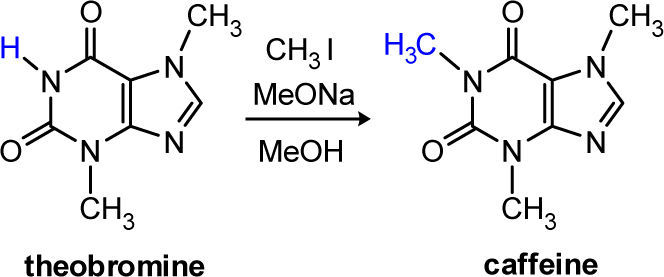
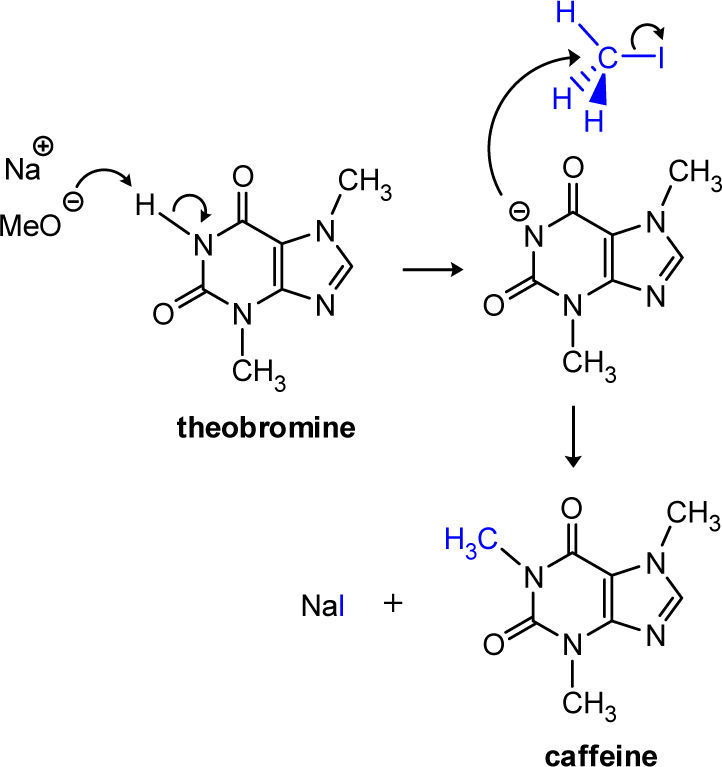
Caffeine is one of the most widely consumed, naturally occurring, mild, and central nervous system stimulants. Its synthesis, by the N-methylation (SN2 substitution) of theobromine is described as an experiment for the undergraduate organic chemistry laboratory. The N-methylation of theobromine is rapid and efficient and its progress is monitored by TLC using authentic standards. The undergraduate student also is able to observe the effect of temperature on the rate of the N-methylation reaction.
La cafeína es uno de los productos más consumido, concurrente en la naturaleza, afable y estimulante del sistema nervioso central. Su síntesis, mediante la N-metilación (sustitución SN2) de la teobromina se describe como un experimento de laboratorio de química orgánica para nivel licenciatura. La N-metilación de la teobromina es rápida y eficiente, y su progreso es monitoreado por Cromatografía en Capa Fina (TLC, por sus siglas en inglés) comparándose con estándares comerciales. El estudiante de licenciatura también será capaz de observar el efecto de la temperatura sobre la velocidad de la reacción de la N-metilación.
Caffeine (a methylxanthine) is a well-known compound that occurs in coffee, tea, kola nuts, mate leaves, guarana paste, cocoa beans, and other related natural products. Caffeine and theobromine (from Theobroma cacoa; theo = god, and broma = food; thus, food of the gods) are the two most abundant methylxanthines in chocolate, both of which have received considerable attention in the food and nutrition fields, in part because of the physiological effects which they elicit (Brown, 2004). Surprisingly, given the widespread use and occurrence of caffeine, there are relatively few reported syntheses thereof described in the literature, and a significant number of these methods date back to the late 19th and the beginning of the 20th century. In 1895 Emil Fisher (Nobel Prize in Chemistry, 1902) described the first synthesis of caffeine from uric acid (Kunz, 2002, and citations therein). Subsequently, Fisher reported the synthesis of caffeine by N-methylation of theobromine with CH3I in the presence of NaOH as the base (Fischer, 1898). Later, Biltz and Damm described the N-methylation of theobromine using dimethyl sulphate (Blitz, 1917). More recent syntheses of caffeine from theobromine describe the use of dimethyl sulfate in the presence of alumina impregnated with KF in acetonitrile (Yamawaki, 1981).
