Severe traumatic brain injury (sTBI) patients often experience stress hyperglycaemia, which can lead to negative outcomes. This study aims to introduce an effective insulin infusion protocol specifically designed for sTBI patients.
MethodsData was collected from all sTBI patients during two periods: 1 October 2019 to 30 April 2020, and 1 June 2020 to 31 December 2020. In May 2020, a new insulin infusion protocol was implemented. Blood glucose management, infection, coagulation, and prognosis were compared in these two periods.
Result195 patients were included, with 106 using the new protocol. The proportion of hyperglycaemia decreased from 40.04% to 26.91% (P<0.05), and the proportion of on-target blood glucose levels increased from 35.69% to 38.98% (P<0.05). Average blood glucose levels decreased from 9.98±2.79mmol/L to 8.96±2.82mmol/L (P<0.05). There was no substantial increase in hypoglycaemia, which remained controlled below 1%. The new protocol positively influenced glucose concentration and dispersion trends. There were no significant differences in catheter-related infections, antibiotic use, mechanical ventilation (MV) duration, length of stay in ICU, Glasgow Outcome Scale (GOS), or mortality. However, the conventional protocol group had a higher coagulation tendency (R-value of thromboelastography 4.80±1.35min vs. 5.52±1.87min, P<0.05), with no difference in deep vein thrombosis (DVT) incidence.
ConclusionOur findings suggest that a customized insulin infusion process for sTBI patients can effectively manage blood glucose. While there is no significant improvement in infection control or prognosis, it may have a positive impact on coagulation without affecting the occurrence of DVT.
Los pacientes con lesiones cerebrales traumáticas graves (sTBI) a menudo experimentan hiperglucemia por estrés, lo que puede llevar a resultados negativos. Este estudio tiene como objetivo presentar un protocolo efectivo de infusión de insulina diseñado específicamente para pacientes con sTBI.
MétodosSe recopilaron datos de todos los pacientes con sTBI en 2 períodos: del 1 de octubre de 2019 al 30 de abril de 2020 y del 1 de junio de 2020 al 31 de diciembre de 2020. En mayo de 2020, se implementó un nuevo protocolo de infusión de insulina. Se compararon la gestión de la glucosa en sangre, la infección, la coagulación y el pronóstico en estos 2 períodos.
ResultadosSe incluyeron 195 pacientes, de los cuales 106 utilizaron el nuevo protocolo. La proporción de hiperglucemia disminuyó del 40,04 al 26,91% (p<0,05), y la proporción de niveles objetivo de glucosa en sangre aumentó del 35,69 al 38,98% (p<0,05). Los niveles medios de glucosa en sangre disminuyeron de 9,98±2,79mmol/L a 8,96±2,82mmol/L (p<0,05). No hubo un aumento sustancial en la hipoglucemia, que se mantuvo controlada por debajo del 1%. Esto influyó positivamente en las tendencias de concentración y dispersión de la glucosa. No hubo diferencias significativas en infecciones relacionadas con el catéter, uso de antibióticos, duración de la ventilación mecánica, tiempo de estancia en la UCI, resultados de la escala de Glasgow (GOS) o mortalidad. Sin embargo, el grupo con el protocolo convencional tenía una mayor tendencia a la coagulación (valor R de tromboelastografía 4,80±1,35min vs. 5,52±1,87min, p<0,05), sin diferencia en la incidencia de trombosis venosa profunda.
ConclusiónNuestros hallazgos indican que un proceso de infusión de insulina personalizado para pacientes con sTBI puede gestionar eficazmente la glucosa en sangre. Si bien no hay una mejora significativa en el control de infecciones o el pronóstico, puede tener un impacto positivo en la coagulación sin afectar la aparición de trombosis venosa profunda.
