To compare the performance of maternal body fat index (BFI) assessed during the first 20+6 weeks among 138 pregnant women in an ultrasound outpatient clinic as a predictor of gestational diabetes mellitus (GDM) later in pregnancy.
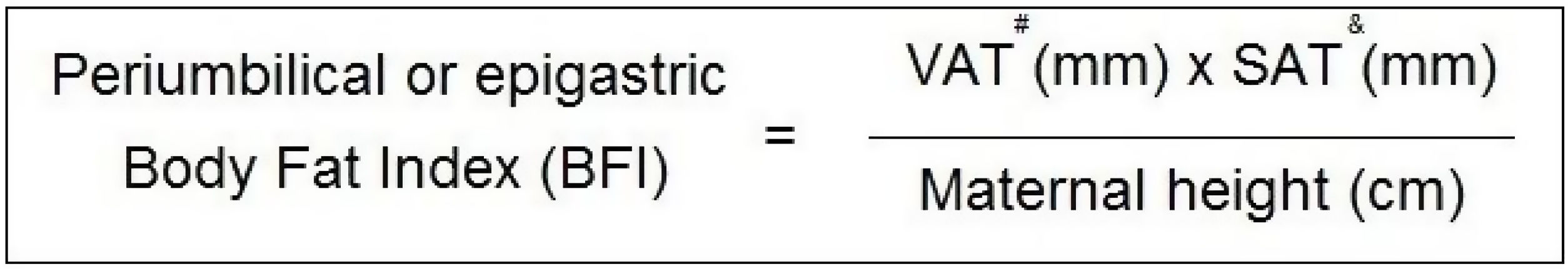
MethodMaternal visceral and subcutaneous fat was measured with a convex ultrasound probe placed in two locations on the maternal abdominal surface: the first in the mid-sagittal epigastric region, visualising epigastric fat, and the second 2cm above the maternal umbilical scar, visualising periumbilical fat. Ultrasound callipers measured the distance from dermal edge to the linea alba and after from the linea alba to the anterior hepatic surface (epigastric fat). Periumbilical fat was measured from the dermal edge to the linea alba and after from the linea alba to the anterior aortic surface. The BFI formula was [visceral adipose tissue (mm)×subcutaneous adipose tissue (mm)]/maternal height (cm).
ResultsThe best thresholds for predicting GDM outcome for epigastric and periumbilical BFI were 1.2 and 4.8, respectively. Odds ratio, sensitivity and specificity were 5.88 (95% CI 1.86–18.6), 80.9%, 58.0% for the epigastric site and 6.31 (95% CI 1.73–22.94), 84.2%, 54.2% for the periumbilical site. Pre-pregnancy body mass index compatible with adult obesity shows inadequate predictive performance for GDM outcome. Only epigastric BFI above 1.2 maintained statistical significance for GDM in the logistic regression analysis, when compared to periumbilical BFI above 4.8.
ConclusionEpigastric BFI above 1.2 during the first half of pregnancy may help identify women at risk of developing GDM later in pregnancy.
Comparar el desempeño del índice de grasa corporal materno (BFI) evaluado durante las primeras 20+6 semanas entre 138 mujeres embarazadas en una clínica ambulatoria de ultrasonido como predictor de la diabetes mellitus gestacional (DMG) más adelante en el embarazo.
MétodoLa grasa visceral y subcutánea materna se midió con una sonda de ultrasonido convexa colocada en 2 lugares de la superficie abdominal materna: el primero en la región epigástrica sagital media, llamada grasa epigástrica, y el segundo 2cm por encima de la cicatriz umbilical materna, llamada grasa periumbilical. Las sondas de ultrasonido midieron la distancia desde el borde dérmico hasta la línea alba y luego desde la línea alba hasta la superficie hepática anterior (grasa epigástrica). La grasa periumbilical se midió desde el borde dérmico hasta la línea alba y luego desde la línea alba hasta la superficie aórtica anterior. La fórmula del BFI fue (tejido adiposo visceral [mm] × tejido adiposo subcutáneo [mm])/altura materna [cm]).
ResultadosEl mejor umbral para predecir el resultado de la DMG entre el BFI epigástrico y periumbilical fue de 1,2 y 4,8, respectivamente. El odds ratio, la sensibilidad y la especificidad fueron 5,88 (IC del 95%: 1,86-18,6), 80,9, 58,0% para el sitio epigástrico materno y 6,31 (IC del 95%: 1,73-22,94), 84,2, 54,2% para el sitio periumbilical, respectivamente. El índice de masa corporal previo al embarazo compatible con la obesidad en adultos muestra un rendimiento predictivo inadecuado para el resultado de la DMG. Solo el BFI epigástrico por encima de 1,2 mantuvo la significación estadística para la DMG en el análisis de regresión logística, en comparación con el BFI periumbilical por encima de 4,8.
