Some concerns persist regarding the safety of semaglutide. The objective of this updated meta-analysis is to assess the risk of acute pancreatitis with the use of semaglutide, assessing the results according to the different administration regimens.
MethodsWe performed an updated meta-analysis of randomised, placebo-controlled studies of semaglutide therapy that report acute pancreatitis. This meta-analysis was performed in line with PRISMA guidelines. A global and stratified analysis according to the therapeutic scheme used was performed using the fixed-effects model.
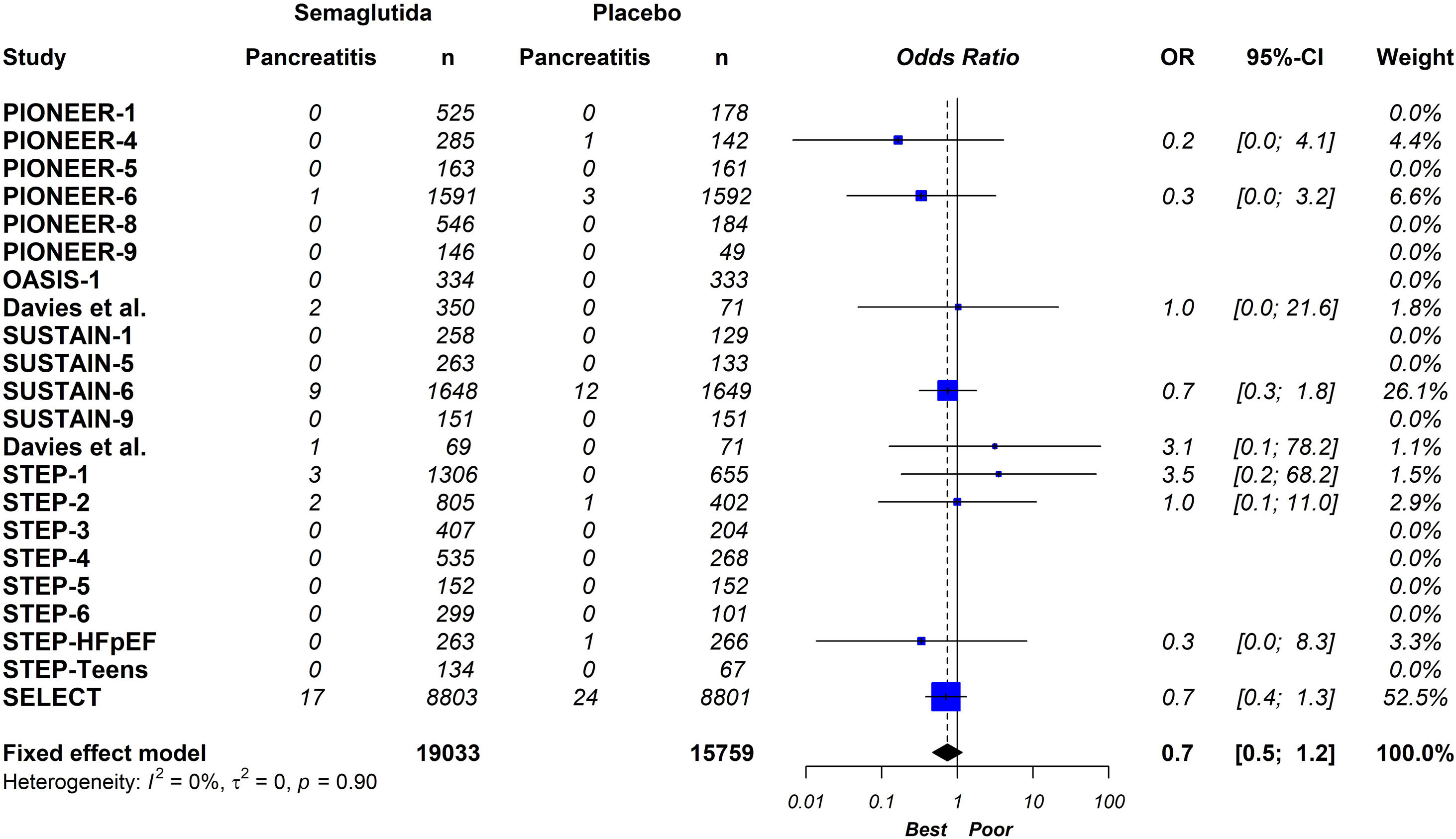
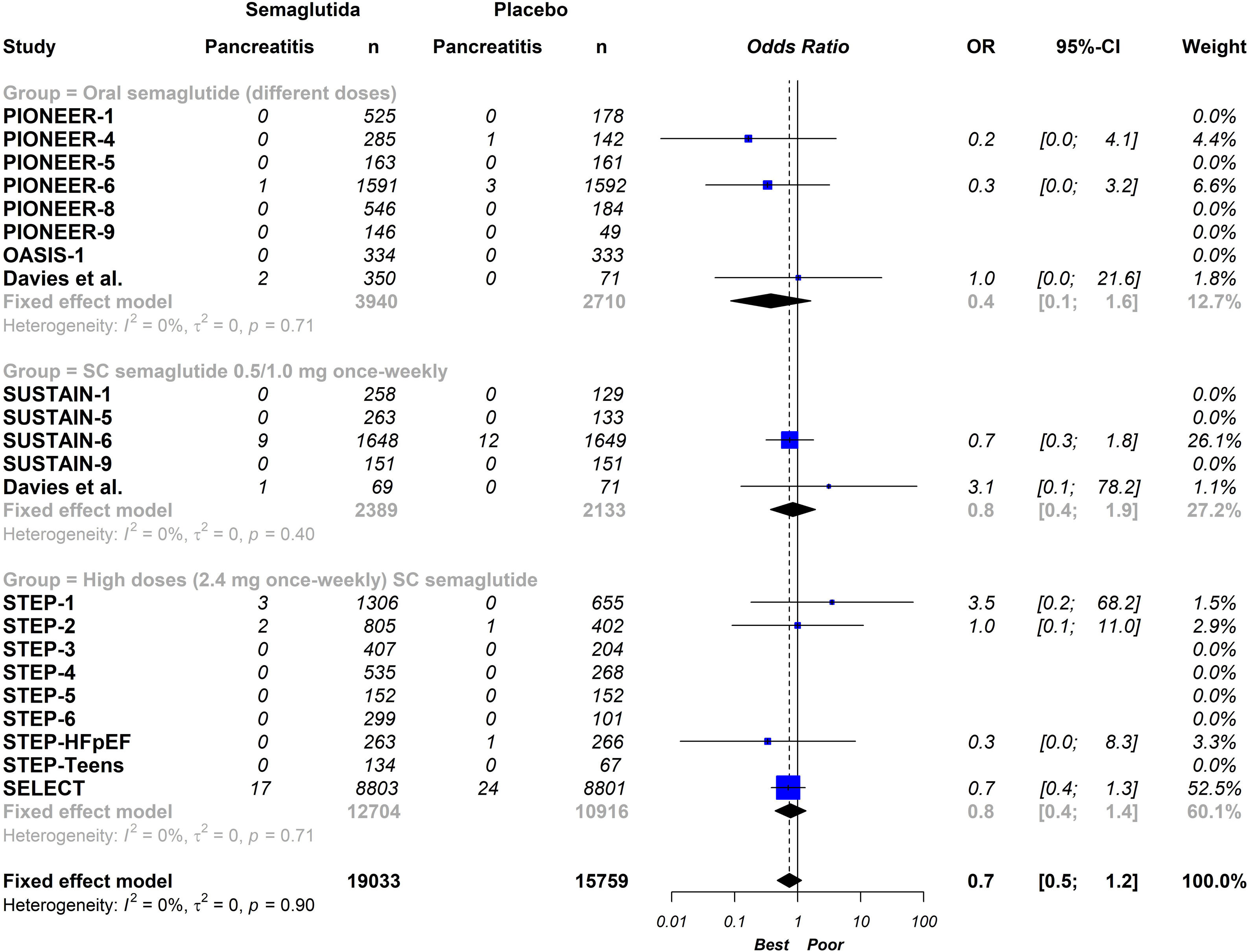
ResultsTwenty-one eligible trials of semaglutide, including 34,721 patients, were identified and considered eligible for the analyses. Globally, semaglutide therapy was not associated with an increased risk of acute pancreatitis (OR 0.7; 95% CI 0.5–1.2, I2 0%). When we analysed the studies according to the different schemes used, the results were similar (group with oral semaglutide: OR 0.40; 95% CI 0.10–1.60, I2 0%; group with low subcutaneous doses of semaglutide: OR 0.80; 95% CI 0.40–1.90, I2 0%; group with high subcutaneous doses of semaglutide: OR 0.70; 95% CI 0.50–1.20, I2 0%; interaction p-value=0.689).
ConclusionThis updated meta-analysis demonstrates that the use of semaglutide is not associated with an increased risk of acute pancreatitis compared to placebo. In the stratified analysis, the results were similar with the different semaglutide regimens analysed.
Algunas preocupaciones con respecto a la seguridad de la semaglutida aún persisten. El objetivo del presente metaanálisis actualizado es evaluar el riesgo de pancreatitis aguda con el uso de semaglutida, valorando los resultados según los diferentes esquemas terapéuticos.
MétodosRealizamos un metaanálisis actualizado de estudios aleatorizados y controlados con placebo, que hayan evaluado el uso de semaglutida e informaran la incidencia de pancreatitis aguda. Este metaanálisis se llevó a cabo de acuerdo con las directrices PRISMA. Se realizó un análisis global y estratificado según el esquema terapéutico utilizado. Se utilizó un modelo de efectos fijos.
ResultadosVeintiún ensayos clínicos fueron identificaron y considerados elegibles para este metaanálisis (34.721 pacientes). A nivel global, el tratamiento con semaglutida no se asoció con un mayor riesgo de pancreatitis aguda (OR 0,7; IC 95%: 0,5-1,2; I2 0%). Cuando analizamos los estudios según los diferentes esquemas utilizados, los resultados fueron similares (grupo con semaglutida oral: OR 0,40; IC 95% 0,10-1,60, I2 0%; grupo con dosis subcutáneas bajas de semaglutida: OR 0,80; IC 95% 0,40-1,90, I2 0%; grupo con altas dosis subcutáneas de semaglutida; OR 0,70; IC 95% 0,50-1,20, I2 0%; valor de p de interacción=0,689).
ConclusiónEl presente metaanálisis demostró que el uso de semaglutida no se asoció con un mayor riesgo de pancreatitis aguda en comparación con el placebo. En el análisis estratificado, los resultados fueron similares con los diferentes esquemas de semaglutida analizados.
Glucagon-like peptide 1 receptor agonists (GLP-1RAs) demonstrate glucose-lowering, weight-reducing, and favourable anti-inflammatory and metabolic effects.1 These emerging drug classes have been proven to reduce cardiovascular events in patients with type 2 diabetes mellitus (T2DM) who are at high cardiovascular risk or have established cardiovascular disease.2 In this context, current guidelines recommend GLP-1RAs as first-line antidiabetic therapies in patients with established cardiovascular disease or high/very high cardiovascular risk.3
Semaglutide is a potent GLP-1RA used in the treatment of T2DM, with demonstrated cardiovascular benefits. It is currently available in both subcutaneous and oral formulations.4 Furthermore, the impact of high doses of semaglutide on weight loss was explored in adults with overweight or obesity in the STEP programme.5 Recently, the cardiovascular benefit of high doses of semaglutide was also observed in patients with overweight or obesity at high cardiovascular risk but without T2DM.6
Despite the proven cardiovascular benefit of semaglutide, certain safety concerns still linger. A debate surrounds the potential association between semaglutide treatment and the risk of acute pancreatitis. This point is relevant since acute pancreatitis is an unpredictable and potentially life-threatening disease.7,8 Previous systematic reviews have explored the risk of acute pancreatitis with these drugs.9–11 However, these studies have evaluated different GLP-1RAs, involved comparisons between different hypoglycaemic drugs without including a placebo arm, did not analyse the different administration regimens of semaglutide, or failed to include the latest published studies.
The objective of this updated meta-analysis is to ascertain the risk of acute pancreatitis with the use of semaglutide, with a specific focus on evaluating the results according to the different administration regimens.
Material and methodsRegistrationThis meta-analysis was performed in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting systematic reviews.12 This systematic review was registered in PROSPERO [CRD42023485460].
Search and selection strategyA literature search was performed that identified randomised clinical trials of semaglutide published up to 12 November 2023. Two independent reviewers (WM and LB) searched the electronic PubMed/MEDLINE, Scielo, Embase and Cochrane Controlled Trials databases using the terms “semaglutide” or “GLP-1RAs” combined with “acute pancreatitis” or “gastrointestinal adverse effects” or “pancreatic adverse effects” and extracted data. Additionally, the authors also conducted a “snowball search” to find other articles.
Eligibility criteriaThis meta-analysis included all studies that met the following criteria: (a) comparisons of efficacy and safety for semaglutide versus placebo; (b) follow-up duration ≥3 months; (c) randomised clinical trials; (d) reporting the incidence of acute pancreatitis. Studies evaluating semaglutide with other antidiabetic drugs without considering a placebo group were excluded.
Quality assessmentPotential risks of bias were evaluated for all included trials, using a tool for randomised trials (RoB 2) developed for this purpose.13 This tool assesses bias in five different domains: bias arising from the randomisation, bias due to deviations from the intended intervention, bias due to missing outcome data, bias in the measurement of the outcome and bias in the selection of the reported result. These domain-level judgements provide the basis for an overall risk-of-bias judgement for the specific trial result being assessed. Each domain was rated as “High”, “Low” or “Some concerns” depending on the judgement of each author following the recommendations. Two authors determined the risk of bias for each article. Any disagreements were resolved with a third reviewer.
Outcome measures and statistical data analysisThe summary effect of semaglutide on the endpoint of acute pancreatitis was estimated. The diagnosis of acute pancreatitis required two of the following three features: abdominal pain consistent with acute pancreatitis, serum lipase and/or amylase activity at least three times greater than the upper limit of normal, and imaging findings characteristic of acute pancreatitis.
Measures of effect size were expressed as odds ratios (ORs) with 95% confidence intervals [95% CI] and the I2 statistic was calculated to quantify trial heterogeneity or inconsistency. Because heterogeneity was low, a fixed-effects model was chosen. To compare mean effects between subgroups, a Z-test was used. Statistical analyses were performed using the R software for statistical computing version 3.5.1 with additional specific packages.14 The level of statistical significance was set at a two-tailed alpha of 0.05.
Publication bias and sensitivity analysesPublication bias and sensitivity analyses were performed. In the first case, a funnel plot using the standard error (SE) by log OR was created. Egger's regression of intercept tests was also performed. In the second case, the analysis consists of replicating the results of the meta-analysis, in each step excluding one of the studies included in the review. If the results obtained are similar, both in the direction and magnitude of the effect and statistical significance, it indicates that the analysis is robust.
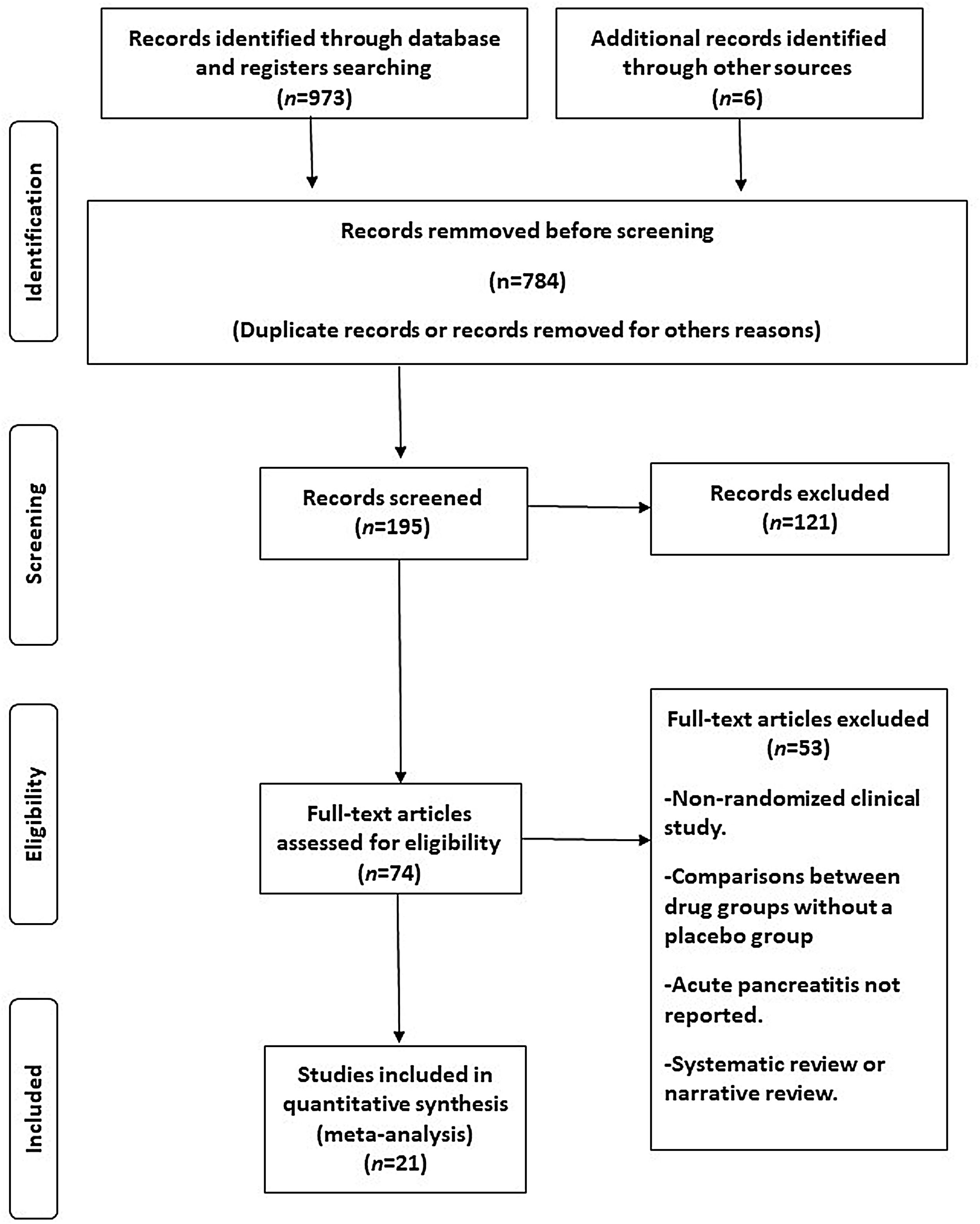
ResultsAfter a comprehensive screening process of the titles and abstracts, 973 articles were initially identified as potentially relevant to our study. Of these, 784 studies were excluded due to duplication or lack of alignment with the study's objectives. After a careful examination of the remaining articles, 174 studies were removed. A flow diagram illustrating the screening process is presented in Fig. 1.
Twenty-one eligible trials of semaglutide, including 34,721 patients, were identified and considered eligible for the analyses.8,15–34 Within these trials, 19,023 subjects were allocated to receive semaglutide and 15,688 subjects were assigned to the respective placebo group.
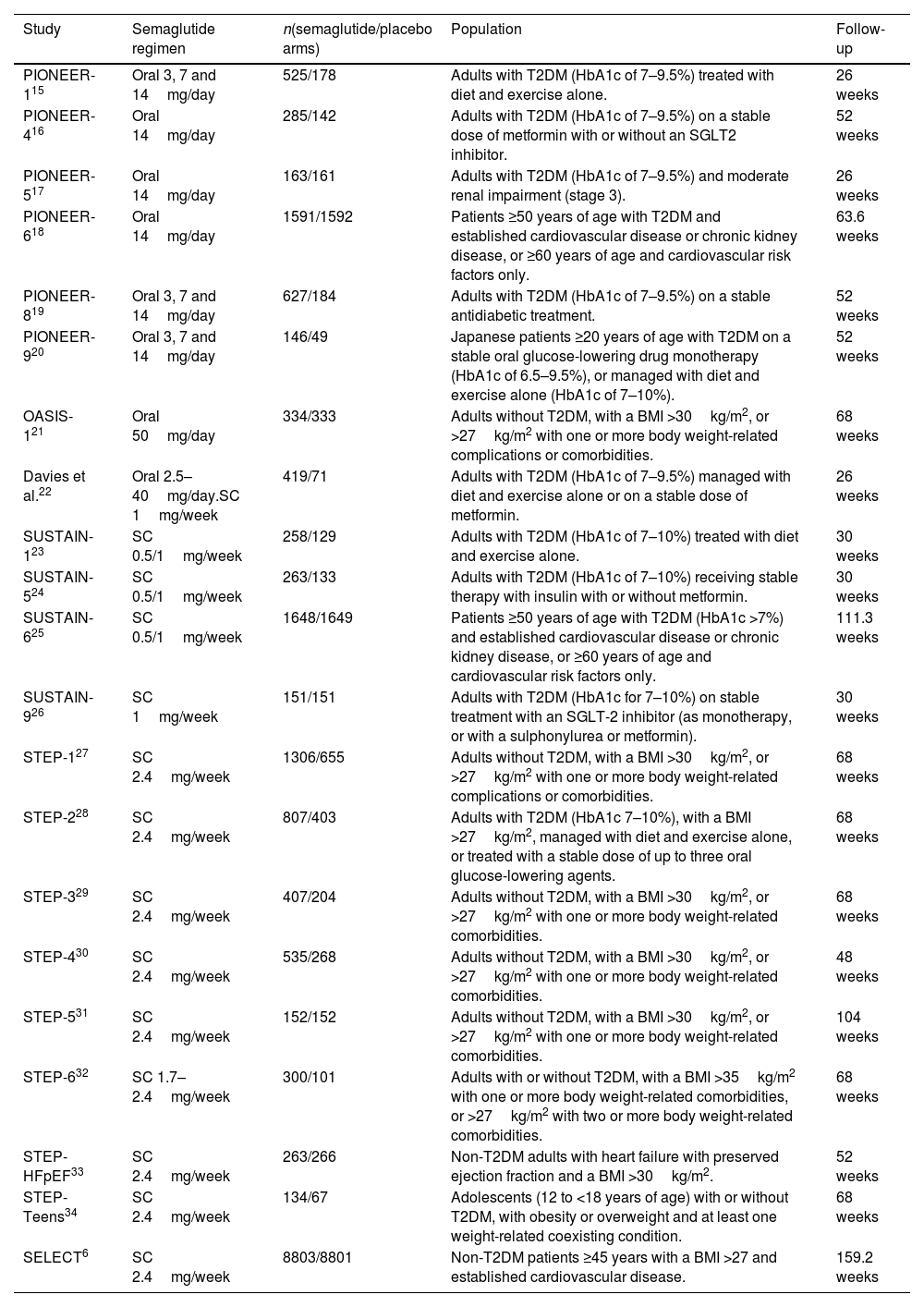
In total, seven studies assessed different oral doses of semaglutide.15–21 Additionally, 4 and 9 studies analysed low (0.5–1mg) and high (1.7–2.4mg) weekly subcutaneous doses of semaglutide, respectively.6,23–34 One study evaluated two active arms: daily oral semaglutide and weekly subcutaneous semaglutide at doses of 0.5–1mg.22 Overall, 12 studies included all patients with T2DM,15–20,22,24–26 while another 7 studies considered this condition as an exclusion criterion (instead studying patients with overweight or obesity).6,21,27–31 Two studies included patients with or without T2DM.32,34 Across all studies, a history of pancreatitis was consistently regarded as an exclusion criterion. The characteristics of the studies included in the analysis can be seen in Table 1.
Characteristics of the included studies.
| Study | Semaglutide regimen | n(semaglutide/placebo arms) | Population | Follow-up |
|---|---|---|---|---|
| PIONEER-115 | Oral 3, 7 and 14mg/day | 525/178 | Adults with T2DM (HbA1c of 7–9.5%) treated with diet and exercise alone. | 26 weeks |
| PIONEER-416 | Oral 14mg/day | 285/142 | Adults with T2DM (HbA1c of 7–9.5%) on a stable dose of metformin with or without an SGLT2 inhibitor. | 52 weeks |
| PIONEER-517 | Oral 14mg/day | 163/161 | Adults with T2DM (HbA1c of 7–9.5%) and moderate renal impairment (stage 3). | 26 weeks |
| PIONEER-618 | Oral 14mg/day | 1591/1592 | Patients ≥50 years of age with T2DM and established cardiovascular disease or chronic kidney disease, or ≥60 years of age and cardiovascular risk factors only. | 63.6 weeks |
| PIONEER-819 | Oral 3, 7 and 14mg/day | 627/184 | Adults with T2DM (HbA1c of 7–9.5%) on a stable antidiabetic treatment. | 52 weeks |
| PIONEER-920 | Oral 3, 7 and 14mg/day | 146/49 | Japanese patients ≥20 years of age with T2DM on a stable oral glucose-lowering drug monotherapy (HbA1c of 6.5–9.5%), or managed with diet and exercise alone (HbA1c of 7–10%). | 52 weeks |
| OASIS-121 | Oral 50mg/day | 334/333 | Adults without T2DM, with a BMI >30kg/m2, or >27kg/m2 with one or more body weight-related complications or comorbidities. | 68 weeks |
| Davies et al.22 | Oral 2.5–40mg/day.SC 1mg/week | 419/71 | Adults with T2DM (HbA1c of 7–9.5%) managed with diet and exercise alone or on a stable dose of metformin. | 26 weeks |
| SUSTAIN-123 | SC 0.5/1mg/week | 258/129 | Adults with T2DM (HbA1c of 7–10%) treated with diet and exercise alone. | 30 weeks |
| SUSTAIN-524 | SC 0.5/1mg/week | 263/133 | Adults with T2DM (HbA1c of 7–10%) receiving stable therapy with insulin with or without metformin. | 30 weeks |
| SUSTAIN-625 | SC 0.5/1mg/week | 1648/1649 | Patients ≥50 years of age with T2DM (HbA1c >7%) and established cardiovascular disease or chronic kidney disease, or ≥60 years of age and cardiovascular risk factors only. | 111.3 weeks |
| SUSTAIN-926 | SC 1mg/week | 151/151 | Adults with T2DM (HbA1c for 7–10%) on stable treatment with an SGLT-2 inhibitor (as monotherapy, or with a sulphonylurea or metformin). | 30 weeks |
| STEP-127 | SC 2.4mg/week | 1306/655 | Adults without T2DM, with a BMI >30kg/m2, or >27kg/m2 with one or more body weight-related complications or comorbidities. | 68 weeks |
| STEP-228 | SC 2.4mg/week | 807/403 | Adults with T2DM (HbA1c 7–10%), with a BMI >27kg/m2, managed with diet and exercise alone, or treated with a stable dose of up to three oral glucose-lowering agents. | 68 weeks |
| STEP-329 | SC 2.4mg/week | 407/204 | Adults without T2DM, with a BMI >30kg/m2, or >27kg/m2 with one or more body weight-related comorbidities. | 68 weeks |
| STEP-430 | SC 2.4mg/week | 535/268 | Adults without T2DM, with a BMI >30kg/m2, or >27kg/m2 with one or more body weight-related comorbidities. | 48 weeks |
| STEP-531 | SC 2.4mg/week | 152/152 | Adults without T2DM, with a BMI >30kg/m2, or >27kg/m2 with one or more body weight-related comorbidities. | 104 weeks |
| STEP-632 | SC 1.7–2.4mg/week | 300/101 | Adults with or without T2DM, with a BMI >35kg/m2 with one or more body weight-related comorbidities, or >27kg/m2 with two or more body weight-related comorbidities. | 68 weeks |
| STEP-HFpEF33 | SC 2.4mg/week | 263/266 | Non-T2DM adults with heart failure with preserved ejection fraction and a BMI >30kg/m2. | 52 weeks |
| STEP-Teens34 | SC 2.4mg/week | 134/67 | Adolescents (12 to <18 years of age) with or without T2DM, with obesity or overweight and at least one weight-related coexisting condition. | 68 weeks |
| SELECT6 | SC 2.4mg/week | 8803/8801 | Non-T2DM patients ≥45 years with a BMI >27 and established cardiovascular disease. | 159.2 weeks |
BMI: body mass index; HbA1c: glycosylated haemoglobin; SC: subcutaneous; SGLT-2: sodium glucose cotransporter-2 inhibitors; T2DM: type 2 diabetes mellitus.
The quality of the studies evaluated can be seen in Supplementary Figure 1.
On a global scale, this updated meta-analysis shows that semaglutide therapy is not associated with an increased risk of acute pancreatitis (OR 0.70; 95% CI 0.50–1.20, I2 0%) (Fig. 2). When analysing the studies based on the different administration schemes, the results remain consistent (group with oral semaglutide: OR 0.40; 95% CI 0.10–1.60, I2 0%; group with low subcutaneous doses of semaglutide: OR 0.80; 95% CI 0.40–1.90, I2 0%; group with high subcutaneous doses of semaglutide; OR 0.70; 95% CI 0.50–1.20, I2 0%; interaction p-value=0.689) (Fig. 3).
The graphical (Supplementary Figure 2) and analytical evaluation (Egger's asymmetry test) do not suggest publication bias (p=0.811). The sensitivity analysis showed the same directionality and magnitude as the overall results when studies were excluded one by one (Supplementary Figure 3).
DiscussionThis updated meta-analysis of randomised, placebo-controlled studies encompasses the entirety of the available evidence examining the association between different semaglutide regimens and the incidence of acute pancreatitis. The findings of this study did not reveal an increased risk of acute pancreatitis with the use of semaglutide. The test for subgroup differences (interaction p-value) indicates a statistically non-significant subgroup effect, suggesting that the regimen used does not modify the effect of semaglutide treatment.
Both oral and subcutaneous semaglutide have been linked with gastrointestinal disturbances, such as nausea, vomiting and diarrhoea.35 However, the data on the association between semaglutide use and the risk of acute pancreatitis remains controversial. Proposed mechanisms of GLP-1RA-induced acute pancreatitis include pancreatic duct gland hyperplasia, pancreatic ductal obstruction leading to proinflammatory reactions, acinar cell hypertrophy, and pancreatic vascular injury.36 Additionally, another mechanism seen with the use of these drugs could be the increased risk of gallbladder or biliary diseases, especially when used in higher doses, for longer periods of time, and for weight loss.37
Previous animal studies suggested a risk of acute pancreatitis after GLP-1RA-based treatment.38 In addition, an initial analysis of the Food and Drug Administration's (FDA) adverse event reporting databases suggested an increased risk for acute pancreatitis with GLP-1RA-based therapy.39 It is important to note that this type of data analysis may not be the ideal method for comparing adverse event rates between medications. Well known limitations, such as incomplete data recording or the presence of reporting biases can impact the reliability of these findings.
In the subsequent years, several observational studies have yielded conflicting results.40–43 Notably, most of the available information is drawn from the subgroup of exendin-4-based drugs, which differ structurally from human GLP-1. Inconsistencies in findings may be attributed to factors such as limited statistical power, insufficient duration of follow-up, or inadequate adjustment for confounding variables. This is particularly relevant given that obesity and T2DM themselves are risk factors for pancreatitis.44,45 Additionally, the prescription of GLP-1RAs is often associated with poor glycaemic control, which could be caused by occult pancreatic diseases.46,47 The treatment selection process introduces intrinsic sources of imbalance and confounding, leading to potential distortions in the results. Therefore, randomised, controlled clinical trials remain the gold standard for such assessment.
Since acute pancreatitis is a relatively uncommon complication, individual clinical studies are usually underpowered to detect differences between groups. In this context, our meta-analysis incorporated all reported cases of acute pancreatitis from clinical trials comparing semaglutide therapy versus placebo. In keeping with our findings, previous meta-analyses that included different GLP-1RAs did not reveal a significant association between the use of these drugs and the incidence of acute pancreatitis.9,11 Unlike our meta-analysis, these works assessed different GLP-1RAs (exendin-4-based drugs and human GLP-1 analogues) and focused on studies that had included patients with T2DM. It is important to note that our meta-analysis analysed different therapeutic regimens of semaglutide, including different routes of administration and doses. While subcutaneous semaglutide, administered as a once-weekly injection, was the first format available for clinical use, recently, semaglutide has been developed into an oral formulation utilising innovative technology.48 The pharmacokinetic and pharmacodynamic distinctions between these formulations may lead to differences in the incidence of adverse events, including acute pancreatitis.49 Additionally, the inclusion of studies evaluating patients with or without diabetes is noteworthy, as the association between the use of the drug and the occurrence of acute pancreatitis could vary. Our study's stratified analysis according to the different therapeutic schemes essentially divided the population based on T2DM status, finding no differences in the results.
Finally, common biochemical markers used for the diagnosis of acute pancreatitis in clinical practice include serum amylase and lipase.50 Some authors have reported that asymptomatic elevations of these biomarkers could be observed after administering GLP-1RAs.51,52 This “subclinical” phenomenon is not necessarily associated with a clinical pancreatic event. Such is the case of some studies with liraglutide.53,54 In this case, liraglutide produced reversible increases in amylase/lipase activity that did not predict the onset of acute pancreatitis. However, more information is necessary to clarify this point.
This meta-analysis has some limitations. Firstly, there was clinical heterogeneity due to the characteristics of the populations and the different follow-up periods. However, statistical heterogeneity was low, and the sensitivity analysis showed robust results. Secondly, the number of events reported was very low. Furthermore, many studies did not report cases of acute pancreatitis in any of the arms analysed. Finally, all studies excluded individuals with a history of pancreatitis. The results could be different if we consider real-life patients who do not meet the strict inclusion criteria observed in clinical trials.
ConclusionThis updated meta-analysis of randomised clinical trials demonstrated that the use of semaglutide in patients with or without T2DM was not associated with an increased risk of acute pancreatitis compared to placebo. In the stratified analysis, the results were similar with the different semaglutide regimens analysed.
Ethical approvalThis article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Authors’ contributionsWM and LB participated in the conception and design of the research. WM, LB and ALC participated in the data collection. The interpretation of the data and the statistical analysis was done by WM and ML. WM and JPN drafted the manuscript. All authors performed a critical review of the final document. All authors have read and agreed to the published version of the manuscript.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestWM, ALC and ML have served as a speaker for Novo Nordisk. The rest of the authors have no conflicts to declare.