Objectives of this study are: a) Develop a theoretical framework based on history and philosophy of chemistry (HPC); b) Facilitate high school (grade 10) students’ understanding of stoichiometry. Study is based on two intact sections of high school students (10 grade, 15-16 year old). Control group instructor used the traditional strategy in which laws of definite and multiple proportions are defined as definitive, irrefutable and applied in the classroom as algorithms. Experimental group instructor used a dialectic constructivist strategy based on the presentation of hypothetical experimental data leading to cognitive conflicts and a critical confrontation of different propositions. Both groups of students were tested on a 6 item test of stoichiometry (1–2 algorithmic and 3–6 conceptual items), after the topic had been taught. Experimental group performed better than the control group, not only on algorithmic items but also items requiring conceptual understanding. Differences in student performance on Items 1, 3, 4 and 5 were statistically significant. HPC perspective developed in this study leads to a critical evaluation of the laws of definite and multiple proportions and their role in chemistry education. Emphasizing these laws as irrefutable, inevitably leads to the memorization of algorithms and formulae in learning stoichiometry.
Los objetivos de este estudio son: a) Desarrollar un marco teórico basado en la historia y la filosofía de la química (HFQ); b) Facilitar a los estudiantes que inician el bachillerato el entendimiento de la estequiometría. El estudio se basa en dos grupos de estudiantes del 10° grado (15 a 16 años de edad). El profesor del grupo de control empleó una estrategia tradicional en la que las leyes de las proporciones definidas y múltiples se definen como definitivas, irrefutables y aplicadas en la clase como algoritmos. El profesor del grupo experimental empleó una estrategia dialéctica constructivista, basada en la presentación de datos experimentales hipotéticos que conducen al conflicto cognitivo y a una confrontación crítica de diferentes proposiciones. Un examen de seis preguntas sobre estequiometría (las 1 y 2 algorítmicas y las 3 a 6 de carácter conceptual) fue aplicado a ambos grupos de alumnos después de haber sido enseñados. El grupo experimental se desempeñó mejor que el grupo de control. La diferencia en los resultados estudiantiles en los Items 1, 3, 4 y 5 fueron diferentes estadísticamente. La perspectiva HFQ desarrollada en este estudio condujo a una evaluación crítica de las leyes de las proporciones definidas y múltiples, así como su papel en la educación química. Al hacer énfasis en estas leyes como irrefutables, nos conduce inevitablemente a la memorización de algoritmos y fórmulas en el aprendizaje de la estequiometría.
Research in science education shows that both high school and freshman students have difficulties in understanding stoichiometry (Agung & Schwartz, 2007; BouJaoude & Barakat, 2003; Dahsah & Coll, 2007; Gabel & Bunce, 1994; Niaz, 1995a; Schmidt, 1997). Besides other factors, stoichiometry is a difficult topic as it also requires a conceptual understanding of various other concepts, such as particulate nature of matter, mole, Avogadro's number, conservation of matter, balancing chemical equations, laws of definite and multiple proportions. Furthermore, most chemistry teachers and textbooks emphasize problem solving techniques based on algorithms that require ‘plug-and-chug’ strategies and little conceptual understanding (Niaz, 1995b; Niaz & Robinson, 1993; Nurrenbern & Pickering, 1987). Based on these considerations, the objectives of this study are to: a) Develop a theoretical framework based on history and philosophy of chemistry; b) Facilitate high school (grade 10) students’ understanding of stoichiometry.