ConclusiónUn BFI epigástrico superior a 1,2 durante la primera mitad del embarazo puede ayudar a identificar a las mujeres en riesgo de desarrollar DMG más adelante en el embarazo.
The increase in obesity among women of reproductive age has resulted in an elevated prevalence of pregnant women with obesity and overweight. A limited group of biometric indexes were used in an attempt to categorise pre-pregnancy obesity that could achieve high sensitivity and specificity to detect adverse pregnancy outcomes. Adult body mass index (BMI) over 30kg/m2 has been used more frequently but with conflicting results among studies that addressed the index as a GDM predictor.1–3 The Child Growth Standard (CGS) index has been used as a predictor for certain outcomes, including the need for a caesarean among pregnant adolescents. Likewise, body surface area (BSA) was employed as a predictive measure for the development of gestational diabetes mellitus (GDM) in a cohort of pregnant women in Finland, all of whom had biometric data since birth. The study revealed a negative correlation between BSA and GDM, indicating that pregnant women with lower BSA scores were more likely to be affected by GDM. The researchers hypothesised that unfavourable conditions since pregnancy, leading to maternal restricted growth, resulted in a reduced count of pancreatic beta cells from birth, thereby elevating the future risk of GDM.4
Maternal abdominal adipose tissue assessed by ultrasound has been shown to be an accurate and low-cost method for predicting abnormal pregnancy outcomes, particularly GDM,5–9 pre-eclampsia and preterm birth,10 metabolic syndrome,5 abnormal arterial blood pressure5,11 and abnormal newborn weight.12 Two maternal abdominal sites were selected to access maternal adipose tissue, the maternal epigastric site5,13 and the periumbilical site6,8 with the first being used during all three trimesters and the second limited to evaluations prior to twenty weeks due to gravid uterus growth.
Recently a new GDM predictive index has been proposed using the amounts of maternal epigastric adipose tissue measured by ultrasound and maternal height, called the body fat index (BFI) as a substitute to pre-pregnancy BMI.14 The reported predictive accuracy for GDM was found to exceed that of pre-pregnancy BMI.
However, the study was conducted late into pregnancy, where the deleterious effects of dysglycaemia are already established. Additionally, the BFI was calculated with scores from the maternal epigastric site rather than both known adipose-related sites. Finally, the study did not report the predictive analysis of the new index for GDM as an outcome, including sensitivity, specificity, or the predictive values. As such, the objective of this study was to evaluate the predictive capacity of the BFI index with GDM as the outcome of interest. Most importantly, we aim to determine BFI index using maternal abdominal fat scores from the epigastric and perumbilical sites, indicating GDM risk thresholds for both sites. Ultimately, our study will assess the comprehensive performance of the new index in comparison to the traditionally utilised pre-pregnancy BMI.
MethodsSampleThe prospective cohort study was carried out at the Ultrasound Department of the Murialdo Teaching Health Center, which offers fetal medicine services to the Public Health System in Porto Alegre City, Brazil, between October 2016 and December 2017. A follow-up evaluation was carried out through a review of hospital charts in five hospitals in Porto Alegre city / Brazil. The follow-up assessment obtained all information regarding the pregnancy and the birth process, as well as reported GDM, Fasting Blood Glucose (FBG) and Oral Glucose Tolerance Test scores (OGTT). The inclusion criteria were pregnant individuals with a gestational age of ≤20+6 weeks. The exclusion criteria were pregnancies with foetal malformations or aneuploidy, scar tissue either of the two maternal abdominal sites used for maternal adipose tissue assessment, twin pregnancies and preexisting type 1 or 2 diabetes mellitus. All women who agreed to participate were given a standardised informed consent document.
