Systemic infiltrative diseases are relatively rare conditions consisting of cell infiltration or substance deposition in multiple organs and systems, including endocrine glands. This article reviews endocrine changes in the main four diseases at epidemiological level: sarcoidosis, Langerhans cell histiocytosis, hereditary hemochromatosis, and systemic amyloidosis. Recommendations to endocrinologists for hormone work-up and management of patients with each of these conditions are provided.
Las enfermedades infiltrativas sistémicas son un grupo de enfermedades relativamente raras que consisten en la infiltración de células o depósito de sustancias en múltiples órganos y sistemas, entre los que se encuentran las glándulas endocrinas. En este artículo se revisan las alteraciones endocrinológicas de las cuatro más importantes a nivel epidemiológico, como son la sarcoidosis, la histiocitosis de células de Langerhans, la hemocromatosis hereditaria y la amiloidosis sistémica. En cada una de ellas se aportarán recomendaciones al endocrinólogo para el estudio hormonal de estos pacientes.
Systemic infiltrative diseases are a group of relatively rare diseases consisting of cell infiltration or substance deposition in multiple organs and systems, including endocrine glands.
Many texts on endocrinology discuss the group of infiltrative diseases as a cause of hormone deficiencies in each of the glands separately. However, addressing these diseases jointly is a complex undertaking, as there are no publications in the literature dealing with them as a group, and there are no clear protocols for either hormone testing or management in these patients.
Although these are low-prevalence diseases, the diagnostic and therapeutic approach to these patients is not uncommon and represents a challenge for endocrinologists in clinical practice. This is more obvious when a patient is being assessed for various hormone deficiencies and the systemic disease that is causing them has yet to be diagnosed.
We present below a narrative review of the available literature on the most common endocrine abnormalities associated with infiltrative diseases. We conducted a literature search in various databases, such as Pubmed, Embase and Scopus, for the most significant related diseases: sarcoidosis, Langerhans cell histiocytosis, hereditary haemochromatosis and systemic amyloidosis. We did not include infectious or metastatic disease. We did include review articles, observational studies, case reports and case series that particularly focused on the endocrine abnormalities in the four diseases listed above.
SarcoidosisSarcoidosis is a multisystem disease characterised by the presence of non-caseating granulomas in the tissues.1 Its aetiology is not fully understood, but activated T lymphocytes are known to be involved. The disease usually appears between 20 and 40 years of age. It has an estimated prevalence of 10 cases per 100,000 population.2 Although in some individuals it may be transient, in others it becomes a chronic disease. Sarcoidosis most often affects the lungs in the form of bilateral hilar lymphadenopathy and interstitial lung disease, but 30% of individuals with this disease develop extrapulmonary manifestations. In addition to endocrine disorders, skin and eye lesions are also typical. Other organs, including the liver, spleen, lymph nodes, heart, bones and organs of the nervous system, may also be affected.3
Calcium and phosphorus metabolismHypercalcaemia is associated with sarcoidosis in 5%–10% of cases.4 It is caused by an increase in the concentration of 1,25(OH)2-vitamin D3 due to extrarenal 1α hydroxylation of vitamin D in granulomas, although excessive production of parathyroid hormone-related protein (PTHrP) and cytokines that resorb bone may play a role in some patients. Local synthesis of 1,25(OH)2-vitamin D3 by macrophages activated by interferon gamma and the activation of the vitamin D receptor (VDR) in these cells constitutes a paracrine system that activates antibacterial mechanisms as part of the normal action of the macrophages. The 1,25(OH)2-vitamin D3 is responsible for increasing intestinal absorption of calcium and phosphate, increasing osteoclastic recruitment and bone resorption, and modulating the increase in bone formation by osteoblasts.3
Hypercalciuria is even more common than hypercalcaemia as it occurs in 40%–50% of patients with sarcoidosis.5 The mechanism by which hypercalciuria occurs is multifactorial, although it is probably due to increased calcium absorption, plus increased bone resorption.
Hypercalcaemia suppresses the PTH secreted by the parathyroid glands. Primary hyperparathyroidism associated with sarcoidosis is very rare.6
Elevated serum calcium can lead to complications such as pancreatitis, nephrocalcinosis, nephrolithiasis, kidney failure and even death.7 Symptomatic hypercalcaemia in the form of dehydration, nephrogenic diabetes insipidus or altered level of consciousness is rare but can occur without treatment.