Theoretical framework of the studyDialectical constructivismThe dialectic approach to constructivism is an attempt to understand reality within the context of complex interrelationships. According to Bidell (1988), “Rather than backgrounding conflict, the dialectic approach seeks to foreground it as the most salient feature of processes grasped in their complexity” (p. 332). The dialectic perspective emphasizes the understanding of psychological phenomena in their interrelationship to one another, rather than as isolated and separate processes as characterized by a Cartesian reductionist approach to science (Pascual-Leone, 1976; Piaget & Garcia, 1989). Pascual-Leone (1976) goes beyond Piaget by postulating dialectical constructivism in which constructive theory attempts to “model or reflect the subject's internal functional organization in order to rationally reconstruct the genesis of the subject's performance” (pp. 90–91), similar to what Lakatos (1971) referred to as the rational reconstruction of scientific theories and models in the history of science. Niaz (2008) has presented a framework that facilitates an understanding of constructivism within a philosophy of chemistry perspective.
Scientific laws as idealizationsIn order to understand the nature of scientific laws let us consider Newton's law of gravitation. According to Lakatos (1970), it is one of the, “… best-corroborated scientific theory of all times … ” (p. 92). Feynman (1967) endorses the view that it is, “… the greatest generalization achieved by the human mind” (p. 14). In spite of such impressive credentials, Cartwright (1983) asks: “Does this law (gravitation) truly describe how bodies behave? ” (p.57) and responds laconically: “Assuredly not” (p. 57). She explains further: “For bodies which are both massive and charged, the law of universal gravitation and Coulomb's law (the law that gives the force between two charges) interact to determine the final force. But neither law by itself truly describes how the bodies behave. No charged objects will behave just as the law of universal gravitation says; and any massive objects will constitute a counterexample to Coulomb's law. These two laws are not true: worse they are not even approximately true” (p. 57). The crux of the issue is that following Galileo's method of idealization, scientific laws, being epistemological constructions, do not describe the behavior of actual bodies. Newton's laws, gas laws, Piaget's epistemic subject — they all describe the behavior of ideal bodies that are abstractions from the evidence of experience and the laws are true only when a considerable number of disturbing factors, itemized in the ceteris paribus clauses, are eliminated (Cartwright, 1999; Matthews, 1994). Chemistry students and teachers generally tend to understand the difference between scientific theories and laws as: a simplistic hierarchical relationship in which hypotheses become theories and theories become laws, depending on the amount of ‘proof behind the idea’ (Lombardi & Labarca, 2007). This suggests that progress in science need not be characterized as a dichotomy between theories and laws, but rather as a “progressive problemshift” (Lakatos, 1970), from one tentative theory to another.
Laws of definite and multiple proportionsChristie (1994) has traced the historical origin of the laws of definite and multiple proportions and presented an interpretation based on a philosophy of chemistry perspective. Various developments in chemistry have presented considerable problems for the law of definite proportions. In the case of the non-stoichiometric compounds (e.g., aluminum oxide), known as the “network solids”, atoms are not bonded in discrete clusters as molecules, but each to several neighbors in the form of a network. Given these difficulties the law of definite proportions, although is still mentioned in the textbooks, it is not used in an explicit sense in modern chemistry (p. 616). In the case of the law of multiple proportions, the problem lies with the word “simple” or “small”, which appears in its statement (Christie, 1994, p. 619). In this context it is important to note that Giere (1999) has presented an alternative account which provides a way of understanding the practice of science without the laws of nature. There are many common elements in the treatments of Cartwright (1983, 1989), Christie (1994), Giere (1999) and Lakatos (1970) with respect to their understanding of laws and theories. All of them would subscribe to the thesis that as scientific knowledge is tentative (Niaz & Maza, 2011), it is advisable not to establish a dichotomous/hierarchical relationship between laws and theories. Niaz (2001) has presented a framework that facilitates an understanding of the laws of definite and multiple proportions within a philosophy of chemistry perspective.