Procedure at inclusionA routine ultrasound evaluation was performed by the author ASR to diagnose foetal growth as described by Hadlock et al.15 The foetal biometry result was compared with the foetal age evaluated based on the last menstrual period or previous obstetric ultrasound. Placental position, foetal heartbeats, amniotic fluid level, and basic foetal anatomy were assessed in all cases. Embryonic phase pregnancies were evaluated endovaginally to determine gestational age, placental position, heartbeat count and to assess the maternal adnexa. For cases between 11+0 to 13+6 weeks, nuchal translucency and the nasal bone were evaluated to estimate the odds of aneuploidy as described by Nicolaides et al.16
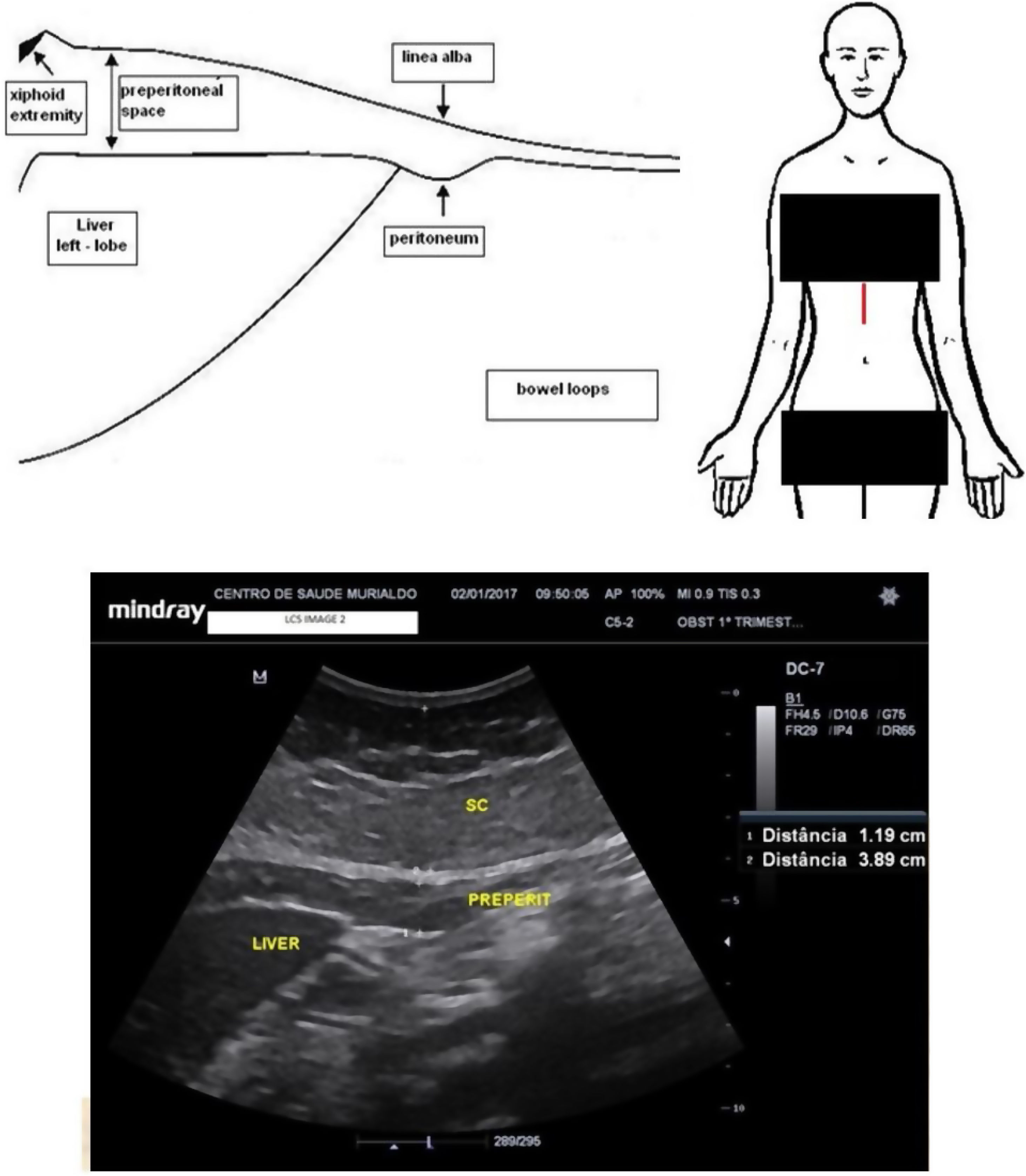
Maternal epigastric fat evaluationMaternal epigastric fat tissue was assessed with a convex probe placed in the middle sagittal epigastric region aiming to measure the visceral and subcutaneous tissues using a method adapted from the studies by Suzuki et al.17 Attention was paid to avoiding excessive pressure that could falsely compress the surfaces of interest. The ultrasound calliper was placed spanning from the deepest anterior liver surface to the linea alba to assess the epigastric maternal visceral adipose tissue (epigastric m-VAT). Thereafter the electronic calliper was moved to span from the linea alba to the superficial dermal edge to determine the epigastric maternal subcutaneous adipose tissue (epigastric m-SAT). Fig. 1 shows the ultrasound measurements
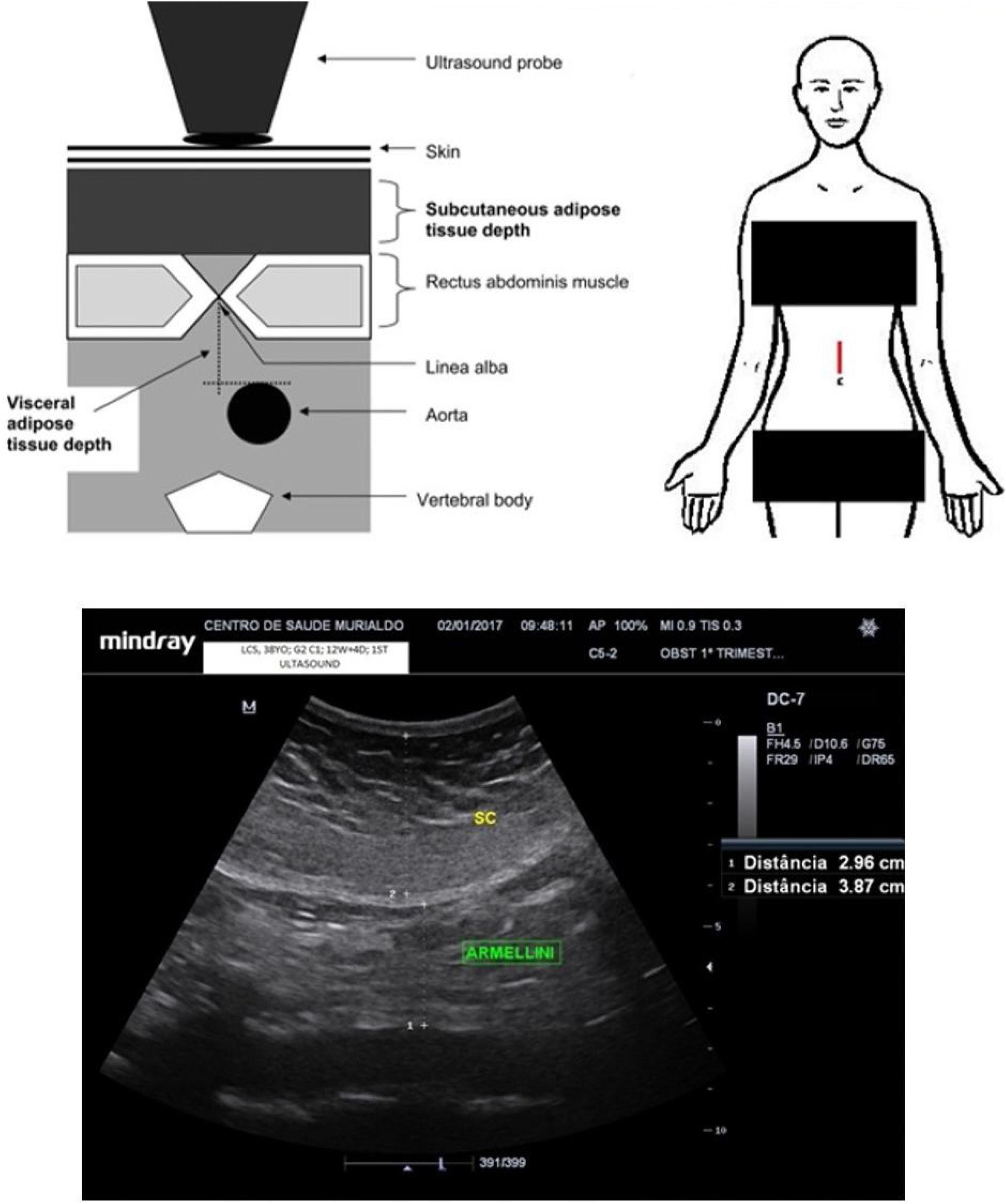
