17 original crystals of [Λ-dinitrobis(ethylenediamine)cobalt]X complexes from the Alfred Werner collection of original samples of the University of Zurich were studied by single crystal X-ray diffraction revealing that the complexes with X=Cl, Br can undergo spontaneous chiral resolution upon crystallization. The main focus of this article was the chiral [Λ- and Δ-dinitrobis(ethylenediamine)cobalt]Cl complexes, which crystallize from racemic solution in the space group P 21 mainly as synthetical twins enriched in one enantiomer, but to a small extent also as pure enantiomorphs. The twinning effect was recognized and correctly described by PhD student Richard Hessen of the Alfred Werner group (PhD thesis 1914). Richard Hessen eventually resolved the [Δ- and Λ-dinitrobis(ethylenediamine)cobalt]Cl complex by the conglomerate salt resolution method. Based on the availability of the pure [Δ- or Λ-dinitrobis(ethylenediamine)cobalt]Cl complex, he carried out seeding experiments, which proved that the [Δ- and Λ-dinitrobis(ethylenediamine)cobalt]Cl complexes can be enriched to a great extent in one enantiomer by spontaneous chiral resolution. Already in the period of time from 1900 to 1904, various PhD students of Alfred Werner's group (Adolf Grün, Edith Humphrey, Ernst Zinggeler, Heinrich Schwarz, and Paul Larisch) prepared the [Δ- or Λ-dinitrobis(ethylenediamine)cobalt]Cl complex. Adolf Grün and Edith Humphrey have prepared enantiomerically enriched and rarely also enantiomorphic crystals of the [Λ- or Δ-dinitrobis(ethylenediamine)cobalt]Cl complex and could have separated crystals by manual crystal picking. Admittedly due to the crystal habits this would have been a difficult endeavour, but this ‘Louis Pasteur method’ was apparently not taken into consideration. Still in a cautiously sounding note one could state that Alfred Werner and his group had missed by this omission the opportunity for spontaneous chiral resolution of the [Δ- and Λ-dinitrobis(ethylenediamine)cobalt]Cl complexes in the period of time from 1900 to 1904. In addition, making this early chiral resolution story even more incredible, we found that Heinrich Schwarz and Paul Larisch applied in these early days of coordination chemistry the S-(D-,d-)camphorsulfonate anion to achieve the separation of the cis- and trans-isomers of the [dinitrobis(ethylenediamine)cobalt] complexes. They did not approach the potentially possible chiral resolution of the [cis-dinitrobis(ethylenediamine)cobalt]+ cation. But based on their synthetic procedure they did indeed accomplish chiral resolution of the cis-isomer and prepared eventually a series of the chiral [cis-,Λ-dinitrobis(ethylenediamine)cobalt]X salts; however all this was in an unintentional manner.
Diecisiete cristales originales de los complejos [Λ-dinitrobis(etilendiamina)cobalto]X, pertenecientes a la colección de muestras originales de Alfred Werner, de la Universidad de Zurich, fueron estudiados mediante difracción de rayos X de monocristal, encontrándose que los complejos con X=Cl, Br pueden resolver su quiralidad espontáneamente a través de la cristalización. El objetivo principal de este artículo fueron los complejos quirales [Λ- y Δ-dinitrobis(etilendiamina)cobalto]Cl los cuales cristalizan desde una solución racémica en el grupo espacial P 21 primordialmente como twins sintéticos enriquecidos en un enantiómero, y también en una pequeña medida como enantiomorfos puros. El efecto twinning fue reconocido y correctamente descrito por el estudiante de doctorado Richard Hessen (tesis doctoral en 1914) perteneciente al grupo de Alfred Wegner. Richard Hessen pudo eventualmente resolver el complejo [Δ- y Λ-dinitrobis(etilendiamina)cobalto]Cl mediante el método de resolución del conglomerado de sal basándose en la disponibilidad de complejo [Δ- o Λ-dinitrobis(etilendiamina)cobalto]Cl puro; llevó a cabo diversos experimentos de sembrado, los cuales concluyeron que los complejos [Δ- o Λ-dinitrobis(etilendiamina)cobalto]Cl pueden enriquecer en gran medida un enantiómero mediante resolución quiral espontánea. Ya en el periodo de 1900 a 1904 varios estudiantes de doctorado del grupo de Alfred Werner (Adolf Grün, Edith Humphrey, Ernst Zinggeler, Heinrich Schwarz, Paul Larisch) prepararon los complejos [Δ- o Λ-dinitrobis(etilendiamina)cobalto]Cl. Adolf Grün y Edith Humphrey prepararon cristales enriquecidos enantioméricamente y, extrañamente, a la vez cristales enantiomórficos de los complejos [Δ- o Λ-dinitrobis(etilendiamina)cobalto]Cl, los cristales pudieron ser separados manualmente. Es cierto, que debido al comportamiento de los cristales, esto debería haber sido un esfuerzo muy dificultoso, sin embargo este ‘método de Louis Pasteur’ aparentemente no fue tomado en consideración. Aun así, hablando con cautela, se podría decir que Alfred Werner y su grupo, mediante esta omisión, perdieron la oportunidad de lograr la resolución quiral espontánea de los complejos [Δ- o Λ-dinitrobis(etilendiamina)cobalto]Cl en el periodo de tiempo de 1900 a 1904. Además, haciendo la historia temprana de la resolución quiral aun más increíble, encontramos que Heinrich Schwarz y Paul Larisch aplicaron en los inicios de la química de coordinación el anión S-(D-,d-) sulfonato de alcanfor para conseguir la separación de los isómeros cis y trans de los complejos [dinitrobis(etilendiamina)cobalto]. Ellos no se acercaron a la potencialmente posible resolución quiral del catión [cis-dinitrobis(etilendiamina)cobalto]+. Pero basados en sus procedimientos sintéticos, efectivamente lograron la resolución quiral del isómero cis y prepararon, eventualmente una serie de sales quirales [cis-,Λ-dinitrobis(etilendiamina)cobalto]X, sin embargo todo esto, de una forma casual.
The microscopic world of molecules is three-dimensional, which comprises the field of stereochemistry. The spatial views of molecular chemistry and the stereochemical properties of molecules are determined by the chemical topologies. These are the crucial factors for the microscopic properties of single molecules and of ensembles of molecules, which is their colligative properties appearing in macroscopic units, such as liquids and solids.
This article deals with the stereochemical properties of chiral coordination compounds. Chiral molecules appear in isomeric forms as mirror and mirror images called enantiomers. Many physical and chemical properties of enantiomers are the same. Our hands for instance are also chiral and the shapes of left and right hands are the same; but our hands are distinguished by the inability to be overlayed, a phenomenon named accordingly as chirality or handedness. Chiral molecules exist thus as left-handed and right-handed forms, which at first glance appear easily distinguishable visually, but cannot for instance be differentiated by various spectroscopic methods, like for instance by IR or UV–vis spectroscopy. The shapes of molecules would allow easy physical distinction of left-handed and right-handed molecules, but unfortunately we cannot make the shapes of single molecules easily visible.
Using however polarized light applying the physical method of polarimetry, we can distinguish the left-handed and right-handed forms of molecules, the enantiomers, as single molecules or as an ensemble of a large number of molecules. Polarimetry is an early spectroscopic method of chemistry applied since the early 19th century. It is very helpful to distinguish enantiomers and can support the chemical resolution process of the enantiomers, but can normally not be used to resolve enantiomers, which remains in synthetic chemistry one of the most difficult tasks.
When chiral molecules are generated by chemical reactions, which do not provide chirally discriminating conditions, the left-handed and right-handed molecules have the same probability to be formed. This leads to 1:1 mixtures of left-handed and right-handed molecules called racemates or racemic mixtures. However, in contrast to the gaseous state, ensembles of molecules in the liquid or solid state show attractive interactions among each other, which are in the solid state physically expressed as lattice energies. They depend in magnitude on the specific arrangement of the molecules in the lattice of the crystal. Favourable stereochemical arrangements are of lower lattice energy and consequently have a higher tendency to appear in the crystalline state. In most cases of chiral molecules, the racemates are of lower lattice energy in the solid state causing in nature a preference for the formation of racemates. Only in rare cases the arrangement of molecules with the same handedness is of lower lattice energy than that of the corresponding racemic mixture. Under these circumstances chiral molecules form crystals containing enantiomers of the same handedness, a phenomenon, which is called spontaneous chiral resolution. Spontaneous chiral resolution of chemical compounds played a pivotal role in the development of stereochemistry, the spatial views of the molecules in three dimensions. In the 19th century the discipline of stereochemistry started in the realm of organic chemistry and provided soon the very fundamental understanding of molecular structures and reactions. For organic chemistry this development was connected to a move from two-dimensional views into the third dimension. Thus organic stereochemistry was discovered, which actually culminated in the discovery of Louis Pasteur in 1848 that carbon based organic compounds can spontaneously resolve in the solid state into crystals of the same enantiomer. At the same time this event marked the discovery of the chirality of molecules. Later in the development of stereochemistry, methods were found to resolve racemates into enantiomers using chiral auxiliaries applying appropriate chemical reactions. One of these separation methods – used particularly some 60 years later in the group of Alfred Werner in the field of coordination chemistry – was the conglomerate crystallization of salts, where ions of enantiomers are separated by formation of diastereomeric salts using a chiral counterion as chiral auxiliary.