Abnormal calcium and phosphorus metabolism can also lead to osteopenia and osteoporosis, to which is added the effect of corticosteroids, the treatment of choice in this disease. For that reason, antiresorptive therapy is essential in these patients; bisphosphonates are the first choice for the primary and secondary prevention of glucocorticoid-induced osteoporosis.
Patients should be advised of the need to minimise exposure to sunlight, limit intake of foods rich in vitamin D and drink plenty of fluids. Prednisolone at a dose of 20−40 mg a day is the drug of choice and is highly effective in restoring normal serum calcium levels. Corticosteroids reduce gastrointestinal calcium absorption and inhibit osteoclastic activity. If hypercalcaemia does not resolve with steroid therapy, primary hyperparathyroidism should be ruled out.3
Ketoconazole is a second-line treatment for hypercalcaemia and is indicated when steroid treatment is ineffective or contraindicated. It is an imidazole antifungal that inhibits cytochrome P450 related to vitamin D hydroxylation.8
Hypothalamic–pituitary axisAround 5% of patients with sarcoidosis show signs and symptoms of nervous system involvement.9 However, subclinical and undiagnosed neurosarcoidosis appears to be far more common.3
Neurosarcoidosis in the sellar region is rare, accounting for only 1% of sellar tumours. Sarcoidosis has a predilection for the cranial nerves, the hypothalamus, the pituitary gland and the pituitary stalk; involvement of the hypothalamus is the most common. These patients experience varying degrees of anterior pituitary dysfunction, associated or not with diabetes insipidus (DI). The most common hormonal abnormalities are hypogonadotropic hypogonadism (89%), DI (65%) and hyperprolactinaemia (49%). The onset of DI without obvious features of pituitary disorder should alert the endocrinologist to the need to rule out hypothalamic sarcoid deposits, especially when magnetic resonance imaging (MRI) shows stalk thickening. Sarcoidosis can also cause secondary hypothyroidism, secondary adrenal insufficiency and growth hormone deficiency.10,11
Contrast-enhanced hypothalamic–pituitary MRI can aid in assessing treatment response.3 In some cases of uncertain diagnosis, biopsy of the lesion is required, as neurosarcoidosis may even mimic pituitary tumours and present with bitemporal hemianopia.12 A characteristic finding is a favourable response to corticosteroid therapy.
Thyroid glandThe thyroid gland is an uncommon site of the disease. The approximate incidence in autopsy series is 4%.13 It is most common in middle-aged women and particularly associated with peripheral and intrathoracic lymphadenopathy. Hypothyroidism resulting from extensive infiltration by granulomas has been reported. There have also been accounts of cases of transient hyperthyroidism due to inflammation of the thyroid gland. There seems to be an association between sarcoidosis and thyroid autoimmune disease, with a reported frequency of 17%.14
The most significant ultrasound findings in patients with sarcoidosis include scattered nodules (1−3 cm), with irregular hypoechoic areas reflecting the formation of granulomas. While 4% of thyroid cancers can induce a sarcoid reaction in the thyroid gland, sarcoidosis as a disease can coexist with papillary thyroid cancer, although the causal relationship remains unclear. Being aware of this association is important in the differential diagnosis of a thyroid nodule associated with lymphadenopathy in a patient with sarcoidosis. Patients with known sarcoidosis who have cervical lymphadenopathy or thyroid nodules should therefore undergo fine needle aspiration (FNA) biopsy for cytology.15
Usually, no treatment is required, unless the patient presents compressive symptoms, in which case surgery may be needed, or hypothyroidism, in which case replacement therapy is indicated.
Adrenal glandsInvolvement of the adrenal glands is rare in sarcoidosis. Adrenal function is usually normal in an adrenocorticotropic hormone (ACTH) stimulation test, with the exception of patients with secondary adrenal insufficiency due to pituitary infiltration.16 In the case of abundant granulomatous infiltration and replacement of glandular tissue by fibrosis, the patient may develop adrenal insufficiency or even adrenal crisis. Addison’s disease associated with sarcoidosis is also unusual but has been reported in the literature.17
Genitourinary systemThe rate of involvement of the male genitourinary system is <0.2% in diagnosed patients, but 5% in post-mortem examinations of these patients.18 Non-caseating granulomas appear mainly in the epididymis, testes and prostate gland; they almost always are asymptomatic or cause a painless increase in testicle size, acute epididymo-orchitis or testicular swelling. Ultrasound images show hypoechoic lesions that can be mistaken for tumours.19 Glucocorticoid therapy can reduce testicular lesions and improve gonadal function.