Maternal periumbilical fat evaluationMaternal periumbilical fat tissue was assessed with a convex probe placed 2cm above the maternal umbilical scar, aiming to measure the visceral and subcutaneous components using a method adapted from the studies by Armellini et al.18 An electronic calliper was placed spanning from the anterior aortic wall to the linea alba to assess the periumbilical maternal visceral adipose tissue (periumbilical m-VAT). Thereafter, the electronic calliper was moved to span from the linea alba to the superficial dermal edge, aiming estimate the periumbilical maternal subcutaneous adipose tissue (periumbilical m-SAT). The mean of the two measurements was calculated for both visceral fat assessments, the first at the end of maternal inspiration and the second at the end of maternal expiration. Fig. 2 shows the ultrasound measurements.
Maternal body fat index (BFI)The visceral and subcutaneous fat scores from both maternal abdominal sites were used to calculate BFI. For this purpose, the formula considered fat measurements in millimetres and maternal height in centimetres as measured during recruitment. Fig. 3 shows the formulae.
Maternal anthropometryPre-pregnancy BMI was calculated using maternal weight recorded before the 12th week of pregnancy with the maternal height measured at the time of recruitment. In cases where the first maternal weight measurement was performed after 12 weeks of pregnancy, the authors utilised a self-reported maternal pre-pregnancy weight to calculate the BMI. The authors considered scores above 30kg/m2 as pre-pregnancy obesity.