Alfred Werner and the stereochemistry of coordination compoundsCoordination compounds, also called complexes, are a class of compounds composed of metal centres with an environment of molecules or molecular fragments called ligands ‘coordinated’, meaning chemically bound, to the metal centres, sometimes bound in a weaker and sometimes in a stronger binding fashion. The ligands are attached to the metal centres in a higher number (called coordination number, usually n≥4). Stronger binding means that the corresponding compounds are more stable and can for instance be isolated as solids. Coordination compounds were principally known since the end of the 18th century with some exceptions of rare species reaching back to the middle ages. However, the complexes of the earlier times of chemistry were often ill defined in their structures. The somewhat ‘mystical’ and in essence two-dimensional Blomstrand and Jorgensen formulae were weak models of structural coordination chemistry and were often insufficient in their explanatory power and could therefore not easily serve well as structural representations (Berke, 2009). Alfred Werner had radically reformed these inadequate views of coordination compounds by putting forward his Coordination Theory (Kauffman, 1972, 1979, 1994, 2003; Werner, 1893), published in 1893, where he gave also the clue to the third dimension in coordination chemistry and started to formulate a respective stereochemistry. The insufficient models of coordination chemistry at the times before Alfred Werner were naturally also accompanied by insufficient comprehension of this field. Full understanding of coordination chemistry arrived thus with Alfred Werner pretty late in time and helped to reform the field. The ‘old’ views of coordination compounds did not match reality very well and could for instance not at all explain the phenomenon of chirality of coordination compounds. It was Alfred Werner's privilege to discover the three-dimensional views in coordination chemistry, which enabled him to conclude on the existence of chirality, which happened in the last decade of the 19th century. Alfred Werner was well trained in organic stereochemistry mainly through his PhD supervisor Arthur Hantzsch at the ETH Zurich, Switzerland (Hantzsch & Werner, 1890; Kauffman, 1966a, 1966b). His switch to the field of coordination chemistry had started already in the group of Arthur Hantzsch, but developed independently further when he eventually had moved to the University of Zurich as an associate professor in 1893 and he had then formulated and published his Coordination Theory (Bailar, 1971; Berke, 2009; Gade, 2002; Kauffman, 1966a, 1966b, 1979; Pfeiffer & Werner, 1928).
Around 1900, Alfred Werner's research group consisted of a major part of PhD students working in the field of coordination chemistry (Kauffman, 2003) and that marks the period of time when his views on the stereochemistry of coordination compounds and his basic theoretical approach to coordination chemistry came effectively into play. Since his respective ideas were then so mature and matched reality so greatly that his predictive and explanatory power was becoming conceptually very strong. He could tackle almost any new problem in the field and indeed could also reflect chirality of coordination compounds (Ramberg, 2003, chap. 9; Von Zelewsky, Hayoz, Hua, & Haag, 1994). He expressed this circumstance in a publication together with A. Vilmos (Werner & Vilmos, 1899) putting forward the notion that chiral coordination compounds could be resolved into enantiomers discussing the specific examples of [Co(en)2(C2O4)]X complexes (C2O4=oxalate, en=ethylenediamine): ‘Das unter dieser Voraussetzung sich ergebende Modell ist jedoch, stereochemisch gesprochen, ein asymmetrisches, d.h. es kann zwei räumliche Anordnungen, die sich verhalten wie Bild und Spiegelbild, geben und nicht zur Deckung gebracht werden können’
In addition, around the same time in 1900, Alfred Werner had professionally to do with chirality, which indicates that he had knowledge and understanding at the frontiers of the field. In 1900 he refereed the PhD thesis of Hans Rehlen. Hans Rehlen had to study the influence of various substituents of organic compounds on their optical rotation effect: ‘Ueber den Einfluss von anorganischen in organische Moleküle eingeführten Atomgruppen auf ihr optisches Drehvermögen.’
When Alfred Werner became definitely aware of the fact that chiral coordination compounds could eventually like the tartaric acid derivatives of Louis Pasteur undergo spontaneous chiral resolution in the solid state, is not clear. As discussed before already spontaneous resolution of chiral compounds is a rare crystallographic phenomenon. But being so firm in the stereochemistry of coordination compounds, it would be very natural to assume that Alfred Werner was able to conclude on the expectation that this phenomenon could be an asset of coordination chemistry. As for the discovery of Louis Pasteur the probability of such a discovery seemed generally very low and could happen only under serendipidous circumstances. As said already crystallographic emergence of spontaneous chiral resolution is based on very small preferences in lattice energies of the chiral crystals. This situation unfortunately prevents any rational perspective for prediction, under which circumstances exactly spontaneous chiral resolution may happen in the various fields of molecular chemistry. As we know now today from various contemporary crystallographic studies on coordination compounds (Bernal, Myrczek, & Cai, 1993; Kostyanovsky, Torbeev, & Lyssenko, 2001), this phenomenon could have been discovered in Alfred Werner's group, since chirally resolving compounds were indeed subject of his research.
The Alfred Werner collection of original samples at the University of ZurichThe Alfred Werner collection at the University of Zurich comprises about 2500 original samples of mainly complexes prepared in the group of Alfred Werner. Alfred Werner had a strict bookkeeping on the samples prepared by his coworkers and registered these in an ordered fashion in a catalogue (see Fig. 1).
The samples are stored in vials and brought in order in boxes and are contained in a cupboard. Fig. 2 shows the box number 21, from which most of the sample vials were taken for the study of this paper.
Nearly all of the complexes shown in the box of Fig. 2 have the yellow to brown colour of the cis-[dinitrobis(ethylenediamine)cobalt]nX (n=1, 2; X=mono- or divalent anions). Among the samples are also the spontaneously resolved chiral crystals of the cis-[dinitrobis(ethylenediamine)cobalt]X (X=Cl, Br) salts, but in addition there are vials with achiral cis-[dinitrobis(ethylenediamine)cobalt]2Y salts (Y=dianionic counterions), trans-[dinitrobis(ethylenediamine)cobalt]X salts and chiral complexes, which were separated by conglomerate crystallizations. It should be mentioned that in addition to box 21 there is one more box (number 22) in the Alfred Werner collection of original samples. Boxes 21 and 22 have about 450 samples altogether. This large number of samples of one type of coordination compound points to the fact that the chemistry of the [dinitrobis(ethylenediamine)cobalt]+ cations played a prominent role in Alfred Werner's research. In some cases of the vials’ labels we not only noted down the name of the student, who prepared the sample, but also the chemical formula and sometimes also respective physical and chemical properties of the sample information, which allowed to take reference to the respective PhD thesis of the PhD student. This was particularly helpful in the cases when the respective work of the coworkers did not get published in scientific journals, indeed with respect to this article this was found not to be a rare case, but in turn this provided us as authors of this article the chance to make quite new and surprising respective chemical discoveries.
The spontaneously resolving chiral complexes cis-[dinitrobis(ethylenediamine)cobalt]X (X=Cl, Br) from the Alfred Werner groupPhD thesesThe cis-[dinitrobis(ethylenediamine)cobalt]X salts (X=Cl, Br) are the one of the rare series of complexes within the thousands of compounds prepared in the Alfred Werner group, which as revealed by X-ray crystallography were found in modern times as spontaneously crystallizing chiral solids crystallizing in the chiral space group P 21 (Bernal et al., 1993; Kostyanovsky et al., 2001). It is worth mentioning that the corresponding iodide salt cis-[dinitrobis(ethylenediamine)cobalt]I crystallizes in a centrosymmetric space and can therefore not appear in spontaneously resolved chiral crystals. The cis-[dinitrobis(ethylenediamine)cobalt]X salts (X=Cl, Br) were subject of the PhD theses of several of Alfred Werner's coworkers in the period of time from 1901 to 1904 and came back later from 1913 to 1914 in a sort of revival of the theme:
Adolph Grün, ‘Triammin- und Aethylendiaminamminverbindungen’, Inaugural-Dissertation, Universität Zürich, 1901.
