Smithson Tennant (1761–1815), a British physician turned chemist and chemical engineer, published very few papers but they were significant enough as to leave a mark in science and technology. He proved that diamond is a pure allotropic form of carbon, established that carbon dioxide was composed of only carbon and oxygen, discovered iridium and osmium in the residue of platinum ore, invented a simple procedure for producing potassium in large quantities, and established the basis of multiple effect evaporation. In addition, he proved the deleterious effect of magnesium oxide and carbonate in agriculture, emery was a variety of corundum, and that potassium nitrate reacted with gold.
Smithson Tennant (1761-1815), un médico inglés convertido en químico e ingeniero químico, publicó muy pocos artículos científicos pero ellos fueron suficientemente importantes para dejar una marca en ciencia y tecnología. Tennant demostró que el diamante es una forma alotrópica pura del carbón, estableció la composición correcta del dióxido de carbono, descubrió el iridio y el osmio en los residuos del mineral de platino, inventó un método simple para fabricar potasio en cantidad, y estableció las bases de la evaporación por efecto múltiple. Además, demostró el efecto nocivo del óxido de magnesio y su carbonato en la agricultura, que el esmeril era una variedad del corundo, así como la acción química del nitrato de potasio sobre el oro.
Smithson Tennant (1761–1815) was born on 30 November 1761 at Selby, Yorkshire, the only child of Mary Daunt, the daughter of a local physician, and the Reverend Calvert Tennant, absentee rector of Great Warley in Essex and fellow of St. John's College, Cambridge. His father, wanting to shape his son as himself, began to teach him Greek when he was only five years of age. Tennant's father passed away in 1772 and his mother in 1781; while riding out with her son she was thrown from her horse and killed on the spot, a fate which would be also that of her only son (Barrow, 1849; Whishaw, 1815).
After his father's death his mother sent Smithson to different grammar schools in Yorkshire, at Scorton, Tadcaster, and Beverley (the oldest state school in England). While at school at Tadcaster, he enjoyed attending the public lectures given by Adam Walker (1731–1821), a popular science traveling lecturer on experimental philosophy (Peacock, 1855). According to John Whishaw (1764–1840), “although Smithson was very young, he put several pertinent questions to the lecturer regarding some of the experiments, and displayed so much intelligent curiosity as to attract the attention of the audience, and give great additional interest to the lecture. Walker requested that he would continue to attend his lectures during the remainder of the course” (Usselman, 2005; Whishaw, 1815).
In 1781, after his mother's death, Tennant enrolled at the University of Edinburgh, where he attended courses on anatomy and surgery, chemistry and materia medica. He was particularly attracted by the chemistry lectures given by Joseph Black (1728–1799) (Usselman, 2005). In October 1782 he entered Christ's College, Cambridge, to study chemistry and botany, He was at first admitted as a Pensioner and afterwards as Fellow Commoner (Peacock, 1855; Usselman, 2004; Whishaw, 1815).
On January 18, 1785, with the support of some of his Cambridge colleagues, the mathematicians Edward Waring (1736–1798) and Isaac Milner (1750–1820), the physician John Jebb (1736–1786), the astronomer Nevil Maskelyne (1732–1811), and the chemist Richard Watson, Bishop of Llandaff, (1737–1816), he was elected, at the remarkably early age of 24, a Fellow of the Royal Society (Whishaw, 1815). During this period Tennant became a close friend of William Hyde Wollaston (1766–1828), a medical student at Gonville and Caius College, with whom he was to start a very successful commercial partnership in 1800 (Usselman, 2004).
In 1788 he took his first medical degree as Bachelor of Physics and afterwards left Cambridge and moved to London. In 1796 he took his degree of Doctor of Physics at Cambridge; being economically independent he chose to pursue his chemistry interests instead of medical practice, a very fortunate decision for science. His substantial chemical contributions led to being awarded the 1804 Copley medal by the Royal Society (Peacock, 1855; Whishaw, 1815).
In 1813, Francis John Hyde Wollaston (1762–1823) resigned his Jacksonian Professorship at Cambridge to become rector of Cold Norton in Essex. William Farish (1759–837), professor of chemistry and natural philosophy, was elected as his successor, vacating his chair as Professor of Chemistry. In May 1813 Tennant was elected to replace him (Usselman, 2005; Whishaw, 1815).
In September 1814 Tennant took a trip to visit the southern provinces of France. On February 15, 1815, he arrived at Calais, with General Baron Bulow (1757–1808), in order to take a boat back to England. The boat departed on February 22 but the strong winds forced it back to port. Tennant and Bulow took advantage of the free time available to take horses and visit Bonaparte's pillar located nearby. On their way back a bridge over which they were riding collapsed; both were thrown, with their horses, into the ditch. Tennant's skull was so severely fractured, that he died within an hour. Tennant was buried a few days later in the public cemetery at Boulogne (Barrow, 1849; Peacock, 1855; Usselman, 2004).
Smithson Tennant published very few scientific papers, but some of them were significant enough to leave a mark in science and technology. In 1791 he communicated to the Royal Society his analysis of fixed air (carbon dioxide) (Tennant, 1791). Antoine-Laurent Lavoisier (1743–1794) had proved that carbon dioxide was a compound of oxygen and carbon, but no one had decomposed the gas into its elements or found their ratio (Lavoisier, 1772a, 1772b). Tennant noticing that carbon was unable to decompose calcium phosphate, in spite of its substantial attraction for oxygen, inferred that the combined affinities of phosphorus for oxygen and of phosphoric acid for calcium were stronger than that of carbon for oxygen. Consequently, it was to be expected that reacting marble (calcium carbonate) with phosphorus under a strong heat would generate calcium phosphate and carbon. The experimental results confirmed his expectations, leading to the correct ultimate analysis of carbon dioxide and the discovery of a new compound, consisting of phosphorus and lime (Peacock, 1855; Tennant, 1791; Whishaw, 1815).
During 1797 Tennant reported the results of his experiments about the combustion of diamond and its composition. He heated powdered diamond with potassium nitrate in a gold tube and obtained carbon dioxide as the only product, proving that diamond “consists entirely of charcoal, differing from the usual state of that substance only by its crystallized form” and that the diamond afforded no more CO2 than an equal weight of charcoal (Tennant, 1797a). In a second paper published in 1797 Tennant proved that under strong heating both gold and platina reacted with nitre (potassium nitrate) to form soluble calxes (Tennant, 1797b).