The most common site of involvement in the female genitourinary system is the uterus; signs include menstrual abnormalities, menstrual bleeding in postmenopausal women and bleeding associated with cervical ectropion.20 Women with sarcoidosis usually do not have difficulty getting pregnant or carrying a pregnancy to term. Uterine sarcoidosis improves with glucocorticoid therapy (Table 1).
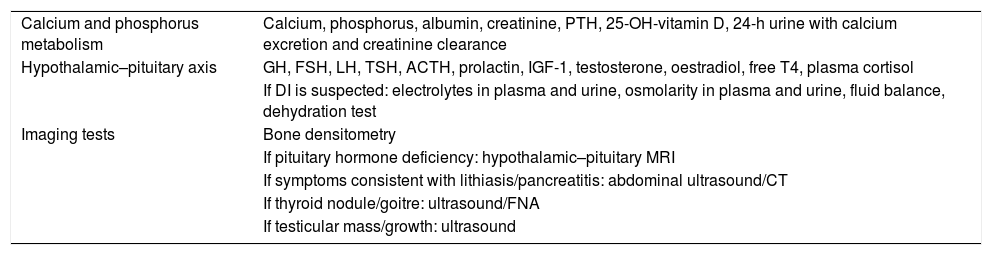
Additional tests to be ordered for the diagnosis of sarcoidosis.
| Calcium and phosphorus metabolism | Calcium, phosphorus, albumin, creatinine, PTH, 25-OH-vitamin D, 24-h urine with calcium excretion and creatinine clearance |
| Hypothalamic–pituitary axis | GH, FSH, LH, TSH, ACTH, prolactin, IGF-1, testosterone, oestradiol, free T4, plasma cortisol |
| If DI is suspected: electrolytes in plasma and urine, osmolarity in plasma and urine, fluid balance, dehydration test | |
| Imaging tests | Bone densitometry |
| If pituitary hormone deficiency: hypothalamic–pituitary MRI | |
| If symptoms consistent with lithiasis/pancreatitis: abdominal ultrasound/CT | |
| If thyroid nodule/goitre: ultrasound/FNA | |
| If testicular mass/growth: ultrasound |
ACTH: adrenocorticotropic hormone; CT: computed tomography; DI: diabetes insipidus; FNA: fine needle aspiration; FSH: follicle-stimulating hormone; GH: growth hormone; IGF-1: insulin-like growth factor 1; LH: luteinising hormone; MRI: magnetic resonance imaging; PTH: parathyroid hormone; TSH: thyroid-stimulating hormone.
Note: prioritise serum calcium and pituitary tests if symptoms are consistent with hormone deficiency. Depending on results, determine follow-up interval according to routine clinical practice.
Langerhans cell histiocytosis (LCH) is a disease characterised by a proliferation of dendritic (Langerhans) cells belonging to the reticuloendothelial system.21 These are mature cells of monoclonal origin capable of infiltrating one or more organs, giving rise to a systemic disease.22
The estimated prevalence of this disease is one to two cases per million population. It can affect all age groups, from neonates to adults. Patients may be asymptomatic or present interstitial lung disease, skin rash, bone infiltration or lymphadenopathy.23
Hypothalamic–pituitary axisOne of the most typical locations is the hypothalamic–pituitary axis. Diabetes insipidus is the most common endocrine disorder in LCH, occurring in 15%–50% of cases.22
In a study by Kaltsas et al., 12 patients with a histological diagnosis of LCH, were followed up for a mean of 11.5 years after being diagnosed with DI. The mean age at diagnosis of DI was 34. In four of the 12 patients, DI was the initial presenting symptom of the disease; in the other eight, DI appeared between one and 20 years LCH was diagnosed, with a median of two years.24
Anterior pituitary gland involvement has also been reported and can occur in 5%–20% of cases, usually associated with the development of DI and prior radiotherapy.22 The most common hormone deficiency after antidiuretic hormone (ADH) deficiency is growth hormone (GH) deficiency, with a mean latency of one year from diagnosis.25 Cases of secondary hypogonadism have also been reported.21
LCH should be considered in the differential diagnosis of patients with apparently isolated DI, and these cases should be monitored closely for symptoms consistent with LCH. One study found that 15% of patients with “isolated” DI had LCH.26
Patients with multisystem disease and craniofacial involvement, particularly in the ear, eye and mouth area, are at increased risk of developing DI in the course of the disease. This risk increases when the disease remains active for a long period of time or reactivates.27
Loss of the posterior pituitary bright spot may be seen on MRI, but this finding is not diagnostic. The gland is thickened in more than 70% of patients with DI and remains thickened five years from diagnosis in 24%.28 The gland can be biopsied for a definitive diagnosis, avoiding complete excision.