Demographic and laboratory dataDuring recruitment, outpatient prenatal charts were reviewed to assess reported GDM, FBG or OGTT results together with maternal ethnicity and age, past term pregnancies and first trimester weight. The labour and delivery hospital chart record was reviewed for references to GDM, FBG and OGTT reports alongside the condition of the newborn, gestational age at delivery, birth weight and mode of birth. The authors identified a GDM-positive case for all pregnant women who retrospectively exhibited abnormal FBG or OGTT results during admission for delivery. According to the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria, FBG results between 92mg/dL and 125mg/dL were considered abnormal.19 Among pregnant women who underwent OGTT, all results above 180mg/dL at hour 1 or 153mg/dL at hour 2 were considered abnormal.
Statistical analysisStatistical analyses were performed in SPSS (Statistical Package for the Social Sciences) 23.0 for Windows. The level of significance was set at p<0.05, with 95% confidence intervals for the odds ratio (OR) estimation. In the bivariate analysis, Student's t-test or the Mann–Whitney test (continuous variables) and Chi-squared (categorical varibles) were used to compare groups. Pearson's Chi-squared test was used to analyse the categorical variables. A Receiving Operating Characteristic (ROC) curve was used to identify the best BFI cutoff with regard to the GDM outcome for the periumbilical and epigastric sites. A binary logistic regression was performed to estimate the OR of the GDM outcome for both BFI measurements and adjusted ORs were estimated under potential confounders related to the GDM outcome.
Data availability statementFull databank was published at Physionet Journal DOI number 10.13026/hfks-3d71.
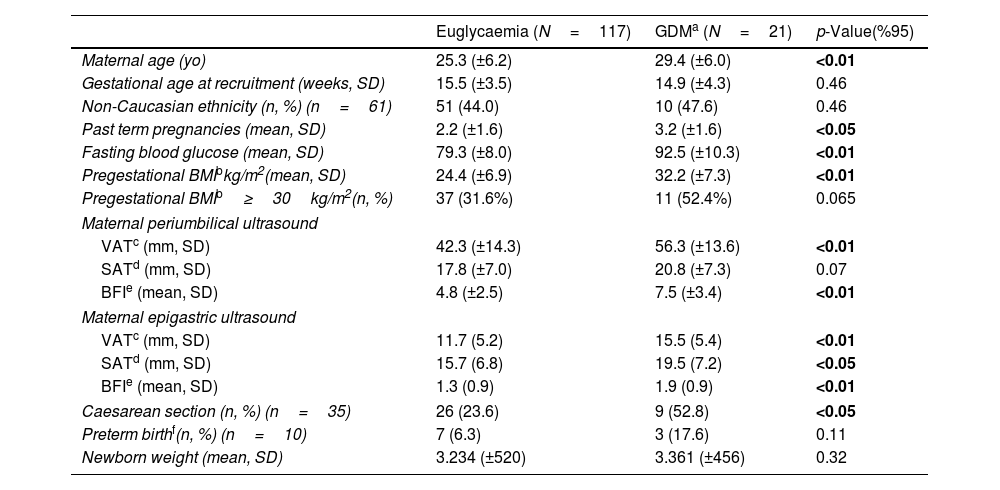
ResultsA total of 159 pregnant women were recruited, with 13.2% (21 cases) lost to follow-up, resulting in 138 individuals in the final analysis. There were 21 cases of GDM (15.2%). Demographic characteristics are shown in Table 1. Maternal age, past term pregnancies, pre-pregnancy BMI and caesarean section were higher among pregnant women who developed GDM. Regarding maternal fat measurements, both epigastric m-VAT and m-SAT sites presented higher scores for GDM cases. The periumbilical site had higher scores only in the m-VAT assessment. Among patients who developed GDM, BFI scores were significantly higher at both sites evaluated.
Sample characteristics.