About 1797 Tennant purchased 500 acres of newly enclosed land near Shipham in Somerset, and this led him to studies in rural economy. Two years later he reported to the Royal Society that the use of magnesium carbonate (or its calcined product) as a fertilizer produced the opposite results; the grains hardly germinated and soon perished. The two magnesium compounds turned the soil barren, even when applied in small amounts. Analytical work on magnesia limestone (dolomite) led him to conclude that both carbonates were actually combined rather than accidentally mixed (Tennant, 1799).
In 1802 he proved that the main component of emery was alumina and that this material was similar to the corundum or adamantine spar of China, and not an ore of iron, as had been previously supposed (Tennant, 1802).
In 1800 Tennant entered into a business partnership with Wollaston, devoted to the purification of platinum ore and the production of malleable platinum, and the manufacture of various organic substances important to the textile industry. In the residues of the platinum purification process Tennant discovered a dark powder, which was not attacked by aqua regia acid. A smart designed set of experiments led him to isolate and characterize the two new metals osmium and iridium (Tennant, 1804).
In 1813 he gave to the Geological Society an account of his analysis of a volcanic substance from the Lipari Islands containing boracic (boric) acid (Tennant, 1811).
In June, 1814, he read his two last communications to the Royal Society, one concerning an improvement of the method proposed by Joseph-Louis Gay-Lussac (1778–1850) and Louis-Jacques Thenard (1777–1857) for producing large quantities of potassium (Gay-Lussac & Thenard, 1808; Tennant, 1814b), and another in which he exposed the reuse of latent heat to evaporate an additional amount of water (Tennant, 1814a). This discovery led what today is the principle of multiple effect evaporation.
Scientific contributionTennant published only eight papers on different chemical and agricultural subjects; nevertheless their importance by far exceeded their number.
Composition of CO2It was long known that fixed air (carbon dioxide) was produced by the combustion of charcoal, and for this reason it was thought highly probable that it was composed of vital air (oxygen) and charcoal (Tennant, 1791). This opinion was confirmed by the experiments of Lavoisier, in which he had discovered that the weight of the fixed air formed during the combustion was nearly equal to that of the vital air consumed by a charcoal in the same process. The small difference of weight detected could be attributed to the production of water arising from the inflammable air (hydrogen) contained in the charcoal. Because oxygen had a stronger attraction for charcoal than for any other known substance, the decomposition of fixed air had not hitherto been attempted. Tennant succeeded in overcoming this difficulty, by means “of the united force of two attractions”, and thus decomposed CO2 and determined its constituent parts in consequence of their separation (Tennant, 1791).
It was long known that the combination of phosphoric acid with calcareous earth could not be decomposed by distillation with charcoal, in spite of the fact that oxygen was more strongly attracted by charcoal than by phosphorus. According to Tennant, oxygen was retained in the phosphate by two attractions (affinities), the one it had for phosphorus, and the one that the phosphorus had for lime. In order to release the oxygen it was necessary to overcome both attractions simultaneously. As these attractions were more powerful than that which charcoal had for vital air, if phosphorus was made to react with fixed air and calcareous earth, the vital air would combine with the phosphorus and the charcoal would be obtained pure. In order to get these substances to react it was necessary to heat them red-hot. According to Tennant, this could be easily carried out in the following manner: Into a glass tube closed at one end and coated with sand and clay to prevent the sudden action of the heat, a small amount of phosphorus was introduced first, followed by some powdered marble. As explained by Tennant, the experiment succeeded more readily if the marble was previously slightly calcined, probably because the part that was reduced to lime, combined immediately with the phosphorus and prevented its reaction with the fixed air. After the ingredients were introduced, the tube was closed, but not completely in order to avoid the free entrance of air, which could inflame the phosphorus, while allowing the heated air within the tube to escape. The tube was kept red-hot for some minutes and then taken from the fire and left to cool down before it was broken. Inspection of the residual material showed that it contained a black powder, consisting of carbon intermixed with a compound of lime and phosphoric acid, and of lime united with phosphorus. The lime and phosphoric acid could be separated by solution in an acid followed by filtration, and the phosphorus by sublimation (Tennant, 1791).
The carbon thus obtained from CO2 appeared to be identical from that obtained from vegetable matter. Deflagration of a small portion of it with nitre in a small retort resulted in the immediate formation of CO2. This experiment proved unequivocally that carbon was one of the constituent principles of fixed air; that is, it had to be present whenever fixed air was produced, and also, that those experiments, where it was supposed that CO2 may be formed without the aid of carbon, had not been conducted with the required precautions (Tennant, 1791).
Tennant did not stop here. Since vital air (oxygen) was attracted by a compound of phosphorus and calcareous earth more powerfully than by carbon, he was curious to find if this element was equally efficient on acids which were assumed to contain oxygen, but were known not to react with carbon.1 He now passed a stream of phosphorus through a compound of marine acid (HCl) and calcareous earth (calcium chloride), and another of hydrofluoric acid and calcareous earth (calcium fluoride), and observed that no reaction took place. Once again, since the strong attraction which these acids had for calcareous earth tended to prevent their decomposition, it might be thought that in this manner they were not more willing to part with vital air than by the attraction of carbon. But this was not the case. Tennant found that phosphorus could not be obtained by passing marine acid through a compound of bones and carbon, held red-hot. The attraction (affinity) of phosphorus and lime for vital air (oxygen) exceeded the attraction of carbon by a greater force than that arising from the attraction of HCl for lime (Tennant, 1791).
Tennant's experiments were repeated and extended by George Pearson (1751–1828). Pearson questioned Tennant's inference that the carbon and phosphoric acids were the necessary result of the affinities present (Pearson, 1792). He believed that “the known fact that phosphorus cannot be produced from bone ashes by heating them in the presence of carbon, only proved that the powers of affinity between respirable air (oxygen) and phosphorus, together with the affinity between the compound formed by their union (namely, phosphoric acid) and quicklime, were not inferior to the joint affinities between the respirable air, in the phosphoric acid, and charcoal, and between the compound of respirable air and charcoal (namely, carbonic acid) and quicklime” Pearson heated elementary phosphorus with the carbonates of sodium, potassium, and calcium in thick white glass tubes and in each case he obtained a phosphate and a black mass containing carbon, with the loss of carbonate (Pearson, 1792). According to Pearson his results indicated that the oxygen in the carbonates combined with the phosphorus to form phosphates, at the same time releasing carbon.