Traumatic brain injury (TBI) is the leading cause of death and disability among all trauma-related injuries globally.1 One-third to half of trauma-related deaths are primarily caused by TBI, affecting 15–20/100,000 individuals annually.1 Severe traumatic brain injury (sTBI) accounts for 8% of all TBI worldwide, with approximately 5.48 million people suffering from severe traumatic brain injury annually.1 Hyperglycaemia frequently occurs in the early stages following TBI and is associated with adverse outcomes, potentially through promoting oxidative stress pathways and inducing neuroinflammation.2 However, glucose is the main energy source for brain cells, and hypoglycaemia exacerbates critical neurocognitive dysfunction and exerts a strong dose-dependent effect on the mortality rate of critically ill patients.2 The optimal blood glucose target for patients with sTBI therefore remains largely unclear.2,3 At the beginning of this century, intensified insulin therapy was reported to improve the prognosis of critically ill patients by maintaining blood glucose levels at 4.4–6.1mmol/L.4 However, the 2009 NICE-SUGAR study suggested that intensified insulin therapy did not improve prognosis but increased the incidence of hypoglycemia.5 In 2018, a review of blood glucose control for TBI reached similar conclusions.2 The latest brain microdialysis research suggests that insulin indeed lowers blood glucose and that the intensified blood glucose control method is associated with brain energy crisis in TBI patients and the deterioration of their prognosis.6 Therefore, the current blood glucose control target for sTBI patients mainly adheres to the recommendations of the American Diabetes Association, which is to control blood glucose levels between 7.8 and 10mmol/L.2,5,7
For critically ill patients, continuous intravenous insulin infusion is the most effective method for achieving glycaemic targets. Paper-based or electronic protocols can be used for glucose management, but there is still a lack of standardized insulin infusion plans worldwide, and the same applies to sTBI patients.7 Computer-based insulin infusion protocols have shown advantages by calculating insulin doses based on current glucose levels and trends, but such commercial options are expensive and complex to operate and have not been widely used.8 Many classical paper-based protocols have been designed, each with advantages and disadvantages. The four most common types are the Yale protocol, Leuven protocol, SPRINT protocol, and NICE-SUGAR protocol.5,9,10 The blood glucose targets of the first three protocols are low and require insulin loading, which can easily cause hypoglycemia,9,10 although some studies suggest that the Yale protocol is associated with better outcomes in certain patient populations.9 The NICE-SUGAR protocol, or Normoglycaemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation, is designed to maintain normoglycaemia in critically ill patients through relatively loose glucose targets and lower monitoring frequency, potentially posing risks of hyperglycaemia (Supplementary Material 1). In contrast, the Yale protocol, emphasizing precision in blood glucose control, employs insulin loading to achieve lower glucose levels in critically ill individuals and has been associated with better outcomes in specific patient populations (Supplementary Material 2). However, during the acute phase of traumatic brain injury, certain nutritional support characteristics, such as high energy demands in the early stages of trauma, can increase resting energy consumption by 1–2 times the baseline prediction. Patients with sTBI may remain in a coma for a long time, and while their gastrointestinal tract is relatively intact, it may have reduced motility, requiring early and continuous enteral nutrition support.11 This requires early, adequate, and continuous insulin infusion and the development of an insulin infusion plan tailored to the characteristics of TBI. To our knowledge, this is the first study report to introduce a convenient and safe paper-based insulin infusion protocol that applies to sTBI patients, combining the advantages of the NICE-SUGAR and Yale protocols.