Edith Humphrey, ‘Ueber die Bindungsstelle der Metalle in ihren Verbindungen und über Dinitrodiäthylenediaminkobaltisalze’, Inaugural-Dissertation, Universität Zürich, 1901.
Robert Stünzi, ‘Beitrag zur Kenntnis der Diacidotetrammninkobaltiake’, Inaugural-Dissertation, Universität Zürich, 1901.
Constantin Popovici, ‘Dinitrodiaethylendiamincobalt-Verbindungen’, Inaugural-Dissertation, Universität Zürich, 1902.
Ernst Zinggeler, ‘Ueber Rhodanokobaltsalze’, Inaugural-Dissertation, Universität Zürich, 1902.
Heinrich Schwarz, ‘Ueber die Beziehung zwischen Metallammoniaken und komplexen Salzen’, Inaugural-Dissertation, Universität Zürich, 1903.
Paul Larisch, ‘Abhängigkeit der Löslichkeit von der Anzahl der Ionen bei den Kobalt-, Chrom-, Rhodium-, Iridium- und Platin-Ammoniaken’, Inaugural-Dissertation, Universität Zürich, 1904.
Curt Rix, ‘Ueber Aethylendiaminkobaltiake’, Inaugural-Dissertation, Universität Zürich, 1904.
Heinrich Seibt, ‘Ueber stereoisomere Difluoro- und Fluoro-ammin-diaethylendiamin-kobaltisalze’, Inaugural-Dissertation, Universität Zürich, 1913.
Richard Hessen, ‘Ueber optisch-aktive Dinitrodiaethylendiamin-Kobaltisalze’, Inaugural-Dissertation, Universität Zürich, 1914.
These PhD theses on cis-[dinitrobis(ethylenediamine)cobalt]X salts should be considered as milestones with regard to the development of chiral octahedral complexes (see head pages of the most important theses in Fig. 3). Apart from the chiral [dinitrobis(ethylenediamine)cobalt]X salts these dissertations report in some cases also on racemic salts from the [dinitrobis(ethylenediamine)cobalt]+ cation possessing anions, which do not give rise to spontaneous chiral resolutions.
Head pages of the most important dissertations of the Alfred Werner group dealing with the [cis-dinitrobis(ethylenediamine)cobalt]X (X=Cl, Br) salts: from left to right and from top to bottom the dissertations of Adolf Grün, Edith Humphrey, Ernst Zinggeler, Heinrich Schwarz, Paul Larisch and Richard Hessen.
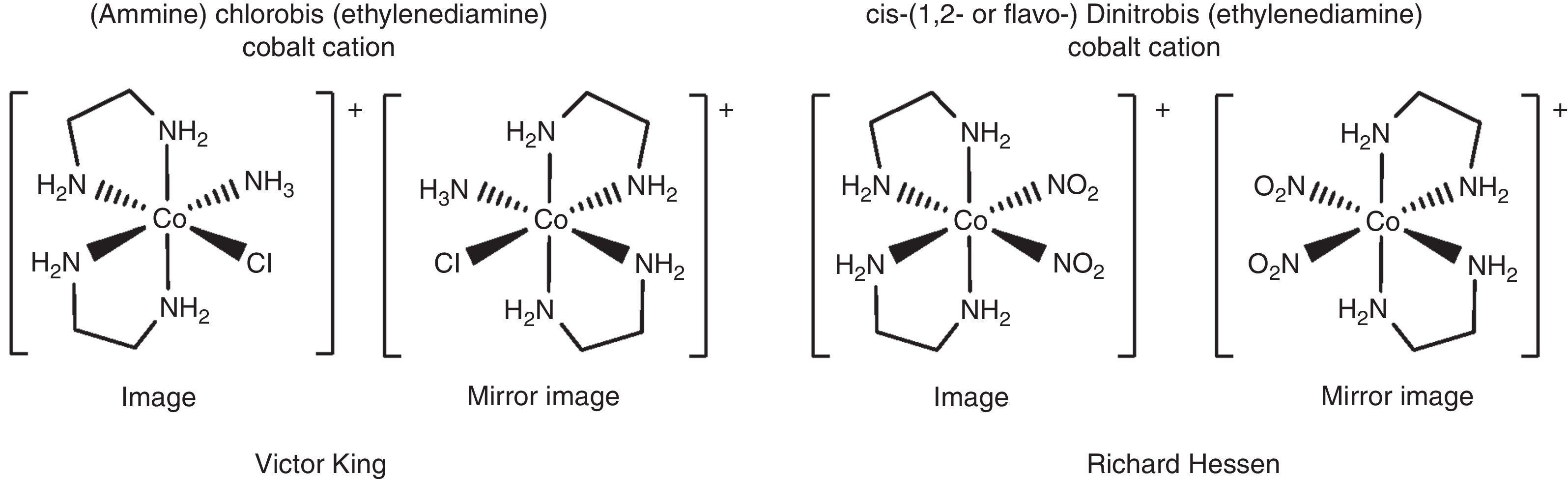
Even though [dinitrobis(ethylenediamine)cobalt]X salts are by far not the only types of complexes, which were studied in Alfred Werner's group. They were indeed a central theme of the group and are running like a thread through Alfred Werner's scientific achievements in coordination chemistry. The [dinitrobis(ethylenediamine)cobalt] cations appear in cis- and trans-isomeric forms, all thoroughly investigated in Alfred Werner's group. Alfred Werner denoted these isomeric forms also as 1,2- or 1,6-forms or in the very early days of his research also as flavo- and croceo-derivatives according to their colours (see Fig. 4, early notations as annotations in brackets).
The cis-configured cations, which are the subject of this report, go together in salts with counteranions, from which only the chloride and bromide are spontaneously crystallizing in chiral crystals. Other salts with mono- or dianionic counterions crystallize in centrosymmetric space groups as racemates showing image and mirror image in the same crystal.
Spontaneous chiral separation of [cis-dinitrobis(ethylenediamine)cobalt]BrSpontaneous chiral separation of [cis-dinitrobis(ethylenediamine)cobalt]Br prepared by Edith Humphrey in the group of Alfred WernerIn the group of Alfred Werner the PhD work of Edith Humphrey dealt with the preparation of various [dinitrobis(ethylenediamine)cobalt]X salts, among them X=Cl, Br (Fig. 4) with the spontaneously separating enantiomeric crystals, but containing also other complexes with X=NO3, X=½SO4 and ½[PtCl4], crystallizing in achiral space groups. The preparations of these complexes are all well described in her PhD thesis. A major aspect of the work of Edith Humphrey could have been the resolution of enantiomorphic crystals of the [cis-dinitrobis(ethylenediamine)cobalt]Br salt by manual crystal picking carried out in the same way as Louis Pasteur had done it for a tartaric acid derivative in 1848. This aspect of her work has been reviewed several times by various authors and had been themed ‘the missed opportunity’, since Edith Humphrey had not attempted separation by the Louis Pasteur method. Based on modern time's work of Ivan Bernal, who carried out an X-ray diffraction analysis of the [cis-dinitrobis(ethylenediamine)cobalt]Br salt, the chance was given that Edith Humphrey's crystals could have been separated into enantiomorphs (Bernal, 1999).