DiamondThe question of the action of heat on diamond had occupied scientists for many years. It was generally believed that diamond was indestructible under the action of high heat. Robert Boyle (1627–1691) was the first to study with care the action of fire on diamond and Isaac Newton (1642–1727) had predicted that diamond could be burned and had thus classified it in the class of combustible substances. In his book Opticks (Newton, 1730) Newton presented a table of the values of the ratio between the refractive power and the density for 22 substances (diamond having the largest value, 14,556) and wrote: “all Bodies seem to have their refractive Power proportional to their Densities…it seems rational to attribute the refractive Power of all Bodies chiefly, if not wholly, to the sulphureous Parts with which they abound…And as Light congregated by a Burning-glass acts upon Sulphureous Bodies to turn them into Fire and Flame…”. The inflammable substances employed by Newton were camphor, oil of turpentine, oil of olives, and amber. These he called “fat sulphureous unctuous Bodies”, and using the same expression with respect to diamond, he said that it was probably “un unctuous body coagulated”. Newton's inference that diamond was combustible was afterward confirmed by repeated experiments.
In 1771 Jean Darcet (1725–1801) heated a diamond in a crucible in Pierre-Joseph Macquer's (1718–1784) furnace and noticed that after 20min the diamond appeared to be enveloped in a small pale flame. When the experiment was repeated for a longer time, it was found that the diamond had been completely destroyed. This was a surprising result because jewelers were accustomed to remove or diminish flaws from diamonds by exposing them to a strong heat for some time and without noting any changes. In a following experiment, done in 1771 by Hilaire-Marin Rouelle (1718–1779), a diamond covered with a paste of chalk and charcoal, was heated in the furnace and seen to vanish completely. This fact suggested that its destruction was due to volatilization rather than to combustion, or decrepitating into fragments too small to be observed.
Lavoisier invited Macquer, Louis-Claude Cadet de Gassicourt (1731–1799) and Mitouart to assist him in performing additional experiments to clarify the doubt. The method adopted was to heat the diamond in such an apparatus that whatever substance was given off could be collected either by distillation or by sublimation in the cooler parts of the apparatus. They were, however, unable to detect any product (Macquer, Lavoisier, Cadet de Gassicourt, & Mitouart, 1772). In the same year (1772) Lavoisier published two memoirs (Lavoisier, 1772a, 1772b) detailing the experiments that the group had carried on the possible destruction of diamond by fire. In the first memoir he reported that diamond was rapidly destroyed when heated in contact with air, at a temperature below that required for fusing silver (962°C). Heating the diamond in a crucible, in the absence of air, it was quite refractory to the action of heat, but after eight days its weight began to decrease and eventually it evaporated completely. The same result was obtained when heated in the presence of carbon powder. The second paper discussed the results of experiments carried on with the aid of a burning glass, which allowed achieving much higher temperatures. When a diamond was subjected to the heat from the Académie's large burning glass, it decrepitated and split up into many fragments, some so small that they could not be seen with the naked eye. Heating the diamond slowly, it disappeared without giving off any smell or visible fumes. When it was burned in a closed vessel by means of the glass in an apparatus designed by Lavoisier, it was found that a part of the air in the vessel disappeared, as was the case with other combustible bodies. The air in which the inflammation had taken place had become partly soluble in water. Addition of limewater turned the liquid milky and precipitated a white powder, which appeared to be chalk because it dissolved in acids with effervescence. The results demonstrated beyond doubt that the higher temperatures attained with a burning glass resulted in the combustion of the diamond, that under the proper conditions the diamond could be reduced to carbon, and that the reaction generated a gas (fixed air) capable of precipitating limewater, having a similar nature to the one produced during fermentations and metallic reductions with carbon (Lavoisier, 1772b).
According to Tennant (1797a) the available information showed that in the absence of air the diamond resisted the effect of a strong heat, but exposed to the action of heat and air, it might be entirely consumed. Most of the experiments were done with the purpose of determining the inflammable nature of the diamond, without paying attention to the products resulting from the combustion and offering no information about the nature of diamond: Was diamond a distinct substance or one of the known flammable bodies? Lavoisier had shown that the process generated fixed air, a result hinting at the possibility that diamond contained charcoal. Tennant believed that the special nature of the diamond deserved further examination of the question. He speculated that conducting the heating in the presence of nitre (potassium nitrate) might allow combustion of the diamond in a moderate heat. His first concern was to eliminate all possibility of generating fixed air from extraneous matter. For this reason he used as a reactor a tube of gold closed at the bottom and connected at the top to a glass tube for collecting the air (gas) produced. First, some nitre was heated in the tube until it became alkaline, and afterwards washed out by water. The resulting solution, treated with limewater, remained perfectly transparent, indicating that no carbon dioxide had been produced. Heating a mixture of diamond particles with nitre resulted in the destruction of the diamond and appearance of a solid residue. The latter was dissolved in water and found to precipitate lime from limewater. Treatment of the residue with acids afforded nitrous oxide and fixed air. Hence the residue appeared solely to consist of nitre partially decomposed and aerated alkali (Tennant, 1797a).
Tennant went on to estimate the quantity of fixed air, which might be obtained from a given weight of diamond (Tennant, 1797a). He carefully prepared a mixture of 2.5 grains (about 0.162g) of small diamonds and a quarter of an ounce of nitre, and kept it in a strong red heat for about an hour and a half. The heat was then gradually increased and the nitre observed to become partly alkaline before the diamond began to be inflamed; by these means almost all the CO2 was retained by the alkali of the nitre. The gas that came over was produced by the decomposition of the nitre and contained so little fixed air as to occasion only a very slight precipitation from limewater. After the tube had cooled down, the alkaline matter was washed out with water, and all the diamonds found to have been destroyed. As an acid would disengage nitrous air from this solution as well as the CO2, the quantity of the latter could not in that manner be accurately determined. To obviate this inconvenience, the CO2 was reacted with calcareous earth, the vessel left undisturbed until the precipitate had settled in the bottom, and the clear supernatant liquid carefully separated and treated with limewater. No precipitate was formed, proving that it did not contain carbon dioxide. The vessel used in this occasion was a glass globe, having a tube annexed to it so that the quantity of the CO2 might be more accurately measured. Hydrogen chloride was streamed in to eliminate the CO2 from the calcareous earth. Measurement of the gas released by 2.5 grains (about 0.162g) of diamonds showed it to occupy the space of a little more than 10.1 ounces (about 0.287L) of water (the gas was measured by the amount of water it displaced). Duplication of the experiment using 1.5 grains (about 0.097g) of diamonds released an amount of gas equivalent to 6.18 ounces (about 0.167L) of water; according to this proportion the bulk of the fixed air 2.5 grains would have been equal to 10.3 ounces of water. This result was very similar to the one obtained when burning an equal weight of coal. Lavoisier used his results to calculate that fixed air (CO2) was composed of nearly 28% wt of charcoal and 72% wt. of oxygen (the correct values are 27.3% and 72.7%); he estimated that the weight of fixed air (at the conditions of the experiment) produced by 2.5 grains of charcoal would be very nearly the bulk of 10 ounces of water (Tennant, 1797a).