| Euglycaemia (N=117) | GDMa (N=21) | p-Value(%95) | |
|---|---|---|---|
| Maternal age (yo) | 25.3 (±6.2) | 29.4 (±6.0) | <0.01 |
| Gestational age at recruitment (weeks, SD) | 15.5 (±3.5) | 14.9 (±4.3) | 0.46 |
| Non-Caucasian ethnicity (n, %) (n=61) | 51 (44.0) | 10 (47.6) | 0.46 |
| Past term pregnancies (mean, SD) | 2.2 (±1.6) | 3.2 (±1.6) | <0.05 |
| Fasting blood glucose (mean, SD) | 79.3 (±8.0) | 92.5 (±10.3) | <0.01 |
| Pregestational BMIbkg/m2(mean, SD) | 24.4 (±6.9) | 32.2 (±7.3) | <0.01 |
| Pregestational BMIb≥30kg/m2(n, %) | 37 (31.6%) | 11 (52.4%) | 0.065 |
| Maternal periumbilical ultrasound | |||
| VATc (mm, SD) | 42.3 (±14.3) | 56.3 (±13.6) | <0.01 |
| SATd (mm, SD) | 17.8 (±7.0) | 20.8 (±7.3) | 0.07 |
| BFIe (mean, SD) | 4.8 (±2.5) | 7.5 (±3.4) | <0.01 |
| Maternal epigastric ultrasound | |||
| VATc (mm, SD) | 11.7 (5.2) | 15.5 (5.4) | <0.01 |
| SATd (mm, SD) | 15.7 (6.8) | 19.5 (7.2) | <0.05 |
| BFIe (mean, SD) | 1.3 (0.9) | 1.9 (0.9) | <0.01 |
| Caesarean section (n, %) (n=35) | 26 (23.6) | 9 (52.8) | <0.05 |
| Preterm birthf(n, %) (n=10) | 7 (6.3) | 3 (17.6) | 0.11 |
| Newborn weight (mean, SD) | 3.234 (±520) | 3.361 (±456) | 0.32 |
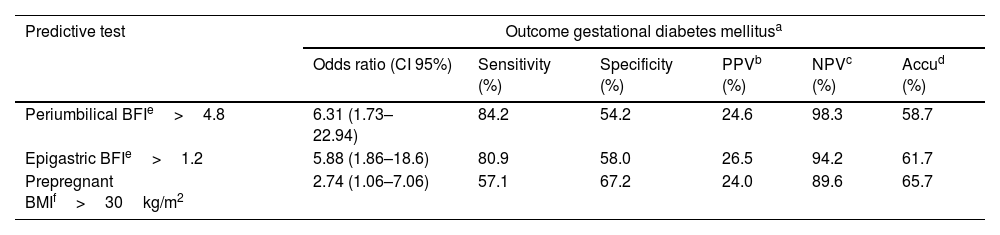
A ROC curve was performed to determine the optimum predictive thresholds for GDM between the BFIs at the two adipose-related sites, giving results of 4.8 and 1.2, respectively (Supporting information file S1). The area under the curve gave scores of 0.74 (CI 95% 0.63–0.85) at the periumbilical site and 0.72 (CI 95% 0.61–0.83) at the epigastric site. Table 2 shows the BFI predictive performance thresholds and the performance of pre-pregnancy BMI compatible with obesity. BFI calculated from the periumbilical site had the highest predictive sensitivity in addition to the highest odds ratio. The highest predictive specificity was achieved with pre-pregnancy BMI above 30kg/m2.
GDM outcome predictive performance from body fat index and prepregnant obesity.
| Predictive test | Outcome gestational diabetes mellitusa | |||||
|---|---|---|---|---|---|---|
| Odds ratio (CI 95%) | Sensitivity (%) | Specificity (%) | PPVb (%) | NPVc (%) | Accud (%) | |
| Periumbilical BFIe>4.8 | 6.31 (1.73–22.94) | 84.2 | 54.2 | 24.6 | 98.3 | 58.7 |
| Epigastric BFIe>1.2 | 5.88 (1.86–18.6) | 80.9 | 58.0 | 26.5 | 94.2 | 61.7 |
| Prepregnant BMIf>30kg/m2 | 2.74 (1.06–7.06) | 57.1 | 67.2 | 24.0 | 89.6 | 65.7 |
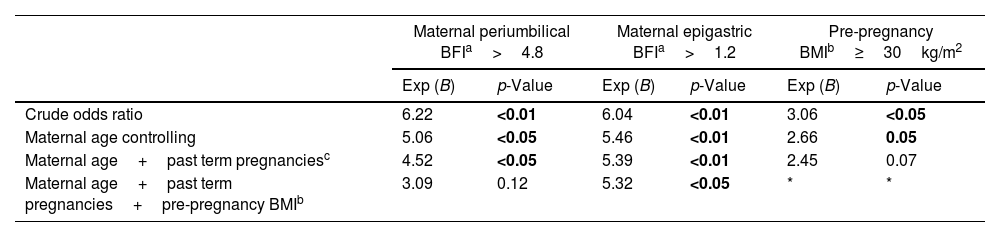
A binary logistic regression was conducted to evaluate the confounding variables associated with the predictive performance of BFI and pre-pregnancy obesity for GDM. As seen in Table 3, the BFI calculated from epigastric site scores maintains the predictive performance when included in the logistic regression analysis. Pre-pregnancy BMI indicating obesity lost its predictive capacity earlier when included in the regression model, in addition to yielding the lowest crude odds ratio.