Caffeine has been an important focus in many undergraduate experimental laboratory protocols, e.g., in analytical chemistry experiments (Singh, 1998; Pawliszyn, 1997; Hill, 1988; Van Atta, 1979; Ferguson, 1998; DiNunzio, 1985; Delaney, 1985; Beckers, 2004; Leacock, 2011;Bergen, 2000; Fenk, 2010; Menguy, 2009; Harmon, 2012; Pavlik, 1973) and isolation from natural (Onami, 1996; Murray, 1995; Hampp, 1996; Mitchell, 1974; Schaber, 2012; Adam, 1996) or commercial products (Neuzil, 1990; Moye, 1972; Hall-Laswick, 1972; Williams, 1992). Surprisingly, however, laboratory experimental procedures for the synthesis of caffeine are quite limited. In 1973, Pavia reported the synthesis of caffeine by methylation of theobromine with dimethyl sulphate (Pavia, 1973), a procedure not suitable for the undergraduate laboratory given the toxicity (carcinogenicity and mutagenicity), volatility (inhalation hazard), and environmentally hazardous nature [It has even been considered as a potential chemical weapon of war! (Rippey, 2005)] of this substance. Since then, no other undergraduate laboratory experiment about synthesis of caffeine has been reported.
Herein we report a modification of the Pavia’s synthesis procedure. The N-alkylation of theobromine using methyl iodide in methanolic sodium methoxide solution allows an efficient and rapid synthesis of caffeine (Scheme 1 and Scheme 2). The reaction goes to completion at room temperature in 90 min, or in 40 min at 60 °C, and the product is free of contaminants giving 90% of yield (similar to the Fischer’s procedure). This experiment is multifaceted and can be used to instruct the students in the bimolecular nucleophilic substitution reactions (microscale organic techniques), the effect of temperature on reaction rates, evaluation of the progress of a reaction by thin layer chromatography, and compound identification by melting points and mixed melting points with authentic materials.
Instructor’s preparation guideThe main focus of this paper is the synthesis of caffeine from theobromine, but this may be complemented by extraction of the required theobromine from cocoa powder using previously published procedures (Pavia, 1973). TLC plates with a fluorescent indicator are necessary. Dichloromethane was used as the preferred solvent (chloroform gave the same result, but is less desirable because of its greater toxicity). It is recommended that the teacher handles methyl iodide, this step has to be done under the fume hood. Gloves, goggles and a face mask are mandatory. The standard solutions of theobromine and caffeine will be prepared by the teacher. Since each group will use a very small amount of each of these solutions, a few millilitres can accommodate many students. To prepare stock solutions of theobromine and caffeine theobromine (Sigma-Aldrich), add 0.2 g of the standard to approximately 50 mL of methylene chloride.
Objectives- a)
To synthetize caffeine from the theobromine by SN2 (microscale technique).
- b)
To monitor a chemical reaction by TLC.
- c)
To identify the effect of temperature on the reaction rate.
- d)
To identify the product by determining the melting point and comparing this value with the authentic standard.
Theobromine (Sigma-Aldrich), caffeine (Sigma-Aldrich), methanol, sodium methoxide, dichloromethane, anhydrous sodium sulphate, and methyl iodide.
EquipmentRotavapor, short wave (254 nm) UV lamp, microscale equipment, lab coat, latex gloves, goggles, hot plate-magnetic stirrer, bucket with reflux pumps, small magnetic bar, tweezers or forceps, capillary tubes, TLC plates, TLC developing chamber, 50 mL beaker, büchner funnel and büchner flask, test tube, glass rod, 1 mL pipette, rubber pipette filler, pasteur pipette and bulb, glass funnel, cotton, filter paper, scissors, spatula, scoopula, rubber tubing (~80 cm long), ring stand, thermometer, sand container (metal or glass), clamp, and a pencil.
Student experimental protocolSynthesis of caffeine was carried out in a microscale apparatus. Students were divided into two groups. Group 1 performed the synthesis at room temperature (Method 1) while group 2 effected the reaction at 50-60°C (Method 2). Reaction was followed by TLC and compared with standards of caffeine and theobromine. Group 1 followed the course of the reaction every 30 minutes while group 2 monitored the progress of the reaction at 40 min (total heating time).