MethodThis study is based on two intact sections of high school students (10 grade, 15-16 year old) in Venezuela. One of the sections was designated as the control group (n = 32) and the other as the experimental group (n = 31). Traditional teaching strategy: Control group instructor used the traditional strategy in which laws of definite and multiple proportions are defined as definitive, irrefutable and applied in the classroom as algorithms. This strategy can be summarized through the following sequence of steps: a) Definition of the two laws; b) Resolution of problems to illustrate the validity of the laws; c) Resolution of additional problems based on algorithms. Constructivist teaching strategy: Experimental group instructor used a constructivist teaching strategy based primarily on the theoretical framework presented above. The basic idea behind this strategy was the presentation of hypothetical experimental data leading to cognitive conflicts and a critical confrontation of different propositions, quite similar to what scientists do in order to achieve consensus. In order to facilitate understanding the instructor used a modified form of the learning cycle (Lawson et al., 1989). The learning cycle facilitates disequilibrium, argumentation and finally facilitates reasoning and is generally based on the following phases: a) Exploration; b) Term introduction; c) Concept application. In this study, the instructor found the exploration and the concept application phases to be more useful. Based on the history and philosophy of science framework (Cartwright, Giere, Lakatos), the instructor avoided defining the laws of definite and multiple proportions, unless the students themselves used these terms. In each of the problems, an attempt was made to achieve a consensus view with respect to the arguments that explained the problem situation well. For most students this was a novel study in which they were not asked to solve algorithmic problems in order to have a right or wrong answer, but rather the instructor evaluated the strength and consistency of the arguments.
Classification and validation of students’ responsesExperimental group students participated in the constructivist teaching strategy for three weeks. Control group students also participated in the traditional strategy for the same period of time. After two weeks both groups of students were evaluated on a semester exam which consisted of six items. Items 1 and 2 were primarily algorithmic problems, requiring application of memorized formulae. Items 3-6 were conceptual problems requiring argumentation, restructuring and reasoning based on alternative interpretations. For algorithmic problems (Items 1 and 2) students responses were classified as: a) Correct, if the student shows all the steps necessary to solve the problem based on the data; b) Incorrect, if the student fails to follow the different steps coherently and does not perform the necessary calculations. For conceptual problems (Items 3-6), students responses were classified as: a) Conceptual (C), if the student explicitly elaborates arguments in a coherent and logical manner from the data given in the problem; b) Partially Conceptual (PC), if the student attempts to present some arguments without a logical and coherent scheme; c) Rhetorical (R), if the students reproduces some elements of the problem situation with no attempt to present arguments in a consistent fashion. In order to validate the classification of students’ responses both authors classified all responses of the experimental group, to Items 3-6 separately, according to the criteria presented above. Following results were obtained: Item 3: 88% agreement, Item 4: 80% agreement, Item 5: 64% agreement, Item 6: 84% agreement. All disagreements were resolved by various rounds of discussions. Based on this experience, the second author classified the responses of the control group students.
Results and discussionItem 1Calcium (Ca) and Oxygen (O2) combine in the ratio of 5:2. If a sample of calcium oxide (CaO) has a mass of 2.050 grams, how many grams of Ca and O2 were necessary to form the compound? [2 Ca (s) + O2 (g) → 2 CaO (s)]
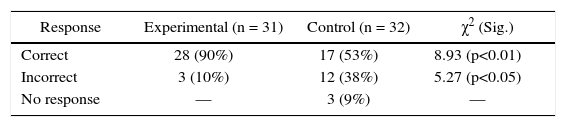
This is a fairly typical algorithmic problem and 90% of the experimental group students responded correctly, in comparison to 53% of control group (Table 1). The difference in the performance of the two groups is statistically significant (p<0.01). Despite control group students’ considerable experience in solving similar problems, experimental treatment facilitated greater understanding even on an algorithmic problem.