Binary logistic regression of body fat index thresholds and prepregnant BMI compatible with obesity as GDM predictors.
| Maternal periumbilical BFIa>4.8 | Maternal epigastric BFIa>1.2 | Pre-pregnancy BMIb≥30kg/m2 | ||||
|---|---|---|---|---|---|---|
| Exp (B) | p-Value | Exp (B) | p-Value | Exp (B) | p-Value | |
| Crude odds ratio | 6.22 | <0.01 | 6.04 | <0.01 | 3.06 | <0.05 |
| Maternal age controlling | 5.06 | <0.05 | 5.46 | <0.01 | 2.66 | 0.05 |
| Maternal age+past term pregnanciesc | 4.52 | <0.05 | 5.39 | <0.01 | 2.45 | 0.07 |
| Maternal age+past term pregnancies+pre-pregnancy BMIb | 3.09 | 0.12 | 5.32 | <0.05 | * | * |
The main finding of this study was the superior predictive capacity for GDM of maternal epigastric BFI above 1.2 compared to periumbilical BFI above 4.8 or pre-pregnancy BMI compatible with obesity. The adjusted OR remained significant at 5.3 (p<0.05) even after controlling for confounders commonly related to GDM such as maternal age, more than two past term pregnancies and pre-gravid BMI. In addition, the epigastric BFI showed a predictive sensitivity of 81% for GDM, with a specificity close to 60%. Nassr et al.14 evaluated maternal epigastric BFI performance as a predictor of GDM as the outcome of interest among 389 pregnant women from the 18th to 24th weeks. The authors found similar results to our study, with a median epigastric BFI of 0.78 (0.42–1.26), a median epigastric m-SAT of 11.0mm (8–14mm) and a median epigastric m-VAT of 12mm (9–16mm). Furthermore, the odds ratio reported was similar to the current study, with a crude value of 7.17 (CI 95% 2.17–23.66), followed by 6.24 (CI 95% 1.86–20.96) after adjustment for common confounders. However, the study was centred later in pregnancy, at a time when the deleterious effects of hyperglycaemia have already been established. Moreover, the epigastric BFI threshold reported as at-risk for GDM was 0.5, encompassing 67% of the sample. Sensitivity, specificity or predictive values were not reported for the epigastric BFI threshold chosen. D’Ambrosi et al.13 reviewed stored maternal epigastric images from the 24th to 28th weeks to evaluate the predictive capacity for GDM of isolated m-VAT and m-SAT. Minimal differences in the two adipose tissues were found between pregnant women who developed GDM compared to women who did not. The mean m-VAT score was 10.1mm among individuals with GDM and 9.7mm among those without. The Logarithmic transformation of the maternal fat scores maintained statistical significance after multivariate analysis only for m-VAT and not for m-SAT. The author highlighted the need for predictive studies that focused on assessments during the first trimester, aiming to maximise the early identification of high-risk GDM cases based on epigastric m-VAT measurements.
Periumbilical BFI showed the highest predictive sensitivity for GDM, reaching 84%; however, the performance was affected by the pre-pregnancy BMI when included in a logistic regression analysis, suggesting that either pre-gravid obesity or maternal height impacts the accuracy of the index. To date, there are no studies reporting periumbilical BFI as a predictor of GDM to compare the study results.
Regarding isolated m-VAT and m-SAT measurements, the current study is in line with previous findings that reported maternal abdominal fat measured by ultrasound as a predictor of GDM during the first twenty weeks of pregnancy. Scores ranging from 19.0mm (±8.0)6 to 22.4mm (±10.1)9 for periumbilical m-SAT and 37.2mm (±16.5)11 to 54.4mm (±1.27)20 for periumbilical m-VAT were reported. Such studies show superior predictive capacity of m-VAT assessment when compared with m-SAT for predicting GDM as an outcome.
Our study shows pre-pregnancy obesity to have a weak predictive capacity, sensitivity and OR for GDM. Likewise, logistic regression analysis resulted in a loss of predictive capacity when including the confounder past term pregnancy early in the model. The result aligns with previous meta-analyses that determined pre-pregnancy obesity to be a poor predictor for abnormal outcomes during pregnancy. Santos et al.1 meta-analysed 39 cohort studies from America, Europe, and Oceania, including 1156 GDM cases with a significant adjusted odds ratio of 4.6 (CI 95% 4.2–5.0, p<0.01). The authors highlighted that the cohorts selected showed moderate to high heterogeneity in the comparative evaluation. The opposite result was found by Rahman et al.2 who meta-analysed 13 cohort studies from developing countries focused on GDM outcomes and pre-pregnancy obesity. The authors found a non-significant OR of 3.54 (CI 95% 2.65–4.73, p=0.63) and highlighted that the cohorts selected showed high heterogeneity at comparative assessment. Population-based studies carried out in Denmark3 and France21 assessed pre-pregnancy obesity and GDM outcomes. After controlling for confounders in logistic regression analyses, the OR were similar at 5.9 (CI 95% 4.8–7.3, p<0.05) and 4.07 (CI 95% 2.3–7.1, p<0.05), respectively. However, despite similar designs, there were different incidences of the GDM outcome, with 1.2% and 6.0% respectively, suggesting a higher loss to follow-up in the first study.