We once again had a close inspection the vial of Edith Humphrey containing crystals of the [cis-dinitrobis(ethylenediamine)cobalt]Br complex stored in the Alfred Werner collection of original samples at the University of Zurich. We examined Edith Humphrey's crystal batch (Fig. 5(left)) and searched again for enantiomorphic crystals. We did not find enantiomorphic crystals with the [cis-dinitrobis(ethylenediamine)cobalt] cation other than those two we had already published in an earlier paper (Ernst & Berke, 2011; Ernst, Wild, Blacque, & Berke, 2011). Anyway we expected that only crystals containing the [cis-dinitrobis(ethylenediamine)cobalt]+ cation in enantiomerically pure form could be enantiomorphs and such crystals are expected to be very rare in any spontaneous crystallization batch of the [cis-dinitrobis(ethylenediamine)cobalt]Br salt (Bernal et al., 1993). Also based on the related structural study and on the crystallization behaviour of the [cis-dinitrobis(ethylenediamine)cobalt]Cl salt to be reported later, this complex tends to undergo synthetical twinning, which was found to lead to enrichment of one of the enantiomers. Twinned such crystals may not necessarily have enantiomorphic shapes. Synthetical twinning should also occur for Edith Humphrey's [cis-dinitrobis(ethylenediamine)cobalt]Br salt. Non-twinned single crystals of the pure enantiomer are expected to show enantiomorphic faces, but they are expected to be formed very rarely in these crystallization batches. Therefore in the case of the [cis-dinitrobis(ethylenediamine)cobalt]Br salt, we can clear Edith Humphrey of the criticism that she would have ‘missed the opportunity’ to achieve chiral resolution by manual crystal picking (Bernal & Kauffman, 1987; Kauffman & Bernal, 1988), because it would have been very difficult by an accidental glance at the crystals to find enantiomorphs of the [cis-dinitrobis(ethylenediamine)cobalt]Br salt. The ‘omission’, which she can be accused of, is more that she apparently was not aware of the possibility for spontaneous chiral resolution of her complex and had the chance to prove the existence of enantiomorphic crystals analytically by measuring optical rotation using a polarimeter, a technique well-established at her times already (Lyle & Lyle, 1964). In modern times we were able to use X-ray diffraction studies, which indeed provided evidence that the investigated crystals were enantiomerically enriched due to the synthetical twinning effect in space group P 21, but we were unable to detect enantiomerically pure crystals by X-ray diffraction analysis. Enantiomeric enrichment in twinned crystals of the [cis-dinitrobis(ethylenediamine)cobalt]Br salt could however definitely be determined via the Flack parameter (Avalos, Babiano, Cintas, Jimenez, & Papacios, 2004; Flack, 1983; Flack & Bernardinelli, 1999). The quantitative estimate of the enantiomeric enrichment could be obtained from the Flack parameters of X-ray diffraction studies for two crystals of the [cis-dinitrobis(ethylenediamine)cobalt]Br salt, which amounted to Δ:λ ratios of 71: 29 and 41: 59, which corresponded to 42 and 18% ee's, respectively.
Photograph of Edith Humphrey's original vials and crystals of [cis-dinitrobis(ethylenediamine)cobalt]Br (left). Photograph of Edith Humphrey's original vials and the weathered crystals of the [cis-dinitrobis(ethylenediamine)cobalt]Cl·2H2O salts (right). Both samples from the Alfred Werner collection of original samples at the University of Zurich.
It should be mentioned at this point that the [cis-dinitrobis(ethylenediamine)cobalt]Cl complex was also prepared by Edith Humphrey, but was crystallized as a dihydrate (Fig. 5 (right)) presumably due to the fact that she applied recrystallization from dilute water solutions (see prescription Fig. 6 (left)). The vial of this complex could be found in the Alfred Werner collection of original samples. The hydrate is apparently not stable and undergoes weathering (photograph of the weathered crystals in Fig. 5 (right)). Due to this circumstance we expected complications in X-ray diffraction studies and refrained from the determination of the crystal structure of this [cis-dinitrobis(ethylenediamine)cobalt]Cl·2H2O compound and preferred to investigate the non-hydrated [cis-dinitrobis(ethylenediamine)cobalt]Cl for spontaneous chiral resolution into enantiomorphic crystals taken from a vial of the contemporary PhD student Adolf Grün (see section “Spontaneous chiral separation of [cis-dinitrobis(ethylenediamine)cobalt]Cl prepared by Adolf Grün in the group of Alfred Werner”).
Edith Humphrey's (left) and Adolf Grün's prescription (right) for the preparation of [cis-dinitrobis(ethylenediamine)cobalt]Cl·2H2O and [cis-dinitrobis(ethylenediamine)cobalt]Cl, respectively, applying recrystallizations from water. Original texts from the corresponding PhD theses.
From the vials of Edith Humphrey in the Alfred Werner collection we then studied two complexes of [cis-dinitrobis(ethylenediamine)cobalt]2X salts possessing dianionic counterions (X=SO4, [PtCl4]) (see Figs. 7 and 8) to see whether these compounds would reveal crystals with spontaneously resolved chiral [cis-dinitrobis(ethylenediamine)cobalt]+ cations. This idea did however not prove true. The X-ray diffraction studies of both complexes revealed crystals of hydrates consisting of racemic mixtures in the unit cell (space groups Cc and C2/c). We can only speculate that this changed crystallization behaviour with respect to [cis-dinitrobis(ethylenediamine)cobalt]Cl may have to do with a changed hydrogen bonding scheme in the crystal lattice (Kostyanovsky et al., 2001) or has to do with the stoichiometry of the salts. The ‘excess’ of cations forces in the solid state two cations getting in closer vicinity to each other, which may be the cause for the energetically prefered crystal packing of optical antipodes.
Models of the molecular structures of [cis-dinitrobis(ethylenediamine)cobalt]2SO4·H2O (left) and of [cis-dinitrobis(ethylenediamine)cobalt]2[PtCl4]·H2O (right) as determined by X-ray diffraction studies from crystals prepared by Edith Humphrey stored in the Alfred Werner collection of the University of Zurich. The full data sets of the X-ray diffraction studies are deposited at the Cambridge Crystallographic Data Centre.
We then had a closer look at the vials of Adolf Grün in the Alfred Werner collection of original samples and found one vial containing also the [cis-dinitrobis(ethylenediamine)cobalt]Cl salt (Fig. 9), which had also been prepared by Edith Humphrey (see Fig. 6 (left)). Adolph Grün was a contemporary of Edith Humphrey in the group of Alfred Werner, at least their times for preparing their PhD thesis were strongly overlapping. Maybe Adolf Grün had started his PhD work in Alfred Werner's group even somewhat earlier than Edith Humphrey, but both finished their PhD work in 1901. It is therefore remarkable that in this research group two PhD candidates had to tackle the synthesis of the same complex in about the same period of time. They perhaps got in competition with their task or what is quite probable in this case that they did not know from each other's work. Quite surprisingly, a closer look into the PhD theses of both candidates showed that the [cis-dinitrobis(ethylenediamine)cobalt]Cl salts were prepared in somewhat different ways (see the relevant text of the PhD theses of Fig. 6). Edith Humphrey applied dilute water solutions for recrystallization, while Adolf Grün applied ‘8–10’ consecutive crystallizations. Apparently this was done from concentrated water solutions, which apparently led to non-hydrated crystals. The preparative procedure to obtain the non-hydrated crystals of Adolf Grün was therefore quite tedious (Fig. 6).
Top: The vial of Adolf Grün containing the [cis-dinitrobis(ethylenediamine)cobalt]Cl complex. Bottom left: The crystals of the vial of Adolf Grün. The crystals (see boxes) show a remarkable striation of the larger crystals indicating a layered growth process by twinning. Each striation stripe is supposed to correspond to a twinning layer. Bottom right: Edith Humphrey's crystals of [cis-dinitrobis(ethylenediamine)cobalt]Br do not show visible striation. The twinning layers of Edith Humphrey's bromide have apparently thicknesses below the visibility range.
From Adolf Grün's vial of [cis-dinitrobis(ethylenediamine)cobalt]Cl we took four crystals, which were investigated by single crystal X-ray diffraction studies. The results with regard to spontaneous chiral resolution are shown in Table 1. The chiral enrichment of the enantiomers in the crystals was again based on twinning analyses within the X-ray diffraction analyses using Flack‘s method (Flack, 1983; Flack & Bernardinelli, 1999).
X-ray diffraction studies and determination of the Flack parameters of four crystals selected from the Grün vial and one crystal was taken from a Hessen vial (conglomerate crystallization) of enantiomerically pure [Λ-,cis-dinitrobis(ethylenediamine)cobalt]Cl of the Alfred Werner collection of original samples at the University of Zurich. Cl counterions are omitted in the drawing of the Δ-,Λ-structural representations. The full data sets of the X-ray diffraction studies are deposited at the Cambridge Crystallographic Data Centre.
| Crystal | Grün 1 | Grün 2 | Grün 3 | Grün 4 | Hessen |
|---|---|---|---|---|---|
| Space group | P 21 | P 21 | P 21 | P 21 | P 21 |
| Flack parameter | 0.497(17) | 0.35(2) | 0.30(3) | 0.029(4) | −0.008(5) |
| R1 | 0.0298 | 0.0247 | 0.0387 | 0.0159 | 0.0188 |
| wR2 | 0.0677 | 0.0524 | 0.1090 | 0.0414 | 0.0469 |
| Major | Δ (∼50%) | Δ (65%) | Δ (70%) | Δ (∼100%) | Λ (∼100%) |
| Minor | Λ (∼50%) | Λ (35%) | Λ (30%) | – | – |
| ee | 0% | 30% | 40% | 100% | 100% |
| Comment | Racemate | Mixt. of enant. | Mixt. of enant. | Enant. pure | Enant. pure |
The [cis-dinitrobis(ethylenediamine)cobalt]X complexes (X=Cl, Br) were grown from racemic solutions leading for the majority crystals to synthetical twinning (Klockmann, 1967) and in addition to prevalence of one enantiomer in the crystals. Naturally the twinned crystals do not possess enantiomorphic faces and may therefore show crystal habits quite different from those of the enantiomorphs (see also section “Spontaneous and conglomerate resolution of [cis-dinitrobis(ethylenediamine)cobalt]Cl carried out by Richard Hessen (PhD thesis 1914) in the group of Alfred Werner”).