a)Method 1. Reaction at room temperatureIn a 5 mL round-bottom flask (equipped with a magnetic bar), suspend 0.05 g of theobromine in 1.5 mL of MeOH. Note that theobromine is not dissolved in methanol. Add 0.03 g of NaOMe and shake gently for one minute by using a plate magnetic stirrer. Assure that theobromine has dissolved completely. Note that the solution turns yellow. Add 0.6 mL of CH3I with a 1 mL syringe. This step has to be done under the fume hood. Gloves, goggles and a face mask are mandatory. Stopper the flask. Start the reaction and use magnetic stirring. Monitor the reaction by TLC at 30, 60 and 90 minutes comparing with authentic standards. Use an eluent system dichloromethane/methanol 95:5. To visualize the spots on your TLC plate, place it underneath a downward facing shortwave UV lamp. Mark all spots that you see, tracing their outlines with a pencil and calculate the retention factor (RF). After completion of the reaction, evaporate the solvent of the round-bottom flask in a rotavapor. Then add 1 mL of CH2Cl2 and stir manually until the product dissolves. Remove the solution with a Pasteur pipette and pour it into a test tube. Add 3 mL of water and shake vigorously. Using a Pasteur pipette, carefully extract the organic phase (bottom) and filter through anhydrous sodium sulphate/cotton, recovering the filtrate in a 10 mL round-bottom flask. Add 4 mL of CH2Cl2 over sodium sulphate for washing, always recovering the liquid in the round-bottom flask. Evaporate the solvent in a rotavapor and observe the formation of a silky white solid (0.048 g, 90%). By determining the melting point and comparing it with the authentic standard (m.p. 234–236°C), identify the product as caffeine.
b)Method 2. Reaction by heatingPrepare a reflux set-up and pre-heat a sand bath. Before starting, assure that the sand bath is ready (at a constant temperature of 50–60°C). In a 5 mL round-bottom flask (equipped with a magnetic bar), suspend 0.05 g of theobromine in 1.5 mL of MeOH (note that theobromine is not dissolved in methanol). Then add 0.03 g of NaOMe and shake gently for a minute by using a plate magnetic stirrer. Assure that theobromine has dissolved completely. Note that the solution turns yellow. Add 0.6 mL of CH3I with a 1 mL syringe. This step has to be done under the fume hood. Gloves, goggles and a face mask are mandatory. Stopper the flask. Start the reaction, allowing it to proceed under continuous magnetic stirring at a constant temperature of 50–60°C for 40 min (reflux set-up). After this time the reaction will be monitored by TLC comparing with authentic standards. Use an eluent system dichloromethane/methanol 95:5. To visualize the spots on your TLC plate, place it underneath a downward facing shortwave UV lamp. Mark all spots that you see, tracing their outlines with a pencil and calculate the retention factor (RF). After completion of the reaction, evaporate the solvent of the round-bottom flask in a rotavapor. Then add 1 mL of CH2Cl2 and stir manually until the product dissolves. Remove the solution with a Pasteur pipette and pour it into a test tube. Add 3 mL of water and shake vigorously. Using a Pasteur pipette, carefully extract the organic phase (bottom) and filter through anhydrous sodium sulphate/cotton, recovering the filtrate in a 10 mL round-bottom flask. Add 4 mL of CH2Cl2 over sodium sulphate for washing, always recovering the liquid in the round-bottom flask. Evaporate the solvent in a rotavapor and observe the formation of a silky white solid (0.048g, 90%). By determining the melting point and comparing it with the authentic standard (m.p. 234–236°C), identify the product as caffeine.
HazardsUltraviolet light is damaging to the skin and eyes. The UV lamps used for visualization of the analytes on fluorescent TLC plates should always face downward, and students should take care not to look directly into them. Methyl iodide is a volatile liquid and exhibits moderate to high acute toxicity by inhalation, and skin contact may cause irritation and dermatitis. Both methanol and sodium methoxide are toxic. Methanol is toxic both by ingestion and inhalation, and therefore reactions with this solvent should be carried out in a fume hood. Sodium methoxide causes topical burns because it is hydrolyzed on the skin to sodium hydroxide. Dichloromethane is least toxic that chloroform (carcinogenic to humans), but it is not without health risks, as its high volatility makes it an acute inhalation hazard. Acute exposure by inhalation has resulted in optic neuropathy and hepatitis. Thus, the use of rubber gloves, face masks, goggles and a well-ventilated fume hood is mandatory.
ConclusionsWe have brought back an old reaction and an extraction process in a new setting. This paper describes a facile, efficient, clean and rapid reaction for the synthesis caffeine, one of the most common naturally occurring compounds in widespread human use. We have shown that the NaOMe can be used as a base and MeOH as a solvent for the synthesis, with the major advantage of: an efficient and rapid synthesis using not toxicity (carcinogenicity and mutagenicity) compounds. This experiment is multifaceted and can be used to instruct students in the bimolecular nucleophilic substitution reactions (microscale organic techniques), the effect of temperature on SN2 reaction rates, evaluation of the progress of a reaction by thin layer chromatography, and compound identification by melting points and mixed melting points with authentic materials.
Financial support from CONACyT is gratefully acknowledged. The authors would like to thank the referee and Joseph M. Muchowski (visiting professor, UNAM) for his valuable comments and suggestions.