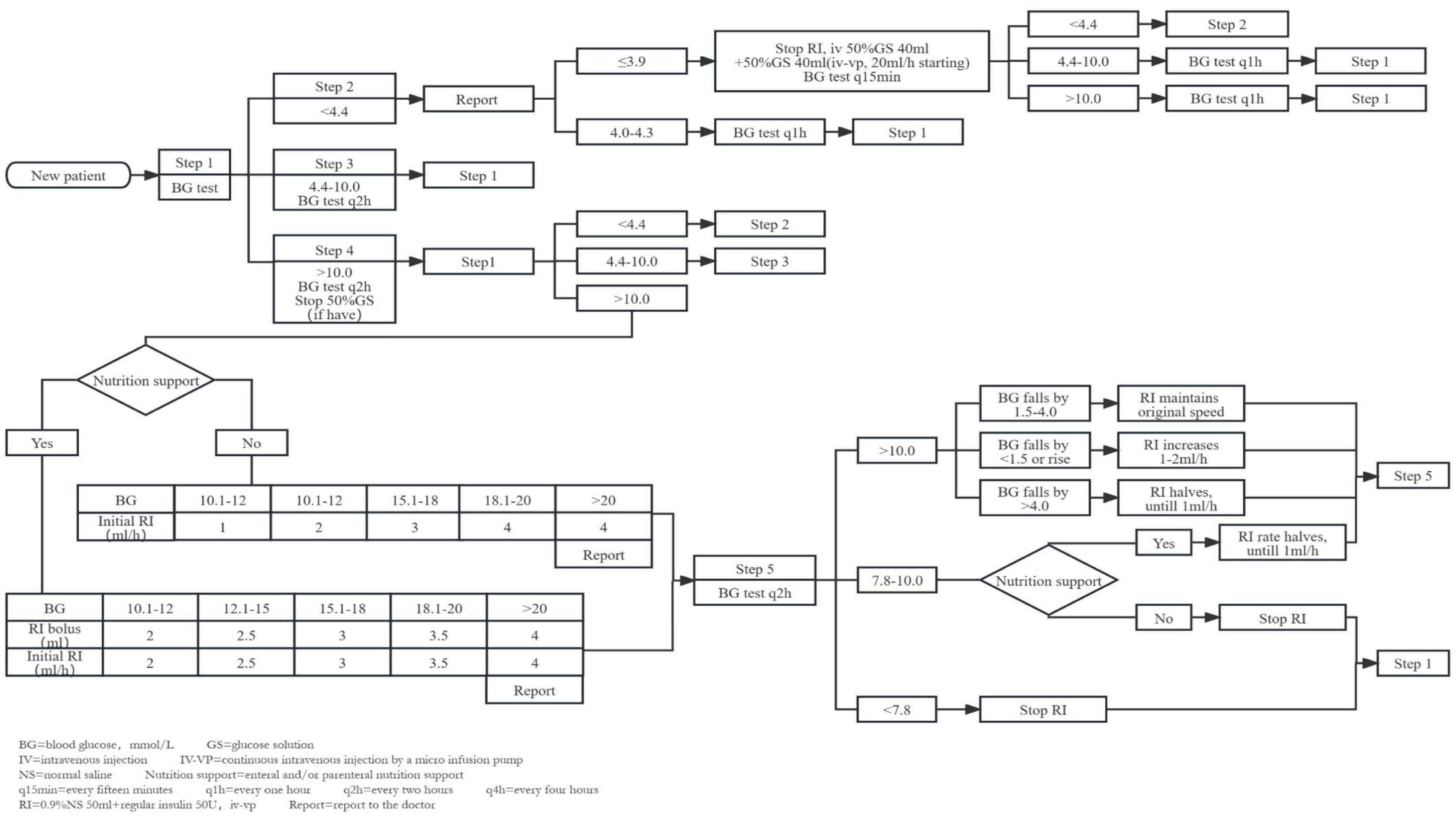
MethodsInsulin infusion protocolIn May 2020, we developed a novel insulin infusion protocol, drawing inspiration from the NICE-SUGAR study's high blood glucose treatment approaches5 and incorporating elements from the Yale protocol.12 In hyperglycaemic cases where nutritional support was provided, we administered an initial insulin bolus. Conversely, for those without nutritional support, we implemented continuous insulin micro-pump infusion. Insulin dosage adjustments were guided by absolute glucose values and glucose fluctuations. Distinct insulin adjustment strategies were applied when enteral and parenteral nutritions were temporarily suspended (Fig. 1).