Item 297.22 grams of Magnesium (Mg) are made to react with 63.96 grams of Oxygen (O2). At completion of the reaction both reactants are consumed. a) How many grams of O2 combine with with 1.00 gram of Mg? b) What is the percentage of Mg and O2 in MgO? c) In what proportion do Mg and O2 combine? [2 Mg (s)+ O2 (g) → 2 MgO(s)]
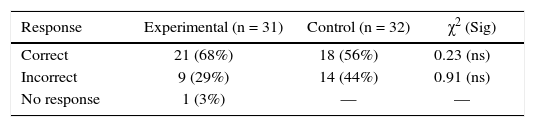
Although Item 2 is quite similar to Item 1, still performance of experimental group (68%) decreased considerably, whereas that of the control group (56%) remained about the same (Table 2). Once again experimental group performed better than control group, the difference however is not statistically significant. Comparing Items 1 and 2, it can be observed that in Item 1 students are provided the proportion in which two elements (Ca and O2) react and asked to calculate the amount of each to prepare a certain amount of the compound (CaO). On the contrary, in Item 2 students are given the amounts of two elements (Mg and O2) and asked to calculate the proportion in which the two would react. Apparently, this difference made Item 2 more difficult.
Item 3Iron (Fe) and Oxygen (O2) form two oxides. One of the oxides having Fe (II) has the formula FeO. This shows that one atom of Fe will combine with one atom of oxygen. In other words, if 100 grams of iron combine with 28.65 grams of oxygen, we would obtain 128.65 grams of FeO. However, it has been found that only 95.00 grams of Fe combine with 28.65 grams of oxygen. Frequently, advances in scientific knowledge are based on such contradictions. How can you explain this contradiction?
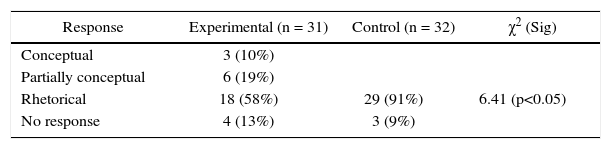
This is a conceptual problem as the response does not depend on memorization of formulae or algorithmic procedures, but rather on argumentation, restructuring and the capacity to develop alternative models/hypotheses, in the face of conflicting information. The problem presents to the students a situation in which the same compound, iron oxide (Fe2+) has a different composition and they are asked to explain this contradiction. Most students found this problem to be difficult, as the problem requires them to think, go beyond pre elaborated responses, resolve a conflict and in a sense overcome the myth of the correct response. Only 3 (10%) students from the experimental group and none from the control group responded conceptually (Table 3). Following are two examples of conceptual responses provided by the experimental group: This problem reports the finding of the same compound with the two elements combining in different proportions. In my opinion, changes must be introduced in the law of definite proportions. In other words, it must be explained as to why the same compound exists with different proportions and when / which are the exceptions to this law (Student #1). We must avoid being carried away by generalities as they are not complied in all cases. One of these cases is that of iron (II) oxide, in which the two elements can form distinct types of iron oxide. The conditions in which a compound is formed can modify the mass relationship of the combining elements, thus forming different compounds from the same elements (Student #12)
Response of Student #1 accepts the existence of the law of definite proportions and at the same time points out that it is not enough to explain the contradiction presented in the problem. This recognition can be considered as the need for alternative explanations. It is interesting to note that this student on first reading the exam question (Item 3) stated: “This question refers to the law of definite proportions, and laws cannot be contradicted. A law is a law and this is not open to discussion” (Reproduced from second author's class notes). This observation is important as during the experimental strategy no mention was made of the laws of definite and multiple proportions. However, it is plausible to suggest that interactions with students from other sections and textbooks, provided students necessary information with respect to these laws. With this perspective, it is important to note that Student #1, based on the contradiction, changed his perspective with respect to what she/he expressed initially. Student #12, refers to the need for going beyond generalities as under different conditions, same elements can form compounds with different composition. Such changes implicitly recognize the need for better explanations that go beyond the accepted laws and are a manifestation of the tentative nature of scientific theories (Lakatos, 1970; McComas et al., 1998). These changes can be attributed to the experimental treatment in which students were constantly asked and encouraged to provide alternative explanations in the face of contradictions.