The use of biometric indexes for comparison between individuals and populations has long been established. During the 1960s, it became evident that normal body weight in kilograms was proportional to the square of height in metres, as originally proposed by Adolphe Quetelet in 1832.22 The more than one hundred-year-old index is routinely recommended by the World Health Organization as a standard estimate for adults to identify individuals at-risk of a variety of abnormal outcomes, including mortality.23 However, the adult BMI thresholds used to determine abnormal pregnancy outcomes can be distorted due to conditions that go beyond pre-gravid maternal biometrics, such as the age of the pregnant woman, genetic and dietary factors, physical activity and socioeconomic conditions.21
The comparison between adult BMI and Child Growth Standards (CGS) was conducted as a predictive measure of the need for a caesarean section among pregnant adolescents exhibiting high weight gain. While demonstrating sufficient predictive capacity, the combined utilisation of both indexes led to significant discrepancies with regard to the prevalence of pre-pregnancy overweight or obesity, with 31% per the CGS and 17% according to the adult BMI.24
Body Surface Area (BSA) was calculated in 1548 pregnant women who presented 190 cases of GDM in a Finnish study.4 Recruited pregnant women had biometric data that allowed access to their BSA in adulthood and immediately after maternal birth from 1987 onwards. The authors concluded that the prevalence of GDM was inverse to BSA at birth, the outcome being the most prevalent (18.1%) among those with the smallest BSA and less prevalent (9.5%) among those with the largest BSA at birth. In addition, the BSA scores in adulthood showed the same inverse relationship to the outcome. The study concluded that low BSA scores are related to impaired foetal pancreas development with fewer functioning beta cells, predisposing the newborn to dysglycaemia later in life. In addition, insulin is recognised as an important foetal growth factor and the same genetic alleles that reduce foetal growth could decrease insulin secretion in adulthood, predisposing to diabetes in later life.
The primary strength of our study is the focus on an early evaluation of a new predictive index for GDM as an outcome in an ultrasound outpatient clinic environment, which simulates most prenatal services. The adequate sample, the prospective design and the full BFI predictive performance thresholds at two different maternal fat sites, establish this study as a guide for future approaches in the area. However, the study is not free of limitations. The pre-pregnancy BMI for some patients was calculated from a self-reported pre-pregnancy weight, which may result in recall bias. In addition, ultrasound m-VAT and m-SAT evaluation was performed by a single foetologist instead of two, which prevents the estimation of the coefficient of interobserver agreement. Finally, FBG or OGTT exams were performed in different laboratories, which can impose a measurement bias.
ConclusionAmong pregnant women screened at an obstetric ultrasound clinic, the predictive capacity for GDM was higher when fat scores located in the epigastric region were used. Body fat index from the periumbilical region or pre-pregnancy BMI compatible with obesity show low predictive capacity for the GDM outcome. However, it is emphasised that GDM prevention involves lifestyle modifications among women desiring pregnancy, aiming to reduce excessive amounts of accumulated visceral fat.
Authors’ contributionsAuthor ASR participated in all study steps from conception to writing the main text. Execution and database creation were performed by DCK and SM. MZG, JRB, RON and JAAM participated in the conception and planning. Author ACS participated in writing the main text.
Data availability statementFull databank published at Physionet Journal DOI number 10.13026/hfks-3d71.
Ethics approval detailsEthical approval was received from Hospital Materno Infantil Presidente Vargas Research Ethics Committee as a Municipality of Porto Alegre representative. General Ethical approval number 1.758.959 issued on 4 October 2016.
FundingFinancial support was provided by the Hospital de Clínicas de Porto Alegre Research and Event Incentive Fund (Fundo de Incentivo à Pesquisa e Eventos, FIPE). Additional support was provided by the Porto Alegre municipal government (ultrasound equipment, research facilities) and the Federal University of Rio Grande do Sul with technical and scientific support.
Conflict of interestThe authors declare no competing financial interests and confirm that there is no support from ultrasound companies. Furthermore, they assert the absence of support from low-calorie food substitutes companies.
A special thanks to Prof. Lisia von Diemen for her indispensable assistance with statistics.