in question. At higher pressures the phenomena were more complex, decomposition took place, the surface of the potassium oxalate became acid and colored, and the salt now contained KCl. His method, which was based on hygrometry, lost validity when the salt reacted with chloral (Troost, 1877b, 1878a).
In a following work Troost described the phenomena that took place when distilling a mixture of chloral hydrate and chloroform (Troost, 1879b). When the temperature reached about 61 °C, the passing vapors condensed into a turbid liquid that afterwards separated into two phases, one of chloroform, and the other of water. Almost all the water combined with the chloral had passed over when about two-thirds of the mixture had distilled (formation of an azeotrope. When the temperature had reached about 67 °C the new liquid phase consisted of anhydrous chloral and chloroform.
Alloys of hydrogen with metalsIn 1868 Thomas Graham (1805-1869) reported that palladium had the remarkable property of absorbing about 982 times its volume of hydrogen and that this characteristic could be explained as a phenomenon similar to dissolution or condensation, which he named occlusion (Graham, 1868). In another paper Graham determined that the density of palladium charged with 800 to 900 volumes of hydrogen was sensibly lower than that of pure palladium, that the tenacity and electrical conductivity of the metal decreased (as occurred with alloys), and that the magnetism increased, as was the case of the combination of palladium with a strong magnetic metal. These results led him to modified his original hypothesis and admit that hydrogen and palladium formed an alloy of equal equivalents (Graham named the combined hydrogen, hydrogenium) (Graham, 1869ab). This new hypothesis was well accepted, although Graham now realized that the actual combination was formed by 0.772 equivalents of hydrogen per equivalent of palladium (assuming H = 1 and Pd = 106.5) (Troost and Hautefeuille, 1874a).
Troost and Hautefeuille believed the phenomenon was more complex and for this reason they decided to further investigate the question of whether hydrogen and palladium formed a real combination or not, and in case of a positive answer, what was the formula of the compound formed (Troost and Hautefeuille, 1874a). From their previous work, they knew that under the action of heat, compounds formed directly by the combination of a fixed substance with a gas decomposed partially, which resulted in a fixed pressure for every temperature level. In the case of bodies that dissolved a gas (e.g. water loaded with CO2), or condensed it (e.g. platinum black loaded with hydrogen), the resulting pressure depended on the initial degree of saturation. Hence, a simple way to answer the question was to measure the pressure of the hydrogen released from palladium, at different tem-peratures.
To do so, Troost and Hautefeuille put an amount of palladium saturated with hydrogen inside a tube communicated with a manometer and with a vacuum pump, which allowed withdrawing measured volumes of hydrogen during a given run. At about 100 °C the results indicated that as long as the volume of fixed hydrogen was above 600 times the volume of palladium, the pressure decreased very rapidly for every withdrawal of hydrogen, a characteristic, which defned a dissolution. When the volume decreased to 600 times, the pressure remained constant; this volume corresponded to ½ equivalent of hydrogen per equivalent of the metal, that is, to the formula Pd2H. From this moment on, the hydrogenated palladium behaved like a definite combination, capable of dissociating in such a manner that the pressure depended only on the temperature. A series of experiments conducted between 20 ° and 180 °C, with a volume ratio of hydrogen/palladium ≤ 600, showed that within this temperature range, the pressure was a function of the temperature alone and that the combination corresponded to the formula Pd2H. The reported data indicated that from 20 ° to 170 °C the dissociation pressure increased from 10 to 1840 mmHg. This pressure became equal to the atmospheric one between 130 ° and 140 °C; a result indicating that to prepare the compound above 130 °C required using compressed hydrogen (Troost and Hautefeuille, 1874a).
In a following publication, Troost and Hautefeuille reported the preparation, properties, and dissociation pressures, of the compounds of hydrogen with sodium and potassium (Troost and Hautefeuille, 1874b). Between 330 ° and 430 °C, the dissociation of potassium hydride, K2H, varied from 45 to 1100 mmHg, while that of sodium hydride, Na2H, varied from 28 to 910 mmHg. They also indicated that in this range of temperatures, lithium and thallium did not combine with hydrogen. At 760 mmHg, lithium heated to 500 °C absorbed 17 times its volume of hydrogen and thallium, only 3 times its volume.
Troost and Hautefeuille used their results to determine the density of the combined hydrogen (hydrogenium) as 0.62 in palladium hydride and 0.63 and sodium hydride. Since these values were close enough for two very different metals, they assumed that the density of hydrogen in any metal hydride should be 0.625 (Troost and Hautefeuille, 1874c).
A following paper resumed most of their activities in this area (Troost and Hautefeuille, 1874d).
In their above researches about the metallic alloys formed by hydrogen, Troost and Hautefeuille had described the characters which distinguished these definite combinations from the solutions of hydrogen in metals. Potassium, sodium, and palladium, combined with hydrogen, while a considerable number of other metals merely dissolved this gas. Additional experiences carried on with iron, nickel, cobalt, and manganese showed striking analogies in the manner in which they behaved with hydrogen at different temperatures. Since the facility with which these metals absorbed or gave off hydrogen gas depended greatly on their physical condition, it was necessary to study them when present as ingots, thin blades, or powder (Troost and Hautefeuille, 1875a).
An ingot of pure nickel gave out, in a vacuum, at a red heat, ⅕ of its volume of hydrogen. Laminæ of nickel, obtained by electrolysis, heated under vacuum to 200 °C, gave out 40 times their volume. Pulverulent nickel gave up 100 times its volume, and remained pyrophoric after the escape of the hydrogen. An ingot of cobalt gave up 1/10 of its volume; electrolytic laminæ of cobalt, heated under vacuum to 200 °C, 35 times their volume, and pyrophoric cobalt powder 100 times. It also remained pyrophoric after the loss of the hydrogen. Soft iron in ingots gave off ⅙ of its volume, and grey castiron more than the half. Electrolytic laminæ of iron gave off 260 volumes. In summary, iron, nickel, and cobalt absorbed directly hydrogen, without seemingly combining with it, just as had been already shown in the case of lithium and thallium. Finely divided iron had a property, which was not shared by nickel or cobalt: it decomposed water slowly at room temperatures and rapidly at about 100 °C. In this respect, iron approximated to manganese (Troost and Hautefeuille, 1875).