One special form of LCH is Hand-Schüller-Christian disease, which presents with the triad of central DI, exophthalmos and osteolytic disease.29
Patients with LCH and DI are at increased risk of developing different forms of hypopituitarism, so they benefit from close and prolonged follow-up for the detection and treatment thereof.
Hormone abnormalities in LCH do not usually respond to systemic treatment of the disease and therefore tend to require replacement therapy.24
Thyroid glandInfiltration of the thyroid gland can also occur. It may present as a solitary nodule or an enlarged thyroid in a context of a multinodular goitre. Most of these patients have normal thyroid function.30 A differential diagnosis should be made with thyroid cancer, and therefore an FNA for cytology is indicated in these patients; this usually shows infiltration by histiocytes with some follicular cells. If involvement is limited to the thyroid gland, thyroidectomy can cure the disease.31
If there are compressive symptoms, surgery is indicated. In patients with systemic involvement, including thyroid involvement, different treatments such as surgery, radiotherapy and chemotherapy will be considered.24 (Table 2).
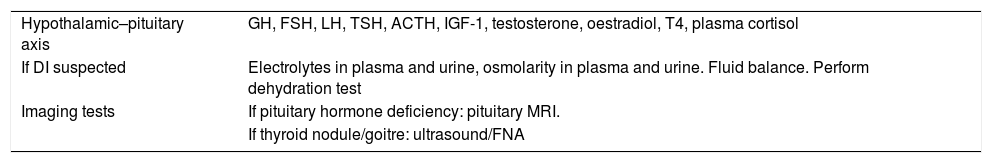
Additional tests to be ordered for the diagnosis of Langerhans cell histiocytosis.
| Hypothalamic–pituitary axis | GH, FSH, LH, TSH, ACTH, IGF-1, testosterone, oestradiol, T4, plasma cortisol |
| If DI suspected | Electrolytes in plasma and urine, osmolarity in plasma and urine. Fluid balance. Perform dehydration test |
| Imaging tests | If pituitary hormone deficiency: pituitary MRI. |
| If thyroid nodule/goitre: ultrasound/FNA |
ACTH: adrenocorticotropic hormone; DI: diabetes insipidus; FNA: fine needle aspiration; FSH: follicle-stimulating hormone; GH: growth hormone; IGF-1: insulin-like growth factor 1; LH: luteinising hormone; MRI: magnetic resonance imaging; T4: tetraiodothyronine; TSH: thyroid-stimulating hormone.
Note: prioritise DI tests if clinical suspicion. If confirmed, a pituitary profile must be ordered. Depending on results, determine follow-up interval according to routine clinical practice.
Hereditary haemochromatosis (HH) is a genetic disorder characterised by an accumulation of iron that causes damage to multiple tissues.32 As many as five types have been reported, all related to mutations in the hepcidin–ferroportin axis. The most common is type 1, caused by mutations in the HFE gene, located on chromosome 6, with an autosomal recessive inheritance pattern. It most commonly affects people of European descent.33 Depending on the type of mutation, signs range from laboratory abnormalities alone to multiple-organ disease in which liver damage, arthritis, cardiomyopathy and endocrine abnormalities may develop. This review will focus on endocrine abnormalities.
PancreasThe earliest studies established the prevalence of diabetes in patients with HH at around 40%–63%.34 The combination of diabetes and abnormalities in skin pigmentation led to this disease being classically referred to as “bronze diabetes”. Later, with the discovery of the genetic mutations associated with the disease, it became possible to diagnose and treat this disorder at an earlier stage, which helped reduce the complications thereof, including diabetes mellitus.