Table 3 shows that 6 (19%) students from the experimental group and none from the control group provided a partially conceptual response to Item 3. Following are two examples from the experimental group: …although elements combine in a definite relation with respect to their masses, I think this depends on the type of elements that react — because there are elements that are in excess and others that are limiting reagents… (Student #31) The relations in which these elements combine in the two cases are different. This means that these are different compounds with the same properties (Student #33).
Partially conceptual responses, on the one hand show a tendency to dissociate from the idea of chemical combination as definitive and constant, but on the other hand do not argue convincingly to understand that the same compound can have different compositions.
Table 3 shows that 18 (58%) students of the experimental group and 29 (91%) of the control group responded with a rhetorical response, and the difference is statistically significant (p<0.05). Following are two examples of rhetorical responses by experimental group students: In my opinion, as we are combining 100 g of iron + 28.63 g of oxygen = 128.63 g of FeO. Thus it can be concluded that this compound does not need additional Fe in order to combine with oxygen to form FeO (Student #2). Perhaps atmospheric conditions were responsible for having left a certain amount of iron without reaction or some iron dispersed into air due to disintegration (Student #3).
Despite the difficulties involved in responding to Item 3, it is suggested that at least some students understood that contradictions are part of the scientific activities and these lead to alternative interpretations and explanations. This understanding is important if we want our students to understand that progress in science is intricately associated with the tentative nature of science.
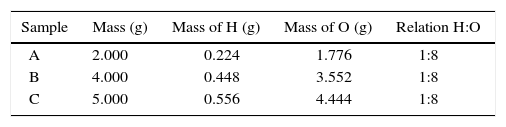
Item 4In the following table data from the chemical analysis of three different samples of water are presented:
- a)
What can you conclude with respect to the chemical composition of the different samples of water?
- b)
Are the samples of water different? Justify your response.
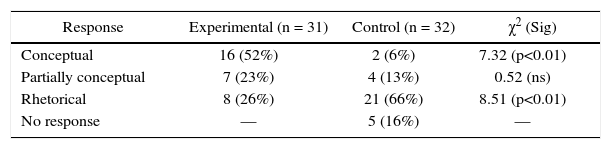
Item 4 provided students with all the relevant information in Table 4, and its most important aspect was the 1:8 relation between hydrogen and oxygen in all three samples. Furthermore, similar tables are presented in textbooks and by teachers to illustrate the validity of the law of definite proportions. Table 4 shows that 16 (52%) students of the experimental group and 2 (6%) of the control group responded conceptually and the difference is statistically significant (p<0.01). Following are two examples of the conceptual responses by the experimental group: Although the samples have different masses, they combine in the same relation (1:8), which means that they belong to the same compound. Masses of hydrogen and oxygen in the different sample may vary, but their relation on forming H2O is the same (Student #1). The three samples present a small amount of hydrogen as compared to oxygen, and these masses combine to establish the same proportion. Analyzing the composition and the proportion, it can be concluded that it is the same compound and it has to be emphasized that water is composed of 2 atoms of hydrogen and one atom of oxygen (Student #6).
Comparison of the performance of experimental and control group students on Item 4 (Conceptual).
| Response | Experimental (n = 31) | Control (n = 32) | χ2 (Sig) |
|---|---|---|---|
| Conceptual | 16 (52%) | 2 (6%) | 7.32 (p<0.01) |
| Partially conceptual | 7 (23%) | 4 (13%) | 0.52 (ns) |
| Rhetorical | 8 (26%) | 21 (66%) | 8.51 (p<0.01) |
| No response | — | 5 (16%) | — |
Response provided by Student #6 is interesting and illustrates what Dori and Hameiri (2003) have referred to as problem complexity of the type: Symbol ←→ Micro transformation. This clearly manifests conceptual understanding as it requires the student to relate the chemical symbol to the concept of atoms.