Spontaneous chiral separation of [cis-dinitrobis(ethylenediamine)cobalt]Cl prepared by Heinrich Schwarz in the group of Alfred Werner (PhD thesis in 1903)Two years after the described work of Adolf Grün and Edith Humphrey, PhD student Heinrich Schwarz prepared in Alfred Werner's group again the [cis-dinitrobis(ethylenediamine)cobalt]Cl salt. Although the main task of his PhD work was to prepare new chromium complexes, Heinrich Schwarz (PhD thesis 1903) described in an appendix to his PhD thesis the syntheses of [cis- and trans-dinitrobis(ethylenediamine)cobalt]Cl complexes, which he separated by manual crystal picking. We found the vial containing the [cis-dinitrobis(ethylenediamine)cobalt]Cl compound in the Alfred Werner collection (vial Fig. 10) and studied several crystals of the vial by single crystal X-ray diffraction. Among the crystals were many of very big size (Fig. 10), which we cut into pieces to analyze their macroscopic inhomogeneity concerning their synthetical twinning structure.
The Flack parameters were used for resolving the twin reflections and from these parameters the enantiomeric excesses of the macroscopic crystals were calculated (Flack, 1983; Flack & Bernardinelli, 1999).
Table 2 demonstrates not only that all the crystals chosen from the Heinrich Schwarz batch had chirality. Very large crystals were cut into pieces and the pieces were investigated by single crystal X-ray diffraction to demonstrate that the crystals consist of macroscopic domains of twinning layers with different chiralities. Two, cut crystals (HS2111 and HS0212) were found to be enantiomorphs of opposite chirality. A microscopic search for enantiomorphic crystals was carried out, which rendered a crystal supposedly possessing enantiomorphic faces (see inserted picture of Fig. 10), and which could well be a single crystal. We anticipated that Heinrich Schwarz's crystal batch consists of more of these enantiomorphs strengthening the notion that the [cis-dinitrobis(ethylenediamine)cobalt]Cl salt has a greater tendency for ‘full’ spontaneous chiral resolution than has the [cis-dinitrobis(ethylenediamine)cobalt]Br salt. The type of twinning of the enantiomerically enriched crystals (HS0711, HS1211, HS2611, HS2711, HS0312, HS0412, HS0912) is generally denoted as synthetical twinning (Klockmann, 1967). For some crystals the weights were additionally determined as denoted in Table 2, because we wanted to see whether there is a dependence between size of the crystals and their enantiomeric enrichment, but apparently there is no such simple relationship. In contrast to Adolf Grün's crystals (Table 1) we found among Heinrich Schwarz's crystals no racemic crystal. This observation may be accidental, but may on the other hand indicate that Heinrich Schwarz and Adolf Grün used somewhat different crystallization procedures. As we will see in section “Spontaneous and conglomerate resolution of [cis-dinitrobis(ethylenediamine)cobalt]Cl carried out by Richard Hessen (PhD thesis 1914) in the group of Alfred Werner” the temperature of crystallization could play a prominent role and maybe there was a slight difference in temperature in the crystallization procedures of the two PhD students causing the given difference in the crystal habits.
X-ray diffraction studies of Heinrich Schwarz's crystals of the [cis-dinitrobis(ethylenediamine)cobalt]Cl salt (Fig. 10) with the main crystal parameters determined. Very large crystals were cut into pieces. The pieces investigated by single crystal X-ray diffraction showing different enantiomeric enrichments. The full data sets of the X-ray diffraction studies were deposited at the Cambridge Crystallographic Data Centre.
| Crystal | HS0711 | HS1211 | HS2111 | HS2611 | HS2711 | HS0212 | HS0312 | HS0412 | HS0912 |
|---|---|---|---|---|---|---|---|---|---|
| Weight (mg) | 0.025 | 0.116 | 0.458 | 2.839* | 7.935* | ||||
| State | Not cut | Not cut | Cut | Cut(1)** | Cut(2)** | Cut(3)** | Cut(1)** | Cut(2)** | Cut(3)** |
| Space group | P 21 | P 21 | P 21 | P 21 | P 21 | P 21 | P 21 | P 21 | P 21 |
| Flack parameter | 0.303(17) | 0.339(13) | −0.012(12) | 0.112(11) | 0.315(11) | 0.044(13) | 0.466(18) | 0.485(13) | 0.060(15) |
| R1 [I>2σ(I)] | 0.0307 | 0.0256 | 0.0201 | 0.0184 | 0.0205 | 0.0222 | 0.0311 | 0.0220 | 0.0259 |
| wR2 [all data] | 0.0752 | 0.0682 | 0.0511 | 0.0488 | 0.0523 | 0.0553 | 0.0679 | 0.0546 | 0.0657 |
| Major (%) | Λ (70) | Λ (66) | Λ | Δ (89) | Λ (69) | Δ | Δ (53) | Δ (52) | Λ (94) |
| Minor (%) | Δ (30) | Δ (34) | – | Λ (11) | Δ (31) | – | Λ (47) | Λ (48) | Δ (6) |
| ee (%) | 40 | 32 | 100 | 78 | 38 | 100 | 14 | 4 | 88 |
A closer inspection of the archive of the PhD theses of the Alfred Werner group brought up a somewhat unexpected observation. As a matter of fact it was up to now generally accepted that the first conglomerate resolution of enantiomeric complexes was carried out in the group of Alfred Werner by PhD student Victor King (PhD thesis of 1911) (Werner & King, 1911; King, 1942). The question arose, why such chiral resolution experiments appeared so late in the group of Alfred Werner, particularly in view of the fact that chirality as a general phenomenon and the method of conglomerate resolution of diastereomeric salts were known for long. Alfred Werner apparently knew about these facts (see also Chapter “Alfred Werner and the stereochemistry of coordination compounds”), since he had postulated earlier that complexes could exist as right-handed and left-handed structures appearing as enantiomers and perhaps also as enantiomorphs.
Much to our surprise we learned from the work of Ernst Zinggeler (PhD thesis 1902) and Paul Larisch (PhD thesis 1904) that they both could separate out one enantiomer of the [cis-dinitrobis(ethylenediamine)cobalt]+ cation successfully by chiral conglomerate resolution using the S-(D-,d-)camphorsulfonate anion as the counterion and producing diastereomeric salts with the [Δ-(d-) and Λ-(l-)cis-dinitrobis(ethylenediamine)cobalt]+ cation. In many cases the diastereomeric salts have drastically distinguished solubility properties and allow separation of the diastereomers by crystallization. Ernst Zinggeler was to prepare in his thesis rhodano complexes of the [bis(ethylenediamine)cobalt] molecular fragment using the [cis-dinitrobis(ethylenediamine)cobalt]+ cation among others as a starting material, which in the synthetic procedure had to be separated from the [trans-dinitrobis(ethylenediamine)cobalt]+ cation. Paul Larisch, as the title of his PhD thesis denotes, was to measure solubilities of a series of complexes among them also the [cis- and trans-dinitrobis(ethylenediamine)cobalt]X salts. In the same way as Adolf Grün and Edith Humphrey, Ernst Zinggeler and Paul Larisch were indeed confronted with the problem to separate [cis- and trans-dinitrobis(ethylenediamine)cobalt]X compounds by crystallization and to produce pure isomers, which Heinrich Schwarz had accomplished by manual crystal picking. It looks like an incredible story that Ernst Zinggeler and Paul Larisch had chosen to use a somewhat obscure looking procedure applying quasi the method of conglomerate separation to separate the [cis- and trans-dinitrobis(ethylenediamine)cobalt]Cl mixture using the S-camphorsulfonate as the anion, an experiment, which however inevitably, but apparently unintentionally, led to chiral conglomerate separation of the rac-[cis-dinitrobis(ethylenediamine)cobalt]X salts producing the diastereomers of the Δ- or the Λ-forms. Ernst Zinggeler and Paul Larisch were apparently not aware of the chirality of the cis-complexes. Their main intention was to probe a new anion as a better separation agent for the cis- and trans-isomers of [cis-dinitrobis(ethylenediamine)cobalt]Cl hoping for enhanced solubility differences of the new salts with the S-camphorsulfonate anion (see cut-out text of Paul Larisch's PhD thesis, Fig. 11). The cis- and trans-dinitro cobalt S-camphorsulfonate salts were indeed found to have a 19-fold difference in water solubilities (almost 35g difference per 100g water); still as an unintentious ‘side effect’ of this synthetic procedure they prepared the enantiomerically pure [cis-,Λ-dinitrobis(ethylenediamine)cobalt][S-camphorsulfonate] complex, and based on this a series of [cis-,Λ-dinitrobis(ethylenediamine)cobalt]X salts.