In a cross-sectional study conducted in patients with HH and a homozygous mutation of the HFE gene that estimated the prevalence of impaired glucose metabolism using oral and intravenous glucose tolerance tests, the authors found prevalences of 23% for diabetes and 30% for carbohydrate intolerance. This indicates a higher-than-expected rate of impaired glucose metabolism before the development of diabetes mellitus.35
The pathogenesis of diabetes in haemochromatosis is not fully understood, but it is accepted that the most important mechanisms are, on the one hand, oxidative damage to pancreatic beta cells leading to a certain insulin deficiency and, on the other, insulin resistance due to the liver damage that develops over the course of the disease. Added to all that is the presence of obesity and predisposition due to family history.36
Although HH is most often associated with type 2 diabetes, there are rare cases of late development of type 1 diabetes37 (Table 3).
Additional tests to be ordered for the diagnosis of haemochromatosis.
| Glucose metabolism | Baseline blood glucose, insulin, C-peptide and glycosylated haemoglobin |
| Hypothalamic–pituitary axis | LH, FSH, testosterone and oestradiol |
| Thyroid gland | TSH and free T4 |
| Calcium and phosphorus metabolism | Calcium, phosphorus, albumin, creatinine, PTH, alkaline phosphatase, 25-OH vitamin D |
| Imaging tests | Bone densitometry |
FSH: follicle-stimulating hormone; LH: luteinising hormone; PTH: parathyroid hormone; T4: tetraiodothyronine; TSH: thyroid-stimulating hormone.
Note: Prioritise glucose metabolism and gonadal axis tests. Depending on results, determine follow-up interval according to routine clinical practice.
Phlebotomy is the gold standard for treating any form of HH, but it has a variable impact on diabetes management. In general, individuals with HH who have not yet developed complications show improvement in their ability to secrete insulin and improvement in their glucose tolerance. However, no such improvement is seen in patients with advanced HH with cirrhosis and diabetes mellitus.38
Pituitary glandThe most common nondiabetic endocrine disorder in HH patients is hypogonadism. The source of the problem is in the pituitary gland, where iron deposits preferentially affect the anterior pituitary gonadotropic cells, leading to their dysfunction and impaired hormone secretion.39 The oldest series, predating the discovery of the genetic diagnosis of HH, reported a prevalence of hypogonadism ranging from 10% to 100% of cases.40
In the largest prospective study of hypogonadism in patients with HH, McDermott et al. followed up 191 patients with haemochromatosis (144 men and 47 women) from 1983 to 2005. The study found a prevalence of hypogonadism of 6.4% in men and 5.2% in women. When comparing patients with HH and hypogonadism to eugonadal individuals, the former group showed a higher prevalence of siderosis and liver cirrhosis, as well as ferritin levels >1500 ng/mL and a trend towards a higher rate of diabetes. This suggested that, in patients with type 1 HH, a hypogonadal state can be considered a sign of advanced disease.41
Although hypogonadism in women with HH has been less studied, it seems that the prevalence is lower than in men, probably due to a lower accumulation of iron resulting from menstrual blood loss.36
With regard to treatment, interestingly, it has been reported that these patients’ gonadal function can improve or normalise if therapy is started early. Therefore, after iron stores are normalised, hormone replacement therapy should be given, knowing that recovery of gonadotropic cell function can take several months.42
Iron deposition has also been reported in somatotropic, lactotropic, corticotropic and thyrotropic cells, but iron accumulation seems to be more pronounced in gonadotropic cells than in these other types of cells.39 In general, panhypopituitarism is relatively rare in these patients.42 Functional tests performed in various studies to assess hormone axes have been inconclusive, especially those related to GH secretion. Secretion of ACTH and thyroid-stimulating hormone (TSH) is usually well preserved. However, further studies are required to more accurately assess these hormone axes by means of functional tests in patients with HH.36
Thyroid glandIt is well known that iron is also deposited in the thyroid gland in these patients.39 However, thyroid abnormalities are very rare. Some cases of both hypothyroidism and primary hyperthyroidism have been reported in patients with HH. However, they are rare and occur in patients with liver cirrhosis and/or other endocrine abnormalities in the final stage of the disease.36