Table 4 shows that 8 (26%) students of experimental group and 21 (66%) of control group provided rhetorical responses and the difference is statistically significant (p<0.01). Following is an example of such responses from the experimental group: Every sample of water has different amounts of hydrogen and oxygen, although they maintain the same relation. What I find strange is the relation 1:8, that is, the three samples are different and still the relation is the same (Student #11).
Most students with a rhetorical response (experimental and control groups) reasoned in this manner, which clearly shows the lack of the ability to understand the symbol ←→ micro transformation, viz., the relation 1:8 has to be translated at the micro level.
Item 5Copper (Cu) forms two oxides. 100.00 grams of copper combine with 12.598 grams of oxygen to produce Cu2O or with 25.196 grams of oxygen to produce CuO. The amount of oxygen that combined in CuO is double that of Cu2O. In other words, the relation of the mass of oxygen that combined in the two compounds is 12.598 : 25.196 or simply 1 : 2. According to these results:
- a)
Can we conclude that if two elements combine to form more than one compound, then the different masses of one of these elements that combine with a fixed mass of the other, would do so in small whole-number ratios?
- b)
Is this conclusion consistent with results presented here? Justify your response.
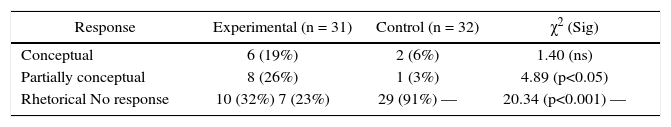
This item deals explicitly with the law of multiple proportions. Control group was given the definition of the law and solved various problems, whereas experimental group did not receive the definition but only solved problems dealing with such situations. Table 5 shows that 6 (19%) students of the experimental group and 2 (6%) of the control group responded conceptually. Following is an example of a conceptual response from the experimental group: “Two atoms can combine to form various compounds and one of the two elements would not change its mass and combine with the other in small whole-number ratios. However, it is possible that this relation may exist as a big whole-number ratio” (Student #1). Table 5 shows that 8 (26%) students of the experimental group and only 1 (3%) from the control group provided a partially conceptual response and the difference is statistically significant (p<0.05). Also 10 (32%) students from the experimental group and 29 (91%) from the control group provided rhetorical responses and the difference is statistically significant (p<0.001).
Comparison of the performance of experimental and control group students on Item 5 (Conceptual).
| Response | Experimental (n = 31) | Control (n = 32) | χ2 (Sig) |
|---|---|---|---|
| Conceptual | 6 (19%) | 2 (6%) | 1.40 (ns) |
| Partially conceptual | 8 (26%) | 1 (3%) | 4.89 (p<0.05) |
| Rhetorical No response | 10 (32%) 7 (23%) | 29 (91%) — | 20.34 (p<0.001) — |
Consider the following compounds formed by Carbon (C) and Hydrogen (H):
- a)
Ethyne (C2H2) and ethylene (C2H4)
- b)
Butane (C4H10) and heptane (C7H16)
- c)
Ethyne (C2H2) and pentane (C5H12)
- i)
Do you think that the three cases mentioned here can be considered as instances of the generalization presented in Item 5?
- ii)
Do you think we need an alternative explanation? Justify your response
This was a difficult and thought-provoking question and could be considered as a follow-up to Item 5, in which the formation of Cu2O and CuO was presented as a instance of the law of multiple proportions (only control group students were provided the definition). In contrast, Item 6 provided students with three examples of fairly well known hydrocarbons, in which only the first case (C2H2 and C2H4) could be considered as an instance of the law of multiple proportions. This item provided an opportunity to observe the difference in the problem solving strategies of two group of students, viz., one that was exposed to an algorithmic definition of the law (control) and the other that was provided an opportunity to think and reason (experimental). Table 6 shows that 1 (3%) student from the experimental group and none from the control group provided a conceptual response. Following is the conceptual response of the experimental group student: The generalization is valid for case (a), as the mass of hydrogen in C2H4 is double that of C2H2. In cases (b) and (c), it all depends on what we may consider as the fixed mass. However, it is not appropriate to generalize in these cases. Each one of such cases should be analyzed and studied in order to find alternative explanations, not only for this problem but for others that we may find in the future (Student #3, before presenting this response, the student did all the relevant calculations for the three cases of hydrocarbons). Note: This student provided a partially conceptual response in Item 5.