In 1797 Guyton de Morveau heated diamond in oxygen under a bell jar, using first a lens 40.59cm diameter with an auxiliary lens. The diamond burnt when the sun's rays were focused on it, but the thick glass of the bell jar cracked and the experiment was abandoned. In 1798 the experiment was repeated with a diamond immersed in an atmosphere of pure oxygen contained in a thin glass globe over mercury, using the Tschirnhausen lens. This time the diamond was completely consumed and transformed into CO2. Guyton found that the diamond yielded more CO2 than an equal weight of charcoal and thus concluded that diamond was pure carbon and charcoal a lower oxide of the element (Guyton de Morveau, 1799). Further experiments by Guyton, Jean Nicolas Pierre Hachette (1769–1834), and others still indicated that charcoal was an oxide of diamond (Guyton de Morveau, 1804).
In 1807 William Allen (1770–1843) and William Hasledine Pepys (1775–1856) studied the composition of carbon dioxide by burning known amounts of diamond and other carbonaceous substances (charcoal, stone coal, graphite, and animal charcoal) in oxygen (Allen & Pepys, 1807). Their results indicated that fixed air contained 28.6wt% carbon, that diamond is pure carbon, that well burnt coal contained no sensible quantity of hydrogen, and that diamond and all carbonaceous differed principally from each other in the state of aggregation of their particles. The last fact explained the variety of temperatures required for the combustion of different flammable substances.
Action of nitre on gold and platinumIt was known that gold did not oxidize when heated in the presence of air, and also that it was not attacked by nitre. Nevertheless, in the course of his investigation on the combustion of diamond, Tennant observed that when nitre was heated in a tube of gold and the diamond was not in sufficient quantity to supply the alkali of the nitre with carbon dioxide, a part of the gold was dissolved. This observation led him to examine in more detail the action of nitre upon gold, as well as on silver and platina (Tennant, 1797b).
The experimental apparatus and technique were similar to the ones used when studying the combustion of diamond. Tennant put thin pieces of gold into the tube together with nitre, and exposed them to a strong red heat for 2 or 3h. After withdrawing the tube from the fire, he found that the remaining nitre, weighing 140 grains (about 9.07g), consisted of caustic alkali and nitre partially decomposed, and 60 grains (about 3.89g) of gold had dissolved. Addition of water to the solid residue precipitated about 50 (about 3.24g) grains of the gold as a black powder. The gold, which was thus precipitated, was principally in its metallic state, and in large part insoluble in marine acid (HCl). The remaining 10 grains of gold communicated to the alkaline solution a light yellow color. Adding diluted sulfuric or nitrous acid to the solution changed its color first to a deeper yellow; and then to green, and afterwards to blue. Tennant explained the change in color as arising from the gradual precipitation of the gold in its metallic form, which, by the transmitted light, is of a blue color. Though the gold was precipitated from this solution in its metallic form, yet there seemed to be no doubt that, while it remained dissolved, it was entirely in the state of calx. Its precipitation in the metallic state was caused by the nitre contained in the solution, which, having lost part of its oxygen by heat, appeared to be capable of attracting it from the calx of gold, Tennant results indicated that if the calx of gold was dissolved by boiling it in caustic alkali, and enough nitre, which “had lost some of its air by heat”, was mixed with it, the gold was precipitated by an acid in its metallic state (Tennant, 1797b).
The next set of experiments was carried out to see if the same phenomena took place when using platinum or silver instead of gold. It was known that heating raw grains of platina with nitre in a crucible reduced the mineral to a powder form. Some scientists like William Lewis (1708–1781) and Andreas Sigmund Marggraff (1709–1782) thought that the nitre only corroded the iron contained in the grains of platina. But by heating nitre with some thin pieces of pure platina in a cup of the same· metal Tennant found that the platina was easily dissolved, the cup being much corroded and the thin pieces entirely destroyed. By dissolving the saline matter in water, the greater part of the platina was precipitated in the form of a brown powder. This powder, which was entirely soluble in marine acid, consisted of the calx of platina, combined with a portion of alkali, which could not be separated by being boiled in water. The platina retained by the alkaline solution, gave it a brown yellow color. By adding an acid to it, a precipitate was formed consisting of the calx of platina, of alkali, and of the acid, which was employed (Tennant, 1797a, 1797b).
Tennant found that silver was little corroded by nitre, but as its action upon that metal was not significant, it did not deserve a more detailed examination.
Lime used in agricultureFrom the time of making his first land purchase in Lincolnshire, Tennant became interested in rural economy and collected many details relative to the modes of cultivation used in different regions of England. Tennant learned from the local farmers that in the neighborhood of Doncaster two kinds of lime were used as fertilizer, which produced very different effects (Tennant, 1799). One of them had to be used sparingly and be spread very evenly over the land because in larger doses it decreased the fertility of soil instead of increasing it. Not only that, when a load of it was left in one place, no growth was observed for many years. A large proportion of the second of kind of lime was never found injurious and the areas, which were covered with it, were extremely fertile. Tennant was surprised by these results and decided to investigate their nature and the peculiar properties shown by both types of manure. In a first series of experiments, he ground the two kinds of lime to a coarse powder and sowed them with seeds of different plants. In both cases the seeds grew equally well and nearly in the same manner as they would in sand. In the second series of experiments, the stones were first burnt to lime, then exposed for some weeks to air to allow a decrease in causticity, and then sown as before. In the lime known to be beneficial, almost all the seeds sprouted and kept growing, as long as they were properly watered. Their roots had many fibers, which penetrated all the way to the bottom of the vessel in which they were planted. Chemical analysis of this sort of lime showed that it consisted entirely of calcareous earth. In the deleterious lime, only a few of the seeds grew, and the plants did not progress beyond the stage of the two seed leaves, unable to feed by themselves. The experiments showed that soil covered by a 2–3mm layer of this sort of lime, completely prevented growth of any seed. Chemical analysis showed that this type of lime contained three parts of pure calcareous earth and two of magnesia, and that on exposure to air it absorbed only 48% of the CO2 it contained before being calcined (Tennant, 1799).