The new insulin infusion protocol for sTBI patients. 1. The protocol mainly consists of the following steps: (A) Categorization of patients based on the first blood glucose measurement into three groups: hypoglycaemia (<4.4mmol/L), acceptable glycaemic range (4.4–10.0mmol/L), and hyperglycaemia (>10.0mmol/L). (B) Hypoglycaemia management process: Hypoglycaemic patients enter the hypoglycaemia management process directly, which includes appropriate interventions such as adjusting nutrition support and insulin dosage to correct the low blood glucose levels. (C) Retesting of blood glucose for patients in acceptable glycaemic range and hyperglycaemia to confirm the need for insulin infusion. (D) Hyperglycaemia management process: Patients with sustained hyperglycaemia for two consecutive blood glucose tests enter the hyperglycaemia management process. Depending on whether nutritional support is provided, an initial insulin bolus may be required, and the insulin maintenance dosage is adjusted according to the range of blood glucose changes. (E) Insulin dosage adjustment with changes in nutritional support: Insulin dosage is reduced when nutritional support is discontinued, with the reduction amount being dependent on the use of enteral or parenteral nutrition. (2) Blood sampling: For patients using vasoactive medications, we collect arterial blood from an arterial line. For other patients, we use capillary blood samples. (3) Monitoring frequency: We employ intermittent blood glucose monitoring, typically every 2h (q2h). If the blood glucose remains within the target range (7.8–10.0mmol/L) continuously for 24h, the frequency is changed to every 4h (q4h). In cases of hypoglycaemia, the monitoring frequency may be increased, such as every 15min (q15min) or every hour (q1h). The doctor has the authority to increase the blood glucose monitoring frequency based on the specific condition of the patient. (4) The protocol is not suitable for oral intake patients.
Following the 2019 European Society for Clinical Nutrition and Metabolism Standard Operating Procedures,13 we have established the following nutrition therapy:
- 1)
Initiate early nutritional support (within 48h) for patients with haemodynamically stable conditions; delay initiation for those with unstable haemodynamics.
- 2)
Assess the patient's gastrointestinal function based on the acute gastrointestinal injury (AGI) grading system. For AGI≤I, administer an initial 20ml/h of standard formulas; for AGI Grades II-III, provide an initial 10ml/h of peptide-based formulas; initiate parenteral nutrition within 3–7 days for AGI Grade IV or in the presence of contraindications to enteral nutrition.
- 3)
During enteral nutrition, nurses should assess the patient's tolerance every 4h. Adjust the enteral nutrition infusion rate and dosage based on the assessment until the therapeutic target value is reached (target calories 25–30kcal/kg/d).
- 4)
If the actual calorie intake cannot reach 60% of the target value after 7–10 days, supplementary parenteral nutrition should be implemented.
Data were collected from all patients with sTBI (GCS≤8) admitted to our neurocritical care unit from 1 October 2019 to 30 April 2020 and from 1 June 2020 to 31 December 2020. Patients with concomitant ketoacidosis and hyperosmolar coma were excluded. During the first phase, the conventional insulin infusion protocol (NICE-SUGAR protocol) was used, and in May 2020, the new insulin infusion protocol was adopted for the second phase.
The primary outcomes were the proportion of hyperglycaemia (>10.0mmol/L), the proportion of on-target glucose measurements (7.8–10.0mmol/L), the proportion of hypoglycaemia (<4.0mmol/L), glucose variability, and the concentration (median and quartile values of mean blood glucose levels in individual patients) and dispersion (calculating the standard deviation for each patient, then reporting the median and interquartile range of standard deviations for the population) of glucose trends.
The secondary outcomes included infection (incidence of catheter-related infections and antibiotic use density), coagulation (TEG R-value of and incidence of DVT), and prognosis (duration of mechanical ventilation, LOS in ICU, GOS after 3 months, and 90-day mortality) -related indicators.
In addition, we randomly selected 30–50 patient-days every month to calculate the accuracy of the process execution, thereby assessing the protocol adherence.
Statistical methodsAll statistical analyses were performed using SPSS 25.0 (IBM Corporation, Armonk, NY, USA). Student's t-test was used to compare the means between the different groups, and the Chi-squared test was used to compare enumeration data. A P-value<0.05 was statistically significant. The incidence of hypoglycaemia was quantified by calculating the percentage of patient days during which at least one blood glucose measurement was below 4.0mmol/L.
ResultsParticipant characteristics195 patients were included in our analysis; 106 patients adhered to the new protocol, and 89 patients followed the conventional protocol. Two patients under the new protocol received an insulin infusion twice during their admission. Our analysis included each insulin infusion, leading to 28,493 blood glucose time points and 2937 patient days.