Adrenal glandsIn the adrenal glands, iron can accumulate predominantly in the zona glomerulosa, where aldosterone is secreted, while not generally affecting the zona fasciculata and the zona reticularis.39 Glucocorticoid function is preserved in practically all patients with HH, and although there may be a significant accumulation of iron in the zona glomerulosa, mineralocorticoid deficiency has only been reported very rarely in the literature.36
Calcium and phosphorus metabolismBone disorders are quite common in HH, but their pathogenesis is not well understood. Iron deposition has been reported in the parathyroid glands, but PTH deficiency is quite rare.43
Several studies have shown a higher prevalence of osteoporosis in patients with HH, estimated to be in the region of 25%–34%, and osteopenia, with a higher prevalence, in the 40%–79% range.44 Bone mineral density seems to correlate with the degree of iron overload and also worsens if there is associated hypogonadism.36 In these cases, the decrease in bone mineral density seems to be more pronounced in the femoral head than in the lumbar spine, which may indicate greater involvement of cortical bone than trabecular bone.45
Studies in vivo and in vitro have demonstrated that iron has a direct toxic effect on osteoblasts. Moreover, iron accumulation seems to inhibit the growth of hydroxyapatite crystals in bone.46 Added to this is the fact that hypogonadism also predisposes individuals to loss of bone mass. These abnormalities can improve if therapeutic phlebotomy is started early.37
Systemic amyloidosisAmyloidosis encompasses a group of diseases characterised by progressive deposition of an insoluble protein substance in the extracellular space of various organs and tissues.47 This process is associated with alteration of cell architecture and organ dysfunction, leading to abnormalities ranging from asymptomatic to potentially fatal. More than 25 types of proteins have been identified as causative agents of amyloid diseases. They characteristically take up Congo red stain and have apple-green birefringence when viewed under a polarised light microscope.48
There are two groups: systemic amyloidosis, in which amyloid protein is produced at a site distant from the deposit and the organ involvement; and local amyloidosis, in which the production and deposition of amyloid protein occurs in the same tissue. The local amyloidosis group refers to generally age-related conditions, such as Alzheimer’s disease and type 2 diabetes, where deposits of amyloid protein are found in neurons and pancreatic beta cells, respectively.49 AL (primary) amyloidosis, which usually occurs in association with any type of B-cell dyscrasia, is the most common type of systemic amyloidosis, with an approximate incidence of 0.8 cases per 100,000 person-years.50 The second most common type of systemic amyloidosis, AA (secondary) amyloidosis, is usually a complication of chronic inflammatory diseases such as tuberculosis, chronic osteomyelitis, bronchiectasis, connective tissue diseases and some cancers.51 The other two forms of systemic amyloidosis are hereditary amyloidosis and dialysis-related amyloidosis.52
The organs most commonly affected in systemic amyloidosis are the liver, kidneys, spleen, heart and gastrointestinal tract, but other organs can also be affected. Amyloid substance can also infiltrate the endocrine glands, but this does not always cause endocrine gland dysfunction.
Pituitary glandPituitary involvement in systemic amyloidosis is not well studied. Amyloid deposition in the pituitary gland is age-related; more than 80% of patients over 80 years of age have amyloid deposits in their pituitary gland. The gland’s hormonal function is normally intact in patients with systemic amyloidosis, but there have been reports of cases of hypopituitarism.53,54 Amyloid deposition also occurs in both functioning (prolactin- and growth hormone-producing) and non-functioning pituitary adenomas.55,56 Pituitary MRI shows a hypointense gland in both T1- and T2-weighted images; this is a typical characteristic of amyloid deposits in the gland.53,54
Thyroid glandAmyloid deposition in the thyroid gland is seen in 30%–80% of patients with systemic amyloidosis. Amyloid goitre, defined as infiltration of the thyroid gland in such an amount that it causes clinical enlargement of the gland, is much rarer.