From these results, Tennant speculated that the source of the problem was the magnesia contained in this lime. To test his hypothesis he carried on additional experiments in which seeds (chiefly of cabbage, known to grow rapidly) were planted in uncalcined and calcined magnesia. The seeds planted in uncalcined magnesia sprouted but their leaves never rose above the surface and almost did not develop a root system. Calcined magnesia proved to be more damaging, as the seeds did not sprout (Tennant, 1799).
The next set of experiments proved, once again, Tennant's ability in designing experiments. In order to learn how long the negative effect of magnesia lasted, he took pieces of mortar made of this species of lime from two houses, which had been built three and eight years before, respectively (and hence long exposed to air). These mortars were reduced to powder and sown as before. Once again, the seeds sprouted, without generating roots. It was also found that both types of mortar had absorbed nearly 45% of the CO2 they contained before calcination.
According to Tennant (1799), it was known that magnesian limestone could be easily distinguished from that which is purely calcareous, by the slowness of its solution in acids. From this property he speculated that the kind of marble called dolomite,2 might also be similar in its composition. In order to test his hypothesis he obtained dolomite samples from different places; one found among the ruins of Rome, thought to have come from Greece; another said to have been thrown up by Mount Vesuvius, and the third was from Iona, one of the western islands of Scotland. Inspection of the crystallized structure, generally observed in the magnesian limestone, led him to postulate that it had not been formed by the accidental union of the two earths, but as a result from their chemical combination. In addition, the difficulty of dissolving probably arose from the attraction of the different component parts to each other.
EmeryThe very hard substance called emery,3 although long used for grinding and polishing, had not been satisfactorily analyzed. In books on mineralogy it was considered as an ore of iron, probably because of its great specific gravity, although emery could not be easily separately from the mineral (Tennant, 1802). Tennant believed it should actually be considered an accidental impurity present in the iron ore. In his book about mineralogy Richard Kirwan (1733–1812) had reported that Johann Christian Wiegleb (1732–1800) had analyzed an emery sample and found it to contain 95.6% of silex (quartz, silicon dioxide), and 4.4% iron; Kirwan did not trust the correctness of this account, and commented that Wiegleb had probably analyzed a different material.
It was known that boiling emery powder in acid did not alter it, except making its color lighter, probably because the acid dissolved part of the iron. For this reason Tennant decided to test the reaction of emery with an alkaline reagent (sodium carbonate), heating the mixture to a red heat in a platinum crucible. Adding water to the cooled product showed that most of the emery was in a powdery state, of lighter color. Repetition of this process, under a stronger heat, showed no additional changes; the turbid alkaline solution was separated by decantation from the precipitated iron red calx and then neutralized with acid. A precipitate of white earth formed, which Tennant found to be almost argillaceous (Tennant, 1802).
According to Tennant (1802), the results of his experiments were so similar to those of corundum (adamantine spar) of China, which had been analyzed a short time before by Martin-Heinrich Klaproth (1743–1817), so as to believe that emery and corundum were in reality the same substance, although usually mixed with a different proportion of iron. In order to obtain a quantity of emery as free from iron as possible, Tennant first ground emery rock (seen to be formed of different strata) to a coarse powder and then separated the iron particles with a magnet. The remaining material, which seemed to have the usual degree of hardness, was then reduced to a fine powder in an agate mortar. This operation, principally done by pressure and not by grinding, hardly changed the weight of the material. A known weight of the emery powder was now heated to red heat with six times its weight of sodium carbonate, which had been previously deprived of CO2 and boiled to dryness in a filter pan. Analysis of the resulting material indicated that it contained 80% argillaceous earth, 3% silex, 4% iron, and 3% undissolved matter (total 90), a result very similar to that reported by Klaproth.
Osmium and iridiumIt was known that native platinum dissolved in aqua regia and that with ammonia and potassium salts the solution produced precipitates that varied in color from pale yellow to dark red-brown. Similar hues characterized the triple salts formed by platinum chloride with sodium hydroxide. Hyppolyte Victor Collet-Descotils (1773–1815) had carried on a large number of experiments in order to determine the nature of the principle responsible for the color variation (Collet-Decostils, 1803). His results indicated that the color of the red salts of platinum were caused by the oxides of a particular metal, which was almost insoluble in acids but dissolved easily when combined with platinum. During oxidation the metal first became blue and then green, although sometimes it appeared as violet. The oxides combined with platinum were soluble in alkalis, were not colored by borax, and when dissolved in acids did not produce precipitates with hydrogen sulfide. They were partially reduced by heat, while partially volatilizing. The sublimation was helped by a stream of oxygen and generated blue vapors. According to Collet-Descostils these properties did not characterize any known metal, hence he believed that the metal, which colored red the platinum salts, was a new one (Collet-Decostils, 1803).
Collet-Descostils experiments were continued by Antoine François Fourcroy (1755–1809) and Louis Nicolas Vauquelin (1763–1829), who investigated the black residue left by the action of aqua regia on native platinum ore and concluded that raw platinum contained five foreign metals, titanium, chromium, copper, iron, and a new metal: «Tout annonçait que le poudre noire contenait un métal nouveau» (Everything points out that the black powder contains a new metal) (Fourcroy & Vauquelin, 1803, 1804). They were unable to determine if the latter was or was not combined with platinum, although it certainly helped its fusion. This new metal had a white-gray color; it was difficult to melt, to oxidize, and to dissolve in acids. Its oxide was green and able to combine with alkalis; in the presence of ammonia salts in yielded a red precipitate and a variety of colored solutions when acted upon by different reagents (Fourcroy & Vauquelin, 1804).
As told by Tennant, he decided to experiment on the black powder, which remained after the solution of platina noticing that it did not, as was generally believed, consist of plumbago (graphite), but contained some unknown metallic ingredients (Tennant, 1804). He was familiar with Collet-Descostils and Fourcroy and Vauquelin's assumption that the black powder contained one new metal, but his observations indicated that in fact the black powder contained two new metals. He dissolved the material using a method similar to that employed by Vauquelin, namely, “the alternate action of caustic alkali and of an acid”: the powder was mixed in a crucible of silver with a large of pure dry sodium hydroxide, kept in a red heat for same time, and then washed with water. The alkali solution had a deep orange or brownish-yellow color. The remaining powder was now digested with hydrogen chloride giving a dark blue solution, which then became of a dusky olive green, and later, on continued heating, attained a deep red color. According to Tennant the alkaline solution contained the “oxide of a volatile metal, not yet noticed and also a small proportion of the second metal. If this solution was kept for some weeks, the latter metal separated spontaneously from it in the form of very thin flakes, of a dark colour”. When the alkali solution was first formed by adding water to the dry alkaline mass in the crucible, a “pungent and peculiar smell was immediately perceived”. This particularly special odor led Tennant to name the new metal osmium. The volatile osmium oxide could be separated by acidification and distillation. It was a colorless body, condensing first to an oily liquid and then solidifying into a semi-transparent mass (Tennant, 1804).