Solubilities of the conglomerates of the cis- and trans-isomers of [dinitrobis(ethylenediamine)cobalt][camphorsulfonate] salts at 16.5°C in water taken from the PhD thesis of Paul Larisch. The enantiomeric cis-isomer is expected to possess the Λ-configuration when the S-camphorsulfonate anion is applied.
Adolf Grün and Edith Humphrey developed special procedures to achieve relatively high purity of the cis-[dinitrobis(ethylenediamine)cobalt]Cl complex, but Ernst Zinggeler and Paul Larisch apparently were not content with the accomplishable purities along the synthetic lines of Adolf Grün and Edith Humphrey, particularly with regard to a complete separation of the [cis- and trans-dinitrobis(ethylenediamine)cobalt]+ isomers. Ernst Zinggeler and Paul Larisch had therefore chosen to use conglomerate formation for separation of the cis- and trans-isomers. Paul Larisch had quantitatively determined the solubilities of several [cis- and trans-dinitrobis(ethylenediamine)cobalt]X salts prepared from the camphorsulfonate salts (see Fig. 12 as taken from Paul Larisch's PhD thesis).
These experiments were successful, because as mentioned already the solubilities of the diastereomeric Δ- or Λ-cis-derivatives with the S-camphorsulfonate anion are very different and are expected to lead to the [Λ-,cis-dinitrobis(ethylenediamine)cobalt][S-camphorsulfonate] complexes as the least soluble conglomerate salt (see also Chapter “Spontaneous and conglomerate resolution of [cis-dinitrobis(ethylenediamine)cobalt]Cl carried out by Richard Hessen (PhD thesis 1914) in the group of Alfred Werner”), while the [cis-,Δ-dinitrobis(ethylenediamine)cobalt][S-camphorsulfonate] and the [trans-dinitrobis(ethylenediamine)cobalt][S-camphorsulfonate] complexes possess high solubilities in water and stayed in solution. This is an incredible story of another missed opportunity of Alfred Werner and his group. Ernst Zinggeler was thus the first one to carry out chiral resolution of chiral complexes in the field of coordination compounds. His experiments were presumably run in 1901 at a time when Adolf Grün and Edith Humphrey were still in the group of Alfred Werner, but apparently these experiments did quite astonishingly not have any impact on the late works of Adolf Grün and Edith Humphrey.
We found the vials of [Λ-,cis-dinitrobis(ethylenediamine)cobalt][S-camphorsulfonate] salts of Ernst Zinggeler and Paul Larisch (Fig. 13) and determined exemplarily the absolute configuration of Paul Larisch's [dinitrobis(ethylenediamine)cobalt][camphorsulfonate] via an X-ray diffraction study, which indeed revealed the Λ-,cis-configuration for the [dinitrobis(ethylenediamine)cobalt]+ cation and the S-configuration for the camphorsulfonate anion (see Fig. 14).
Original vials [Λ-,cis-dinitrobis(ethylenediamine)cobalt][S-camphorsulfonate] salts of the Alfred Werner collection at the University of Zurich prepared by the PhD students Ernst Zinggeler (left vial) and Paul Larisch (right vial) in the group of Alfred Werner (PhD theses of 1902 and 1904, respectively).
Structural model of [Λ-,cis-dinitrobis(ethylenediamine)cobalt][S-camphorsulfonate] obtained by a X-ray diffraction study, which demonstrates the Λ-,cis-configuration of the [dinitrobis(ethylenediamine)cobalt]+ cation and the S-configuration of the camphorsulfonate anion prepared by Paul Larisch in the group of Alfred Werner. The full data set of the X-ray diffraction study was deposited at the Cambridge Crystallographic Data Centre.
It should be mentioned at this point that Richard Hessen also prepared the [Λ-,cis-dinitrobis(ethylenediamine)cobalt][S-camphorsulfonate] complex as an intermediate system to transform eventually this compound via conglomerate crystallization into several [Λ-,cis-dinitrobis(ethylenediamine)cobalt]X salts (see Chapter “Spoontaneous and conglomerate resolution of [cis-dinitrobis(ethylenediamine)cobalt]Cl carried out by Richard Hessen (PhD thesis 1914) in the group of Alfred Werner”). Ernst Zinggeler and Paul Larisch also prepared various [Λ-,cis-dinitrobis(ethylenediamine)cobalt]X salts from the [Λ-,cis-dinitrobis(ethylenediamine)cobalt][S-camphorsulfonate], but these derivatives could not be identified anymore in the Alfred Werner collection. We found however the [trans-dinitrobis(ethylenediamine)cobalt][S-camphorsulfonate] salt of Paul Larisch (Fig. 15) confirming his successful endeavour of separating cis- and trans-isomers of the [dinitrobis(ethylenediamine)cobalt]+ cations. To summarize this section, Ernst Zinggeler and Paul Larisch had deliberately chosen to use a chiral auxiliary for non-chiral purposes: the separation of cis- and trans isomers, what one might denote also as an excessive misuse of a precious chiral agent. Only the final success of their endeavours eventually could justify this somewhat mysterious approach.
Spontaneous and conglomerate resolution of [cis-dinitrobis(ethylenediamine)cobalt]Cl carried out by Richard Hessen (PhD thesis 1914) in the group of Alfred WernerFrom 1910 onwards, the group of Alfred Werner became very active in attempts to resolve chiral complexes via conglomerate resolutions. The awareness that complexes could be chiral and could exist in solution and in solid state as separable enantiomers (for octahedral complexes showing the Δ and Λ forms) matured in Alfred Werner's perception of coordination chemistry long before that time, since in the years before 1900 Alfred Werner had already set the grounds for such ideas creating his three-dimensional views of coordination chemistry (Ernst & Berke, 2011; Ernst et al., 2011), which was part of ‘Alfred Werner's Coordination Theory’ (Berke, 2009; Werner, 1893). In addition, the section “Conglomerate resolution of [cis-dinitrobis(ethylenediamine)cobalt]Cl carried out by Ernst Zinggeler and Paul Larisch in the group of Alfred Werner (PhD theses 1902 and 1904)” witnessed that chiral auxiliaries, like the S-camphorsulfonate salt (Werner, 1918), were available for chiral conglomerate resolutions in the group of Alfred Werner.
The stereochemical views of Alfred Werner reached in those days of Alfred Werner's career, at the University of Zurich (around 1910), such a high level of abstraction and matched reality so well that the presumption of enantiomers of coordination compounds became an inevitable asset to his views of Coordination Theory. As expressed in the introductory part of this paper already, it is somewhat of an enigma why Alfred Werner's group then did not start to deal with his chirality endeavours in coordination chemistry already in the years of around 1900. Alfred Werner also started to publish on the asymmetric cobalt atom only in 1911. When Alfred Werner and his group could then also give experimental proof to his chirality concept of coordination compounds, Alfred Werner's Coordination Theory got then quite comprehensive and was generally accepted, and due to its experimental verification, the distinction with the Nobel Prize (1913) seemed to be in reach (Kauffman, 1968; Werner, 1912, 1918; Werner & King, 1911). As said already, polarimetry (Lyle & Lyle, 1964) as an analytical tool for tracing chirality of compounds was known for long and had reached at Alfred Werner's time a high level of sophistication in its application, which became then also heavily applied in Alfred Werner's laboratory.