Basic clinical characteristics of the two groups of patientsThere were no differences in basic information such as gender, age, BMI, glycosylated haemoglobin, and initial blood glucose level between the two groups. Moreover, disease severity as assessed by the APACHE II score was comparable. There were no significant differences in risk factors for poor glucose control, such as nutritional route, enteral nutrition formulas, glucocorticoids, vasoactive drugs, haemodialysis, or blood transfusion (Table 1). All patients were comatose and required early invasive mechanical ventilation, but their gastrointestinal tracts were intact.
Baseline information and characteristics of patients.
| Conventional protocol | New protocol | Statistics | |
|---|---|---|---|
| Number of patients | 89 | 106 | |
| Men/women | 69/20 | 73/33 | NS |
| Age (years) | 56.94±16.08 | 56.07±18.74 | NS |
| BMI (kg/m2) | 22.99±3.30 | 22.96±3.75 | NS |
| HbA1c (%) | 6.94±3.77 | 5.81±0.79 | NS |
| Admission glucose (mmol/L) | 9.88±3.59 | 8.87±3.66 | NS |
| APACHE-II score | 19.43±6.99 | 18.54±6.57 | NS |
| No/enteral feeding/enteral+parenteral | 15/61/13 | 18/70/18 | NS |
| ENS(SP)/ENS(TPF)/ENE(TP-HE) (500ml) | 181/763/387 | 269/1049/467 | NS |
| Glucocorticoids | 42 (47.19%) | 47 (44.34%) | NS |
| Vasopressor therapy | 61 (68.54%) | 66 (62.26%) | NS |
| Hemodialysis | 0 | 0 | NS |
| Blood transfusion | 22 (24.72%) | 26 (24.53%) | NS |
Data are presented as numbers as mean±SD.
BMI: body mass index; APACHE-II: acute physiology and chronic health evaluation II; ENS(SP): enteral nutritional suspension (short peptide); ENS(TPF): enteral nutritional suspension (total protein fibre); ENE(TP-HE): enteral nutritional emulsion (total protein-high energy).
There were no statistically significant differences in gender, age, BMI, HbA1c, glucose on admission, APACHE-II score, nutritional route, enteral nutrition formulas, blood transfusion, glucocorticoids, vasopressor therapy, or haemodialysis between the two groups.
There was a significant decrease in the proportion of hyperglycaemia (>10.0mmol/L) from 40.04% to 26.91% following the new protocol, along with a significant increase in the proportion of on-target blood glucose levels (7.8–10.0mmol/L) from 35.69% to 38.98% (P<0.05). Additionally, the mean blood glucose level decreased significantly from 9.98±2.79 (mmol/L) to 8.96±2.82 (mmol/L) in the new protocol group (P<0.05). However, there was no substantial increase in hypoglycaemia, which remained successfully controlled below 1% (Table 2). The introduction of the new protocol did not have a significant impact on the variability of glucose levels. However, notable changes were observed in the concentration and dispersion of glucose trends. The median and quartile values of the mean blood glucose levels were 8.47 (7.62–9.40) mmol/L compared to 9.21 (8.18–11.3) mmol/L before the protocol implementation (P<0.05). Similarly, the median and quartile values of the standard deviations were 1.81 (1.32–2.86) mmol/L compared to 2.09 (1.61–2.99) mmol/L previously (P<0.05) (Table 2).
The primary outcomes and protocol adherence.
| Conventional protocol | New protocol | Statistics | |
|---|---|---|---|
| Blood glucose (mmol/L) | |||
| >10.0 | 4924 (40.04%) | 4358 (26.91%) | P<0.05* |
| 7.8–10.0 | 4389 (35.69%) | 6313 (38.98%) | P<0.05* |
| <4.0 | 8 (0.63%) | 16 (0.96%) | NS |
| MBG (mmol/L) | 9.98±2.79 | 8.96±2.82 | P<0.05* |
| IQR of MBG (mmol/L) | 9.21 (8.18–11.3) | 8.47 (7.62–9.40) | P<0.05* |
| IQR of SD (mmoL/L) | 2.09 (1.61–2.99) | 1.81 (1.32–2.86) | P<0.05* |
| Coefficient of variation (%) | 22.8 (18.3–27.5) | 20.9 (17.9–25.3) | NS |
| Protocol adherence | 2253 (92.18%) | 3746 (97.30%) | P<0.05* |
Data are presented as numbers as mean±SD or as median (25–75th percentile).