According to Tennant, the acid solution also contained both metals, particularly the one mentioned by Collet-Descostils and Fourcroy and Vauquelin. Tennant suggested naming the last metal iridium, from the remarkable diversity of colors, which it gave, while dissolving in HCl. Tennant used the latter solution to obtain iridium chloride in a pure state. To do so, he evaporated the solution, dried the resulting crystalline mass on a blotting paper, and dissolved the solids in water. Evaporation of the solution yielded individual octahedral crystals, which dissolved in water giving a deep red colored solution. Pure iridium was obtained by simply heating the crystals; this process eliminated the oxygen and the HCl. Tennant reported some of the properties of iridium: It could not be melted by any degree of heat he applied, it did not combine with sulfur or arsenic but did so with lead, copper, silver, and gold (Tennant, 1804).
Tennant's publication also reports his experiments on the properties and reactions of iridium, osmium and osmium oxide (Tennant, 1804).
In 1804 Wollaston announced the discovery of an additional new metal, rhodium, in the soluble parts of platinum metal (McDonald, 1960, 1962; Wollaston, 1804). The name was given to describe the rose color of the dilute solution of the salts containing it. Wollaston studied the solution of native platinum in aqua regia using a similar procedure as that of Tennant, but directed his attention to the liquid phase remaining after the addition of sal ammoniac. He first neutralized the solution with soda and then introduced in it bars of iron to recover the dissolved platinum, as well as other substances which he believed were present. The new precipitate was a fine, very black powder. He suspected that something new was present in it, remaining in the solution after the precipitation of platinum by sal ammoniac, which was neither platinum nor Tennant's iridium. He soon came to the conclusion that a new metal was present, which he called palladium after the asteroid Pallas that had just been discovered by Heinrich Wilhelm Olbers (1758–1840) (McDonald, 1960; Wollaston, 1805).
This discovery completed the identification of all the metals belonging to he platinum group.
Boric acidAccording to Tennant (1811) boracic (boric) acid was not as widely distributed as other substances; it appeared to be confined to a few geographical locations. In 1811 the geologist Leonard Horner (1785–1864) showed him a collection of materials ejected by volcanic activity in the Lipari Islands (part of the Aeolian islands located in the Tyrrhenian Sea off the north coast of Sicily). Tennant inspected this material and reported his results to the Geological Society: In addition to the usual substances found in these volcanic productions (sulfur and saline sublimations on the lava) he observed several pieces of a scaly shining appearance, resembling boracic acid. The largest of these had been cut of a rectangular shape, and was about 7 or 8 inches in length, and 5 or 6 inches breadth, as if it had been taken from a considerable mass. A crust of sulfur covered one side of most of these pieces, and the scaly part itself was yellowier than pure boracic acid. In order to find if the yellow color was due to the presence of sulfur, he heated the scales in a glass tube and noticed the distillation of water, followed by sublimation of sulfur. The residue was found to be pure boric acid.
PotassiumIn 1807 Humphry Davy (1778–1819) reported to the Royal Society the isolation of potassium by decomposing a fragment of slightly moistened potassium hydroxide by means of a voltaic cell. The globules of metallic potassium separated at the negative pole were preserved under naphtha (Davy, 1808). Davy's procedure was expensive and allowed the preparation of only very small amounts of the metal, a fact that made difficult the study of its properties and reactions. To obviate this problem Gay-Lussac and Thenard developed a new process based on the reaction between potassium hydroxide and metallic iron (Gay-Lussac & Thenard, 1808). Iron turnings were heated to whiteness in a curved gun barrel, which was covered with infusible cement clay to preserve it from the action of the air at a high temperature. Then, melted potassium hydroxide was passed slowly over the ignited iron; the hydroxide decomposed into a mixture of potassium vapors and hydrogen. The metal was condensed in a cold copper receiver and afterwards transferred into a vessel containing naphtha oil.
According to Tennant, although the Gay-Lussac–Thenard procedure allowed production of large amounts of potassium, it required the construction of a special furnace capable of admitting the gun barrel containing the iron fillings, and a short piece of barrel containing the alkali had to be adapted to the former by grinding, to make it airtight. Since the latter piece was out of the furnace, it required another source of heat to melt the alkali and let it flow into the longer barrel. To avoid the need of separate fires, the passage of the alkali was carried on in England by making a small perforation between the two barrels. The very hot alkali was poured into the smaller barrel, which was then closed with a ground stopper (Tennant, 1814a).