Table 6 shows that 6 (19%) students from the experimental group and only 1(3%) from the control group provided a partially conceptual response, and following are two examples from the experimental group: In each case there exists a different relation, as hydrogen in order to form different compounds varied its mass (Student #1, all relevant calculations were presented). Note: Apparently, this student did not follow-up on his/her response in Item 5, where she/he had suggested the possibility of big whole-number ratios. We can observe that in each one of the compounds, the proportion of the same combining elements is different. All these relations are in small whole-number ratios (Student #22, all relevant calculations were presented).
It is interesting to note that students in this group did all the relevant calculations and then concluded that all whole-number ratios were small (i.e., C2H2 and C2H4) and simply ignored the cases in which this was not the case (viz. C4H10 & C7H16 and C2H2 & C5H12). One student (#16), however, recognized that the generalization presented in Item 5, can perhaps only be applied in case (a).
Table 6 shows that 14 (45%) students from the experimental group and 23 (72%) from the control group provided rhetorical responses and following are two examples: Yes, because in the previous cases 2 atoms combined, and in the present case various atoms did so (Student #5, no calculations were performed). The generalization is valid for this problem, as different compounds are formed from the same elements. Thus it is not necessary to look for an alternative explanation (Student #12, all relevant calculations were presented).
In general, rhetoric responses of both experimental and control group students did not perform all the calculations and concluded that the generalization from Item 5 could be applied to all three cases presented in Item 6. Results obtained also show that none of the students from control or experimental group, solved all four items (3, 4, 5, 6) conceptually. Three students from the experimental and none from the control group solved three items conceptually. Four students from the experimental and none from the control group solved two items conceptually. Nine students from the experimental and four from the control group solved one item conceptually. This clearly shows the better performance of the experimental group not only in algorithmic but also conceptual items.
Conclusions and educational implicationsControl group students in this study were exposed to traditional problem solving strategies in which algorithms based on formulae were used. In contrast, experimental group students participated in argumentation, cognitive conflicts and historical reconstruction of events, which facilitated the capacity to develop alternative models/hypotheses in the face of conflicting information. This experimental teaching strategy was based on an epistemological, historical and philosophical framework, which required considerable time and effort on part of both the students and the instructor. Results obtained in this study show the better performance of the experimental group not only on algorithmic (Items 1 and 2) but also all conceptual items (Items, 3, 4, 5, & 6). Despite Christie's (1994) critique, textbooks still emphasize the law of definite proportions. One study has reported that of the 27 general chemistry textbooks analyzed (all published in U.S.A.) only three explained chemical combination, stoichiometry and other related concepts without enunciating or referring to the law (Niaz, 2001).This study shows that experimental group students were better prepared to understand and interpret the formation of non-stoichiometry compounds. Based on the theoretical framework and the results obtained, this study has important educational implications. If scientific laws are idealizations, then they do not describe the behavior of actual bodies, and hence may not be very helpful in understanding the world of experience (Giere, 1999). This perspective leads to a critical evaluation of the laws of definite and multiple proportions and their role in chemistry education. Emphasizing these laws inevitably leads to the use of algorithms and formulae in learning chemistry and especially stoichiometry. Thus, stating the laws and definitions first and then asking students to work out exercises as illustrations of the laws, is counter-productive (Stinner, 1992). This constitutes an important guideline towards the understanding of the history and philosophy of chemistry (cf. Niaz & Maza, 2011).