Amyloid goitre is characterised by progressive, firm, nontender, diffuse growth of the thyroid gland. It can cause compressive symptoms such as dysphagia, dyspnoea and dysphonia. Its firm consistency and rapid growth mean that it can be mistaken for a malignant lesion. There may also be local or regional lymphadenopathy, which could increase suspicion of malignancy. FNA should be performed if a malignant lesion is suspected. In rare cases, thyroid infiltration may be the first sign of systemic amyloidosis.57,58
Ozdemir et al. compiled data on thyroid function from a total of 149 cases of patients with amyloid goitre published in the literature. Thyroid function was abnormal in 34% of the cases; the most common abnormality was hypothyroidism (14.8%), followed by hyperthyroidism (4.7%), subacute thyroiditis (4%) and euthyroid sick syndrome (11.4%).49
Several ultrasound patterns have been reported in patients with impairment of the thyroid gland due to amyloidosis. The most common finding is unilateral or bilateral enlargement of the thyroid gland along with increased echogenicity.59 The amyloid deposit may also be visualised as multiple solid hyperechoic or hypoechoic lesions, cysts or nodules.60 Echogenicity may be homogeneous or heterogeneous. Findings on computed tomography (CT) and MRI are not well defined. Both a decrease and an increase in attenuation on CT, as well as a low intensity on MRI, have been reported.61
FNA is a safe method for diagnosis of amyloid infiltration of the thyroid gland.62 However, it is not always possible to demonstrate amyloid material because the deposit can be focal inside the gland. Cytological examination reveals thick, waxy-looking extracellular material mixed with follicular cells with a benign appearance.63
Aetiological treatment of the associated disease can improve thyroid impairment. Surgical treatment is of course unavoidable if compressive symptoms occur or if the patient prefers to undergo surgery for cosmetic reasons. The increased risk of bleeding in surgery due to deposition in small veins and the coagulation disorders which can affect in these patients must be taken into account.
Adrenal glandsThe adrenal glands can also become infiltrated in systemic amyloidosis. However, usually, there are no symptoms consistent with cortisol deficiency as this would require extensive destruction of the adrenal cortex.64 Nevertheless although it does not cause symptoms, a poor cortisol response to stimulation with adrenocorticotropic hormone (ACTH) has been reported.65 Several studies on the function of the adrenal axis in these patients have shown that half of the patients without symptoms consistent with adrenal insufficiency had a poorer response to stimulation tests. There are even four reported cases of death from Addisonian crises, in which post-mortem examinations showed amyloid deposition in the adrenal glands.66 Based on the studies published, the importance of evaluating this hormone axis is obvious. It is advisable to perform an ACTH stimulation test to determine adrenal reserve, bearing in mind that a suboptimal cortisol response to ACTH stimulation may be secondary to a low level of cortisol-binding globulin caused by strong proteinuria. In such cases, ACTH and renin levels must be determined to rule out primary adrenal insufficiency.
GonadsIn women, reported cases of amyloid deposition in the ovaries are extremely rare.67 However, there have been reports of amyloid deposition in the testes, associated in some cases with azoospermia and hypergonadotropic hypogonadism, in both AA and AL amyloidosis.68 One study even assessed testicular biopsy as a tool for the diagnosis of systemic amyloidosis in patients with confirmed renal amyloidosis and considered it a valid method more sensitive than rectal biopsy69 (Table 4).
Additional tests to be ordered for the diagnosis of amyloidosis.
| Thyroid gland | TSH and free T4 |
| Adrenal glands | ACTH, baseline plasma cortisol, 1-24 ACTH stimulation test |
| Gonads | If symptoms of hypogonadism (males): FSH, LH, testosterone |
| Imaging tests | If thyroid growth/nodules: ultrasound/FNA |
| If abnormal adrenal function: abdominal CT/MRI |
ACTH: adrenocorticotropic hormone; CT: computed tomography; FNA: fine needle aspiration; FSH: follicle-stimulating hormone; LH: luteinising hormone; MRI: magnetic resonance imaging; T4: tetraiodothyronine; TSH: thyroid-stimulating hormone.
Note: prioritise adrenal axis tests. Depending on results, determine follow-up interval according to routine clinical practice.
It is important to be aware of these conditions because, although they are not very common, they are associated with a large number of endocrine abnormalities. In each of them, early diagnosis and treatment of the most prevalent hormone deficiencies are essential. Moreover, it is always possible that the endocrine abnormality represents the onset of the disease, representing a diagnostic challenge. Endocrinologists must therefore position themselves as key to the multidisciplinary management of these patients.
Some possible limitations of this review were that some of the articles cited were old and had few participants because of the low prevalence of these diseases. Further research is needed to arrive at a more in-depth understanding of the pathogenic role of these and other infiltrative diseases in various endocrine disorders.
FundingNo sponsorship of any kind was received for producing this article.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Muñoz Moreno D, Miguélez González M, González Fernández L, Percovich Hualpa JC. Revisión sobre enfermedades infiltrativas sistémicas y patología endocrinológica asociada. Endocrinol Diabetes Nutr. 2021;68:312–320.