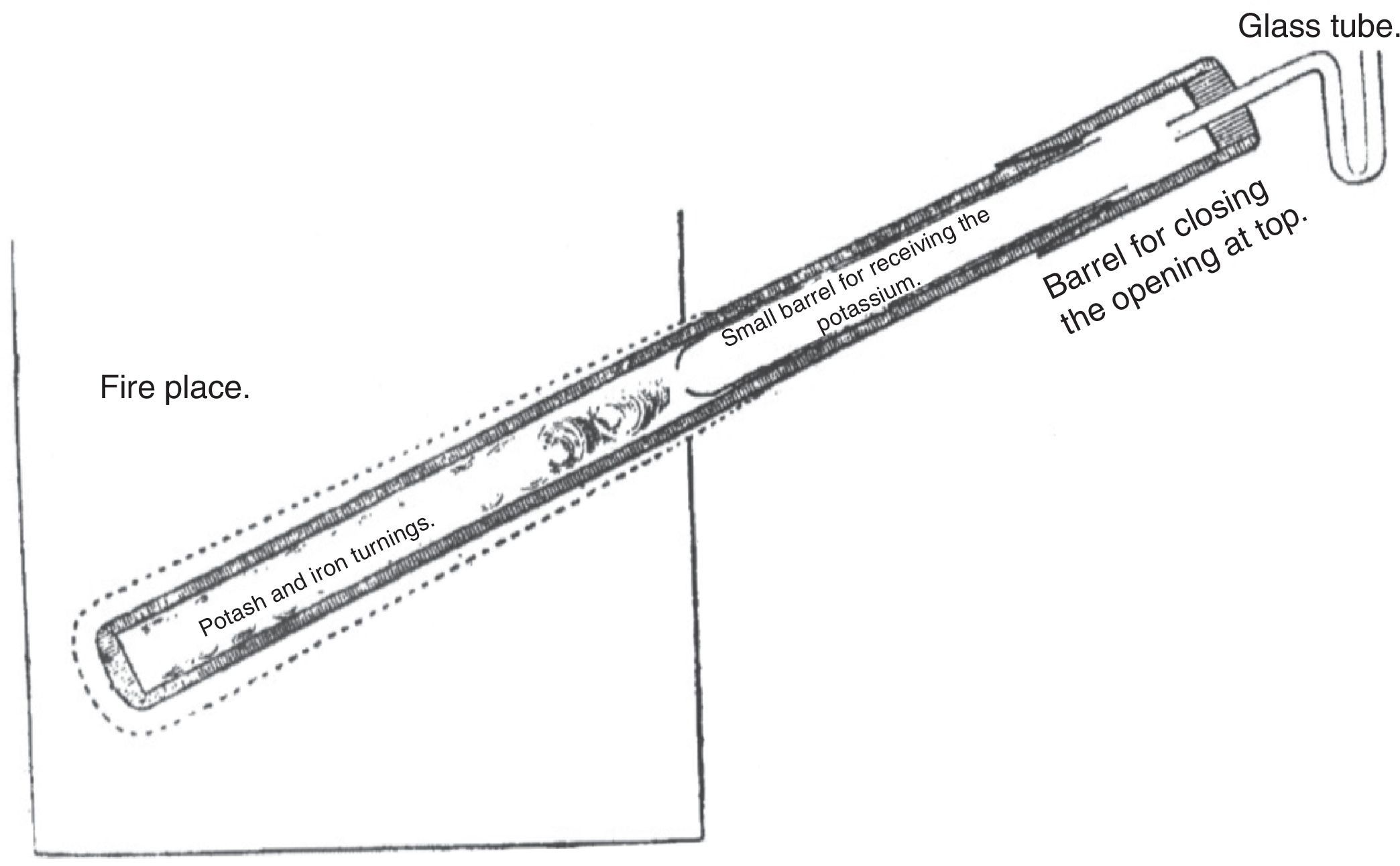
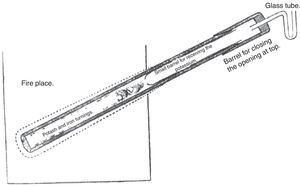
After some experimentation, Tennant found that there was no need to heat the iron turnings and the hydroxide separately. The mixture was put inside a piece of gun barrel closed in the lower end by welding and in the upper end by a cork provided with a glass tube to allow the escape of the air. The lower part of the barrel was now heated while keeping the upper part away from the heat source; as a result, the potassium sublimated and condensed in the upper part. The potassium so produced was found to be impure; it was probably a mixture of potassium and potash carried over. This limitation was eliminated by a very simple modification of the equipment: A smaller and thinner iron tube, provided with a very small orifice at its bottom, was inserted into the upper half of the barrel and both pieces were covered by a cap, which fitted the gun barrel sufficiently to be sealed with cement. The top of the cap was closed with a cork provided with a U tube to allow escape of the gas. The orifice of the insert was small enough to admit passage of the potassium vapors and prevent the entrance of potash (see Fig. 1) (Tennant, 1814a).
Tennant's apparatus for manufacturing potassium (Tennant, 1814a).
Tennant provided some practical recommendations for those wanting to build his apparatus: (1) the external barrel should be 45cm long, and the internal one about 17.5–20cm; (2) the internal tube should protrude outside the external one by about 2.5cm to allow its easy withdrawal; (3) the closing cap should be only fastened with sealing wax, and covered with linen or blotting paper kept wet; and (4) the glass U tube should contain only a drop of mercury, which being moved by the passage of the air showed that the vessels were perfectly tight.
Double distillationAccording to Tennant (1814b) in 1761 Black was the first to show that the quantity of heat required to raise the temperature of water from 50°C to its boiling point at atmospheric pressure [4.187(100–50)=209.4kJ/kg] was about 20% of that which was afterwards required for converting the boiling water into saturated steam at the same pressure (2257kJ/kg, hence the correct ratio is about 9.3%). Black's results were afterwards confirmed by James Watt (1736–1819) (Smeaton, 1971). According to Black, since saturated steam and boiling water had the same temperature, the additional heat that had been absorbed was latent and, as such, merely employed in supporting the gas state assumed by the water. If the steam was condensed, this latent heat reappeared in the same amount that had been absorbed for producing the phase change; this amount was so large that it could be employed for heating or evaporating other bodies.