The first conglomerate resolution of chiral complexes was carried out in Alfred Werner's group by PhD student Victor King (PhD thesis 1911, Fig. 17) (Ernst & Berke, 2011; Ernst et al., 2011) separating the cis-[amminechlorobis(ethylenediamine)cobalt]+ cation (Fig. 17) as camphorsulfonate salts. Many resolutions of chiral complexes followed then in the group of Alfred Werner. Among those was also the resolution of [Δ- and Λ-dinitrobis(ethylenediamine)cobalt]Cl carried out mainly by PhD student Richard Hessen again via diastereomeric salt formation using the silver S- and D-camphorsulfonates. In Richard Hessen's nomenclature the [Δ-(d-) and Λ-(l-)dinitrobis(ethylenediamine)cobalt]Cl compounds are called the compounds of the ‘d-’ and ‘l-Reihe’ (see also Figs. 16 and 17). The two vials of Fig. 16 mark the successful resolution into the enantiomers. On the labels of the vials we find also polarimetric information. As a X-ray study of one crystal of the ‘l-Reihe’ vial of Fig. 16 revealed, the complex was determined to be optically pure [Λ-cis-dinitrobis(ethylenediamine)cobalt]Cl complex (see Table 1).
The PhD thesis of Richard Hessen reviews at a very high level the stereochemical knowledge of chiral organic compounds and their resolution methodologies accumulated since the discovery of chirality by Louis Pasteur in 1848. In addition he also gave detailed information on the conditions of spontaneous chiral resolution, where the main point was that the crystallization temperatures play a decisive role marking a transition temperature, above which a spontaneous chiral resolution can no longer be achieved and racemates are obtained. For instance for Louis Pasteur's sodium potassium tartarate this temperature lies at 3°C. Richard Hessen does not report the transition temperature for the spontaneous resolution of the dinitrobis(ethylenediamine)cobalt]Cl compound, but from his resolution experiments one can conclude that this temperature should not lie far above 24°C. Since he had the optically active complexes in hand, he had also realized that the racemic crystals of [dinitrobis(ethylenediamine)cobalt]Cl have a much higher solubility than the corresponding enantiomeric forms and he reported in his thesis that under ‘normal circumstances’ the racemates of compounds have lower solubilities. He observed that the crystals of the [dinitrobis(ethylenediamine)cobalt]Cl compound have a striated surface appearance showing a ‘twinning striation’ (see Fig. 9, bottom (left)), which he interpreted in terms of the crystals being racemic by ‘intergrowth of d- and l-crystals’. On this basis he devised seeding experiments taking a(n) (over)saturated solution of rac-[dinitrobis(ethylenediamine)cobalt]Cl and seeded this with [Δ-(d-) or Λ-(l-)dinitrobis(ethylenediamine)cobalt]Cl crystals, which he denoted as a spontaneous resolution experiments. In these cases of experiments Richard Hessen did not get purely enantiomeric crystals, but strong enrichment of one enantiomeric form, the form of the seeding crystal. The crystals showed the feature of full transparency and were nicely shaped plates (Fig. 18).
ConclusionsThis study on the chirality of crystals of samples from the Alfred Werner collection of original samples of the University of Zurich revealed that the [dinitrobis(ethylenediamine)cobalt]X complexes (X=Cl, Br) undergo spontaneous resolution upon crystallization. Emphasis of our investigation was placed on the chiral [Λ- and Δ-dinitrobis(ethylenediamine)cobalt]Cl derivatives, of which 14 original crystals were studied by single crystal X-ray diffraction. The [dinitrobis(ethylenediamine)cobalt]Cl complex crystallizes from racemic solutions in the space group P 21, mainly as synthetical twins, but to a small extent also as pure enantiomorphs. The twinning effect of these crystals was already recognized and correctly described by PhD student Richard Hessen of the Alfred Werner group (PhD thesis 1914). In the period of time from 1912 to 1914 Richard Hessen eventually resolved the [Δ- and Λ-dinitrobis(ethylenediamine)cobalt]Cl complex by conglomerate salt resolution. Based on the availability of the pure [Δ- or Λ-dinitrobis(ethylenediamine)cobalt]Cl complexes he also carried out seeding experiments, which proved that the [Δ- and Λ-dinitrobis(ethylenediamine)cobalt]Cl complexes can be resolved to a great extent by spontaneous chiral resolution. Enantiomerically enriched twinned crystals were obtained, which are difficult to be recognized as enantiomorphs by visual inspection. In the period of time from 1900 to 1904, PhD students of the Alfred Werner group (Adolf Grün, Edith Humphrey, Ernst Zinggeler, Heinrich Schwarz, Paul Larisch) had also dealt with the [dinitrobis(ethylenediamine)cobalt]Cl complex. Adolf Grün and Edith Humphrey had prepared unintentionally enantiomerically enriched complexes and rarely also enantiomorphic crystals. From a modern times point of view this procedure seemed quite difficult for the [dinitrobis(ethylenediamine)cobalt]Cl complex, since recognition of chiral crystals by visual inspection is indeed difficult. But they did not apply manual crystal picking for chiral resolution according to Louis Pasteur. Alfred Werner and his group had thus missed the opportunity to become the ‘Louis Pasteurs’ of coordination compounds. Heinrich Schwarz and Paul Larisch used the S-(D-,d-)camphorsulfonate anion to separate the cis- and trans-isomers of the [dinitrobis(ethylenediamine)cobalt] complexes with the ‘side effect’ that the [dinitrobis(ethylenediamine)cobalt]Cl got chirally resolved. Nevertheless, spontaneous resolution of the [Δ- and Λ-dinitrobis(ethylenediamine)cobalt]Cl complex is principally experimentally accomplishable. In addition, making this early chiral resolution story even more incredible, we found that Heinrich Schwarz and Paul Larisch had applied in these early days of coordination chemistry the S-(D-,d-)camphorsulfonate anion to achieve merely the separation of the cis- and trans-isomers of the [dinitrobis(ethylenediamine)cobalt] complexes. They did not approach the potentially possible chiral resolution of the [cis-dinitrobis(ethylenediamine)cobalt]+ cation. But based on their synthetic procedure they did indeed accomplish chiral resolution of the cis-isomer and prepared eventually a series of the chiral [cis-,Λ-dinitrobis(ethylenediamine)cobalt]X salts; however all this was in an unintentional manner.
Conflict of interestThe authors declare no conflict of interest.
Deposition of X-ray dataCrystallographic data for the seventeen structures were deposited with the Cambridge Crystallographic Data Centre as supplementary publication nos. CCDC 1412902-1412918. Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK, (fax: +44-(0)1223-336033 or e-mail: deposit@ccdc.cam.ac.Uk).
The authors are indebted to Professor emerit. Clive Küenzle, former Vice-Rector of the University of Zurich, stating permission from the Head Office of the University of Zurich to take samples from the Alfred Werner collection. In addition, the authors are very grateful to Dr. Helmut Schmalle, former X-ray crystallographer at the Department of Chemistry, University of Zurich, for very helpful discussions on synthetical twinning of enantiomorphs.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.
