MBG: mean blood glucose; IQR: interquartile range; SD: standard deviations.
The proportion of hyperglycaemia (>10.0mmol/L) decreased from 40.04% to 26.91%, while the proportion of on-target blood glucose levels (7.8–10.0mmol/L) increased from 35.69% to 38.98% (P<0.05). Moreover, the mean blood glucose level significantly decreased from 9.98±2.79mmol/L to 8.96±2.82mmol/L (P<0.05). However, there was no significant increase in hypoglycaemia, which remained successfully controlled below 1%. Although the new protocol did not have a significant impact on the variability of glucose levels (20.9% vs. 22.8%), significant changes were observed in the concentration and dispersion of glucose trends. The median and quartile values of the mean blood glucose levels were 8.47 (7.62–9.40) mmol/L compared to 9.21 (8.18–11.3) mmol/L before the protocol implementation (P<0.05). Similarly, the median and quartile values of the standard deviations were 1.81 (1.32–2.86) mmol/L compared to 2.09 (1.61–2.99) mmol/L previously (P<0.05). The new protocol had higher protocol adherence (97.30% vs. 92.18%, P<0.05).
The incidence of catheter-related infections and antibiotic use density were comparable between the two groups. The duration of MV and LOS in ICU were similar. In terms of long-term prognosis, there was no difference in 90-day mortality or GOS after 3 months. However, the conventional group exhibited a hypercoagulable state, as evidenced by a difference in TEG R-value compared to the new protocol group (4.80±1.35min vs. 5.52±1.87min, P<0.05). Despite this, there were no notable differences in the incidence of venous thromboembolism between the two groups (Table 3).
The secondary outcomes.
| Conventional protocol | New protocol | Statistics | |
|---|---|---|---|
| CAI | 14 (15.73%) | 9 (8.49%) | NS |
| AUD | 74.37 | 74.91 | NS |
| R value of TEG (min) | 4.80±1.35 | 5.52±1.87 | P<0.05* |
| DVT | 22 (24.72%) | 38 (35.85%) | NS |
| Duration of MV | 9.71±15.31 | 10.22±8.95 | NS |
| LOS in ICU (days) | 13.48±16.41 | 14.35±12.29 | NS |
| GOS | 2.85±1.23 | 2.84±1.05 | NS |
| Mortality | 15 (16.85%) | 11 (10.38%) | NS |
Data are presented as numbers as mean±SD.
CAI: catheter associated infection; AUD: antibiotics use density; TEG: thrombelastography; DVT: deep vein thrombosis; MV: mechanical ventilation; LOS in ICU: length of stay in ICU; GOS: Glasgow outcome scale.
There was no significant difference between the two groups in terms of infection control measures, such as the incidence of catheter-related infections and antibiotic use density. The duration of MV and LOS in ICU were similar. In terms of prognosis, there was no difference in duration of MV, LOS in ICU, GOS after 3 months, and 90-day mortality. The TEG R-value differed between the two groups, with the conventional group presenting a hypercoagulable state (4.80±1.35min vs. 5.52±1.87min, P<0.05), but there was no difference in the incidence of DVT.
The accuracy of the process execution increased from 92.18% to 97.30% (P<0.05) (Table 2).