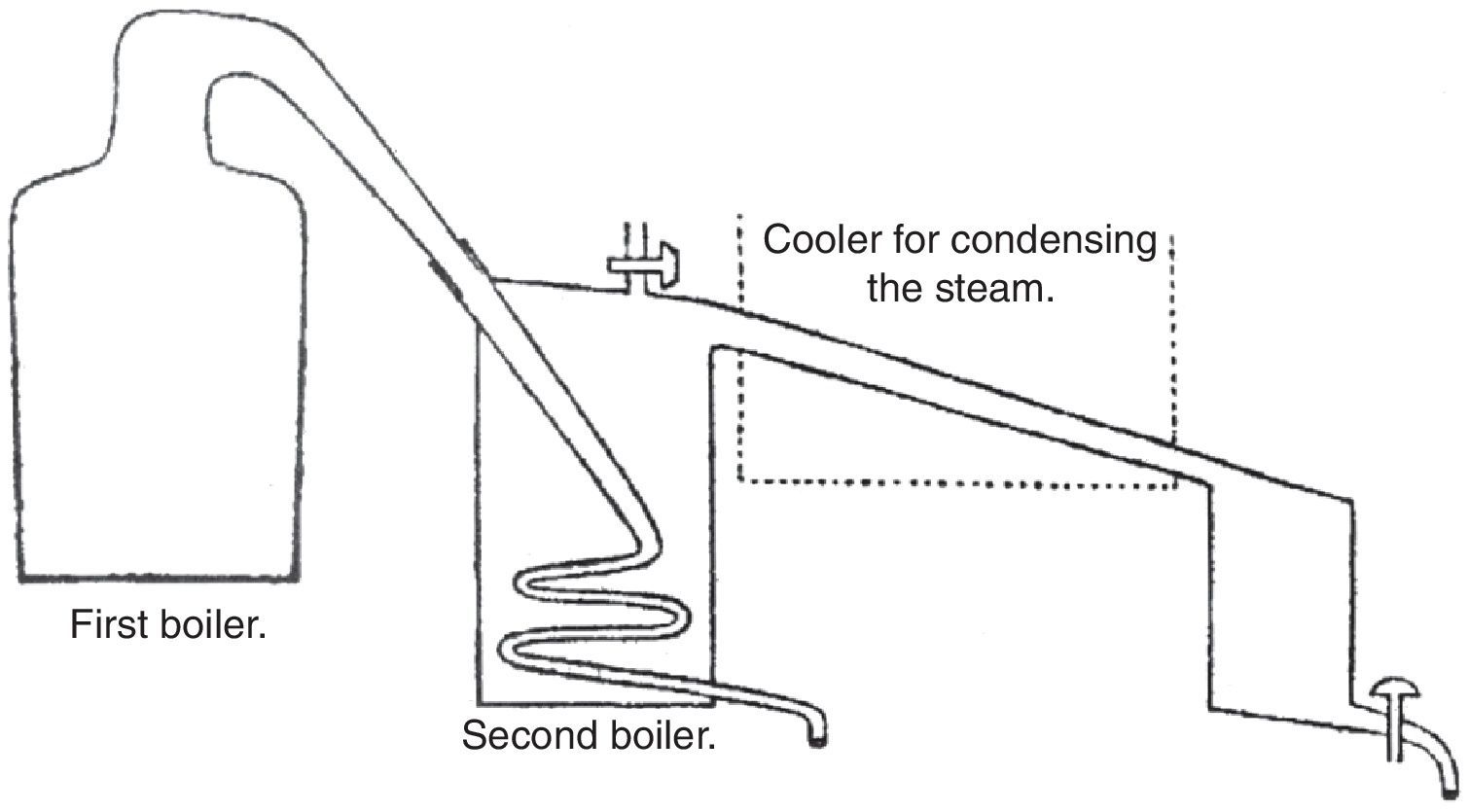
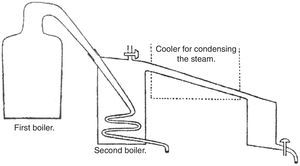
Tennant added that since water could not be heated beyond its boiling point, the change phase took place at constant temperature; in other words, the steam generated passed through liquid water without condensing. Now, if the steam was allowed to condense the heat released could be used to convert more water into steam and distilled over without the need for additional fuel (the observant reader will note that Tennant was establishing the principle used in multiple effect evaporation). Although this was not the usual process, it could be carried on in the following manner (Fig. 2).
Tennant's apparatus for reusing the latent heat (Tennant, 1814b).
Since the temperature required for vaporizing any fluid depends on the external pressure, reducing that pressure may lower it. Vapor will then be generated below the ordinary boiling point and might therefore be distilled over by steam or the usual heat. Tennant indicated that to produce this effect, it was necessary that a vessel having a receiver connected with it, should be made air tight and the steam made to pass through the vessel through a worm or spiral tube of metal, as shown in Fig. 2. The vacuum was now easily produced by applying heat to the vessel until steam issued from the opening in it, and in the receiver when they were to be immediately closed and the heat removed. The water distilled over was collected in the receiver, which was kept cool for that purpose.
Tennant wrote that although he had constructed an apparatus of this kind chiefly for the purpose of explaining the theory of latent heat, it was possible to use in a practical application whenever it was important to economize the consumption of fuel. As an example he mentioned that when drinking water was scarce on board a ship, common practice was to complement the supply by distilling water from the ship boiler. Now, if the steam from the boiler were made to pass through the apparatus just described, the quantity produced would be nearly doubled. Tennant mentioned that some experiments he had conducted about the subject showed that it was possible to distil an additional 75% of drinking water; the increase could be further increased if the second distilling vessel was properly insulated.
Though salt water does not boil at so low a degree of heat as fresh water, yet upon trial with sea water the difference was found to be quite insignificant, compared with that of the steam formed under the usual pressure and in vacuum and did not sensibly affect the result of the process. The only doubt as to the propriety of taking such a vessel to sea would arise from the degree of danger, which there is of experiencing a need of fresh water. Tennant thought that this risk was not great, but on the other hand, there was the important object of saving the lives of the people in the ship, whenever there was a lack of water.
When steam is passed through a tube surrounded with water, it is well known that it becomes condensed on the sides of the tube so long as the water is at a lower temperature than that of the steam; but since the latent heat given out in the condensation of steam soon raises the temperature of the water to 212°F, all transfer of heat ceases at that temperature and the steam then passes uncondensed. But since the temperature at which water may vaporize depends on the pressure of the atmosphere, the temperature of the surrounding water may be kept permanently lower, by reducing that pressure, so as to constantly lead to condensing the vapor of the first distillation; and being evaporated by the simple transfer of the same original quantity of heat, it may be received as an additional liquid product of the same process, by a suitable arrangement of the apparatus.
Interesting enough, at about the same time that Tennant published this memoir, Edward Charles Howard (1774–1816) was granted a patent (Howard, 1813) where he claimed that the refining of the sugar should be carried on by mixing it with a proper proportion of the finings, in a vessel having a perforated bottom, through which steam is allowed to enter until the sugar is fully dissolved and heated to 200°F. The most important feature of Howard's invention consisted in using the fact that the boiling point of a fluid decreases as the pressure is lowered. The syrup was now put in a closed vessel heated in the outside by steam while vacuum was applied to the vessel with the help of an air pump. The operating pressure was about 25mmHg. This method of concentrating sugar solutions avoided all danger of overheating; it reduced the amount of sugar lost through caramelization, and the solid sugar presented a boldness and brilliancy of crystal, which had never been attained before. It addition, it saved fuel. Design of the vacuum evaporator and other accessories resulted in a substantial improvement in the economic balance of sugar production and made Howard very rich.
Conflict of interestThe author declares no conflict of interest.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.
At that time many acids had been shown to consist of a peculiar basis and respirable air (oxygen). On the ground of analogy it was also assumed that other acids were composed in a similar manner.
Named after its discoverer, the French geologist and mineralogist, Déodat de Gratet de Dolomieu (1750–1801).
Emery is a very hard rock type, composed largely of the mineral corundum (aluminum oxide), mixed with other species such as iron and titanium oxides. Corundum is a crystalline form of aluminum oxide with traces of iron, titanium and chromium. It is the second hardest natural mineral known.