![Box number 21 of the Alfred Werner collection of the University of Zurich containing 237 vials with the cis-[dinitrobis(ethylenediamine)cobalt]+ cation as their main feature in common. Box number 21 of the Alfred Werner collection of the University of Zurich containing 237 vials with the cis-[dinitrobis(ethylenediamine)cobalt]+ cation as their main feature in common.](https://static.elsevier.es/multimedia/0187893X/0000002600000004/v1_201509250118/S0187893X15000397/v1_201509250118/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Head pages of the most important dissertations of the Alfred Werner group dealing with the [cis-dinitrobis(ethylenediamine)cobalt]X (X=Cl, Br) salts: from left to right and from top to bottom the dissertations of Adolf Grün, Edith Humphrey, Ernst Zinggeler, Heinrich Schwarz, Paul Larisch and Richard Hessen. Head pages of the most important dissertations of the Alfred Werner group dealing with the [cis-dinitrobis(ethylenediamine)cobalt]X (X=Cl, Br) salts: from left to right and from top to bottom the dissertations of Adolf Grün, Edith Humphrey, Ernst Zinggeler, Heinrich Schwarz, Paul Larisch and Richard Hessen.](https://static.elsevier.es/multimedia/0187893X/0000002600000004/v1_201509250118/S0187893X15000397/v1_201509250118/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Isomers of the [dinitrobis(ethylenediamine)cobalt]+ cation. Isomers of the [dinitrobis(ethylenediamine)cobalt]+ cation.](https://static.elsevier.es/multimedia/0187893X/0000002600000004/v1_201509250118/S0187893X15000397/v1_201509250118/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)


![Photograph of the original vials of Edith Humphrey containing the cis-dinitrobis(ethylenediamine)cobalt]2SO4·H2O and the cis-dinitrobis(ethylenediamine)cobalt]2[PtCl4]·H2O from the Alfred Werner collection of original samples at the University of Zurich. Photograph of the original vials of Edith Humphrey containing the cis-dinitrobis(ethylenediamine)cobalt]2SO4·H2O and the cis-dinitrobis(ethylenediamine)cobalt]2[PtCl4]·H2O from the Alfred Werner collection of original samples at the University of Zurich.](https://static.elsevier.es/multimedia/0187893X/0000002600000004/v1_201509250118/S0187893X15000397/v1_201509250118/en/main.assets/thumbnail/gr7.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Models of the molecular structures of [cis-dinitrobis(ethylenediamine)cobalt]2SO4·H2O (left) and of [cis-dinitrobis(ethylenediamine)cobalt]2[PtCl4]·H2O (right) as determined by X-ray diffraction studies from crystals prepared by Edith Humphrey stored in the Alfred Werner collection of the University of Zurich. The full data sets of the X-ray diffraction studies are deposited at the Cambridge Crystallographic Data Centre. Models of the molecular structures of [cis-dinitrobis(ethylenediamine)cobalt]2SO4·H2O (left) and of [cis-dinitrobis(ethylenediamine)cobalt]2[PtCl4]·H2O (right) as determined by X-ray diffraction studies from crystals prepared by Edith Humphrey stored in the Alfred Werner collection of the University of Zurich. The full data sets of the X-ray diffraction studies are deposited at the Cambridge Crystallographic Data Centre.](https://static.elsevier.es/multimedia/0187893X/0000002600000004/v1_201509250118/S0187893X15000397/v1_201509250118/en/main.assets/thumbnail/gr8.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Top: The vial of Adolf Grün containing the [cis-dinitrobis(ethylenediamine)cobalt]Cl complex. Bottom left: The crystals of the vial of Adolf Grün. The crystals (see boxes) show a remarkable striation of the larger crystals indicating a layered growth process by twinning. Each striation stripe is supposed to correspond to a twinning layer. Bottom right: Edith Humphrey Top: The vial of Adolf Grün containing the [cis-dinitrobis(ethylenediamine)cobalt]Cl complex. Bottom left: The crystals of the vial of Adolf Grün. The crystals (see boxes) show a remarkable striation of the larger crystals indicating a layered growth process by twinning. Each striation stripe is supposed to correspond to a twinning layer. Bottom right: Edith Humphrey](https://static.elsevier.es/multimedia/0187893X/0000002600000004/v1_201509250118/S0187893X15000397/v1_201509250118/en/main.assets/thumbnail/gr9.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Vial and crystals of [cis-dinitrobis(ethylenediamine)cobalt]Cl of PhD student Heinrich Schwarz taken from the Alfred Werner collection of the University of Zurich. The inserted picture shows a crystal found in the vial of Heinrich Schwarz supposed to be enantiomorphic. Vial and crystals of [cis-dinitrobis(ethylenediamine)cobalt]Cl of PhD student Heinrich Schwarz taken from the Alfred Werner collection of the University of Zurich. The inserted picture shows a crystal found in the vial of Heinrich Schwarz supposed to be enantiomorphic.](https://static.elsevier.es/multimedia/0187893X/0000002600000004/v1_201509250118/S0187893X15000397/v1_201509250118/en/main.assets/thumbnail/gr10.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Solubilities of the conglomerates of the cis- and trans-isomers of [dinitrobis(ethylenediamine)cobalt][camphorsulfonate] salts at 16.5°C in water taken from the PhD thesis of Paul Larisch. The enantiomeric cis-isomer is expected to possess the Λ-configuration when the S-camphorsulfonate anion is applied. Solubilities of the conglomerates of the cis- and trans-isomers of [dinitrobis(ethylenediamine)cobalt][camphorsulfonate] salts at 16.5°C in water taken from the PhD thesis of Paul Larisch. The enantiomeric cis-isomer is expected to possess the Λ-configuration when the S-camphorsulfonate anion is applied.](https://static.elsevier.es/multimedia/0187893X/0000002600000004/v1_201509250118/S0187893X15000397/v1_201509250118/en/main.assets/thumbnail/gr11.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Solubilities of the cis- and trans- isomers of [Co(NH3)4(NO2)2]X (X=Cl, SO4) and [dinitrobis(ethylenediamine)cobalt]X salts (X=NO3, I) complexes at different temperatures in water taken from the PhD thesis of Paul Larisch, p. 61. Solubilities of the cis- and trans- isomers of [Co(NH3)4(NO2)2]X (X=Cl, SO4) and [dinitrobis(ethylenediamine)cobalt]X salts (X=NO3, I) complexes at different temperatures in water taken from the PhD thesis of Paul Larisch, p. 61.](https://static.elsevier.es/multimedia/0187893X/0000002600000004/v1_201509250118/S0187893X15000397/v1_201509250118/en/main.assets/thumbnail/gr12.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Original vials [Λ-,cis-dinitrobis(ethylenediamine)cobalt][S-camphorsulfonate] salts of the Alfred Werner collection at the University of Zurich prepared by the PhD students Ernst Zinggeler (left vial) and Paul Larisch (right vial) in the group of Alfred Werner (PhD theses of 1902 and 1904, respectively). Original vials [Λ-,cis-dinitrobis(ethylenediamine)cobalt][S-camphorsulfonate] salts of the Alfred Werner collection at the University of Zurich prepared by the PhD students Ernst Zinggeler (left vial) and Paul Larisch (right vial) in the group of Alfred Werner (PhD theses of 1902 and 1904, respectively).](https://static.elsevier.es/multimedia/0187893X/0000002600000004/v1_201509250118/S0187893X15000397/v1_201509250118/en/main.assets/thumbnail/gr13.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Structural model of [Λ-,cis-dinitrobis(ethylenediamine)cobalt][S-camphorsulfonate] obtained by a X-ray diffraction study, which demonstrates the Λ-,cis-configuration of the [dinitrobis(ethylenediamine)cobalt]+ cation and the S-configuration of the camphorsulfonate anion prepared by Paul Larisch in the group of Alfred Werner. The full data set of the X-ray diffraction study was deposited at the Cambridge Crystallographic Data Centre. Structural model of [Λ-,cis-dinitrobis(ethylenediamine)cobalt][S-camphorsulfonate] obtained by a X-ray diffraction study, which demonstrates the Λ-,cis-configuration of the [dinitrobis(ethylenediamine)cobalt]+ cation and the S-configuration of the camphorsulfonate anion prepared by Paul Larisch in the group of Alfred Werner. The full data set of the X-ray diffraction study was deposited at the Cambridge Crystallographic Data Centre.](https://static.elsevier.es/multimedia/0187893X/0000002600000004/v1_201509250118/S0187893X15000397/v1_201509250118/en/main.assets/thumbnail/gr14.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Vial and crystals of [trans-dinitrobis(ethylenediamine)cobalt][S-camphorsulfonate] of Paul Larisch from the Alfred Werner collection of original samples at the University of Zurich. Vial and crystals of [trans-dinitrobis(ethylenediamine)cobalt][S-camphorsulfonate] of Paul Larisch from the Alfred Werner collection of original samples at the University of Zurich.](https://static.elsevier.es/multimedia/0187893X/0000002600000004/v1_201509250118/S0187893X15000397/v1_201509250118/en/main.assets/thumbnail/gr15.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Two vials of PhD student Richard Hessen containing the [Δ-(d-) and Λ-(l-)dinitrobis(ethylenediamine)cobalt]Cl compounds obtained by conglomerate chiral resolution with S- and R-camphorsulfonate salts. Vials from the Alfred Werner collection of the University of Zurich. Two vials of PhD student Richard Hessen containing the [Δ-(d-) and Λ-(l-)dinitrobis(ethylenediamine)cobalt]Cl compounds obtained by conglomerate chiral resolution with S- and R-camphorsulfonate salts. Vials from the Alfred Werner collection of the University of Zurich.](https://static.elsevier.es/multimedia/0187893X/0000002600000004/v1_201509250118/S0187893X15000397/v1_201509250118/en/main.assets/thumbnail/gr16.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

![Crystals of [Λ-dinitrobis(ethylenediamine)cobalt]Cl obtained by Richard Hessen via seeding of a saturated solution of the racemic [dinitrobis(ethylenediamine)cobalt]Cl complex with crystals of the [Λ-dinitrobis(ethylenediamine)cobalt] chloride. Crystals of [Λ-dinitrobis(ethylenediamine)cobalt]Cl obtained by Richard Hessen via seeding of a saturated solution of the racemic [dinitrobis(ethylenediamine)cobalt]Cl complex with crystals of the [Λ-dinitrobis(ethylenediamine)cobalt] chloride.](https://static.elsevier.es/multimedia/0187893X/0000002600000004/v1_201509250118/S0187893X15000397/v1_201509250118/en/main.assets/thumbnail/gr18.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)