DiscussionAlong with metabolic characteristics common to critically ill patients, sTBI patients exhibit unique features, including feeding disorders due to a high prevalence of comatose patients with a GCS≤8 and dysphagia.14 Moreover, this patient population also experiences poor gastrointestinal tolerance due to impaired brain–gut axis regulation and weakened gastric and intestinal motility due to deep sedation, hypothermia therapy, etc.14,15 Importantly, our patients had an intact gastrointestinal system, and more than 80% could receive enteral nutrition. However, to ensure the effectiveness of enteral feeding, the feeding pump had to be gradually increased in speed and supplemented with prokinetic drugs and post-pyloric feeding. Parenteral nutrition was used sparingly and in combination with enteral nutrition only when enteral nutrition did not meet 60% of the patient's needs after 7 days.13 No patients received total parenteral nutrition, which could help to reduce hyperglycaemia. Additionally, due to the need for a higher mean arterial pressure (≥85mmHg) to maintain cerebral perfusion pressure, over 60% of patients were given vasopressors, including those with central diabetes insipidus who received posterior pituitary hormone. An increasing body of evidence suggests that haemodynamic instability significantly impacts glucose and lipid metabolism and nutritional support methods.13 Given these unique features, it is necessary to develop a specialized insulin infusion protocol for sTBI patients. The new protocol combines the characteristics of the NICE-SUGAR and Yale protocols and includes actively administering insulin boluses and more maintenance doses when nutritional support is available. This significantly reduces the proportion of hyperglycaemia and increases the proportion of on-target blood glucose measurements. When nutritional support is stopped, insulin dose reduction is based on whether enteral or parenteral nutrition is used to avoid hypoglycaemia. In line with the blood glucose assessment system,16 we also evaluated the coefficient of variation, and concentration and dispersion of blood glucose trends. We found that the new protocol could effectively control blood glucose within the target range.
In terms of secondary outcomes, we evaluated clinical indicators such as infection, coagulation, and prognosis. In our study, the new protocol did not reduce infections or improve outcomes, which is consistent with the literature.2 Studies have shown that hyperglycaemia is associated with coagulation disorders and the activation of a hypercoagulable state after TBI, as stress-induced hyperglycaemia increases the expression of soluble tissue factor and the generation of thrombin-antithrombin complexes.17 We found that the conventional protocol group had a lower R-value, indicating a higher tendency to coagulate than the new protocol group, but there was no significant difference in the incidence of DVT between the two groups.
The notable increase in protocol adherence likely contributed to our successful glucose management. To enhance compliance with the protocol during the COVID-19 pandemic, we utilized online videos and presentations to introduce the new protocol to all nursing staff. Offline training was provided by nursing team leaders who addressed daily inquiries. Online, the designer summarized common issues and offered one-on-one assistance to individuals who made protocol errors, analysing the underlying reasons and promoting compliance. By combining capillary and arterial blood sampling and adjusting monitoring frequency, we ensure adherence to blood glucose monitoring requirements while improving nurses’ workflow.16 Placing the protocol on each nurse's desk further enhances convenience and timeliness in protocol adherence.
Indeed, there were some limitations to this study. First, the retrospective design limits its ability to establish causality, and further prospective studies are needed. Moreover, insulin doses were adjusted based on blood glucose values, not the type of enteral nutrition formula and the rate of enteral nutrition infusion, to achieve better blood glucose control. Finally, as the study did not include any TBI patients who required haemodialysis, the protocol may not apply to this patient population.
ConclusionIn conclusion, sTBI patients have unique metabolic characteristics and require specialized blood glucose management protocols. The new protocol can effectively manage blood glucose in sTBI patients. It was found to have no impact on the incidence of infections or patient outcomes, while potentially contributing to improved coagulation. However, there was no observed effect on the incidence of DVT.
ParticipantsThe authors would also like to thank the patients for participating in this study, and thank Dr. Xinkun Shen and Dr. Liangmiao Chen for their help in the case discussion.
Approval of the research protocolThe research protocol has been approved by the Institutional Review Board of Third Affiliated Hospital, Wenzhou Medical University (a comprehensive tertiary Grade A hospital). A copy of the written consent is available for review by the Editor of this journal.
Informed consentThe informed consent of the patients has been obtained for this study, and the signed consent forms are attached as accompanying documents.
FundingThis work was supported by Wenzhou Science and Technology Bureau Project Fund (No. Y20180628) and Zhejiang Provincial Medical and Health Science and Technology Project (No. 2021KY1082).
Conflict of interestAll authors contributed to the discussion, writing and reviewing the manuscript and all authors have approved the final manuscript. All authors declare no conflict of interest.
The following are the supplementary data to this article: