Protocol for prescribing hormone replacement therapy in isolated growth hormone (GH) deficiency includes magnetic resonance imaging of the brain. There is controversy on the frequency of structural pituitary abnormalities and on the importance of abnormal MRI findings on prognosis and response to GH replacement.
MethodsA descriptive, retrospective study of children of both sexes aged 0–14 years, who had undergone brain MRI, diagnosed with isolated GH deficiency at a tertiary hospital in the past 14 years, aimed at reporting the frequency of abnormal MRI findings in isolated GH deficiency, and to establish whether differences exist in height diagnosis and evolution according to MRI findings. MRI findings were also compared with the findings reported in healthy children in order to establish incidence.
Results96 patients were studied, of whom 74/96 (77%) reached adult age. Abnormal MRI findings were seen in 11.5% of them (8/11 of pituitary origin). No brain or pituitary tumor was seen in any case. Patients with abnormal images had a mean age at treatment start of 8 years, a target height of −0.8SD, and a final height of 1.04SD, while patients with normal MRI findings had an age at treatment start of 10 years old, a target height of −1.44SD, and a final height of −1.75SD, with statistically significant differences.
ConclusionsPatients with abnormal MRI findings show a more favorable response to GH replacement therapy.
El protocolo de prescripción de hormona sustitutiva en el déficit aislado de hormona del crecimiento (GH) incluye la realización de una resonancia cerebral. Hay controversia sobre la frecuencia de anomalías hipofisarias y la importancia de los hallazgos anormales de resonancia magnética en el pronóstico y la respuesta al tratamiento de GH.
MétodosEstudio descriptivo retrospectivo de niños de 0 a 14 años, de ambos sexos, con imágenes de resonancia magnética cerebral, que fueron diagnosticados de deficiencia aislada de GH en un hospital terciario en los últimos 14 años, para describir la frecuencia de las anomalías en la resonancia magnética y establecer si existen diferencias en el diagnóstico de talla y evolución de acuerdo con los hallazgos de la resonancia magnética. Además, comparamos los hallazgos en la resonancia con los hallazgos publicados en niños sanos, con el fin de establecer la incidencia.
ResultadosSe estudiaron 96 pacientes, alcanzando 74/96 (77%) la edad adulta. El 11,5% tenía imágenes de resonancia magnética anormales (8/11 de origen hipofisario). En ningún caso se observó tumor cerebral o hipofisario. Los pacientes con imágenes anormales mostraron una edad media al inicio de tratamiento de 8 años, talla diana de −0,8 DE y una talla final de 1,04 DE; mientras que los pacientes con imágenes de resonancia magnética normal muestran una edad de inicio de tratamiento de 10 años, una talla diana de −1,44 DE y una talla final de −1,75 DE, con diferencias estadísticamente significativas.
ConclusionesLos pacientes con alteraciones en la resonancia magnética muestran una respuesta más favorable al tratamiento sustitutivo de GH.
One cause of childhood short stature is growth hormone (GH) deficiency, which can be treated with recombinant human GH.1
Childhood onset GH deficiency may be isolated or part of a multiple pituitary hormone deficiency syndrome. The former is usually idiophatic; however, meticulous evaluation of auxological, clinical and magnetic resonance imaging (MRI) findings is warranted to guide appropriate treatment of children with GH deficiency.1,2 Although most MRI studies do not show obvious abnormalities, a significant proportion reveal structural pathology.1 In addition, MRI could be useful in predicting the response to GH treatment based on severity of GH deficiency.2
Normal GH secretion is pulsatile. Therefore, clinicians cannot rely on baseline GH levels. Thus, they stimulate the pituitary somatotrophs pharmacologically or physiologically to observe how GH secretion responds.1 In general, severe GH deficiency is considered to be a peak response less than 5ng/ml and partial deficiency when it is between 5 and 7ng/ml.3 Although growth hormone stimulation tests are considered the mainstay for this diagnosis, there is limited evidence-base for cut-off values used to distinguish GH deficient and GH sufficient subjects.1,4
Furthermore, these tests have significant limitations as they do not mimic a normal secretory pattern of GH, there is an arbitrary definition of normal levels, a low specificity (up to 60% of normal children present a deficient GH response). In addition, there is variability in outcomes, depending on the type of stimulus, type of trial, psychosocial factors, age, body mass index (BMI), pubertal stage, and use of sex steroid priming.4 Because normal children may not respond to a specific stimulus test (high rate of false positives), the absence of response should be confirmed with a second test with a different stimulus. These tests are also expensive, uncomfortable, and sometimes involve an element of risk to the patient.1,5
Having said that, it would be interesting to use more reliable tests in order to establish a better and more reliable diagnosis.
Therefore, GH stimulation tests results are usually considered in the clinical context while taking account of other test results such as magnetic resonance and growth rate.3 Furthermore, hypothalamic/pituitary imaging characteristics and GH stimulation tests are useful for directing mutational analysis of candidate genes for isolated GH deficiency.6 Accordingly, Pampanini et al. and Kochi et al. suggest magnetic resonance imaging of pituitary region as an important diagnostic and prognostic tool in childhood-onset GH deficiency.7,8
Magnetic resonance imaging of the sellar area includes sagittal and coronal sequences of hypothalamic-pituitary region after gadolinium injection; contrast enhancement increases the sensitivity of MRI for visualizing the pituitary stalk. It is essential to do this test in the presence of severe GH deficiency to rule out or confirm structural abnormality.8
It is the most sensitive technique to assess size and pituitary structure, in addition it may detect hypothalamic–pituitary lesions. Classical structural abnormalities associated with GH deficiency include an attenuated or absent pituitary stalk, small or absent anterior pituitary, or truncated and ectopic posterior pituitary.9,10 Interestingly, these lesions are common both, in isolated GH deficiency (61.2%) and multiple pituitary hormone deficiency (90.1%), and are closely related to peak GH levels less than 3mcg/l and lower serum IGF1 and IGFBP3 levels.11 However, they are not present in those patients small for gestational age.12 Preliminary data from Bressani et al. describe a variety of patterns of treatment responses according to magnetic resonance imaging data. They recommend further studies to correlate endocrine data and magnetic resonance imaging.13
MRI may be of prognostic benefit in childhood-onset GH deficiency. Previous research suggests that patients with abnormal magnetic resonance imaging and isolated GH deficiency are more likely to develop additional pituitary hormone deficiencies, while those with normal MRI are less likely to suffer additional hormonal deficits.14 Furthermore, they associate high frequency of midline central nervous system such as, optic nerve hypoplasia (9%), Chiari type 1 malformations (20%), and medial deviation of carotid arteries (37%) with GH deficiency. This supports the theory of embryologic defect as the cause of pituitary abnormalities.15
Furthermore, the severity of GH deficiency correlates with the degree of structural abnormality on MRI. Thus, while more severe GH deficiency and multiple pituitary hormone deficiencies are often related to ectopic neurohypophysis and interrupted pituitary stalk; less serious isolated GH deficiency or normal variant short stature are usually related to hypoplastic hypophysis.13,14
Pituitary height and volume, measured on MRI, is proportional to GH secretion in short stature.2 So, pituitary volume could be another potential diagnostic value for growth hormone deficiency and idiopathic short stature. The latter is associated with a smaller pituitary gland volumes in 65.6% and 34.8%, respectively, compared with healthy children.2,9,13,16
Arslanoğlu et al., report that the specificity of pituitary dysmorphology, in determination of GH deficiency, is 100% and its sensitivity in differentiation of isolated GH deficiency and multiple pituitary hormone deficiency is 95%.17 Tillmann et al. claim a specificity of 54% and a sensitivity of 79%, indicating that this could not be used to confirm GH deficiency. However, the presence of either ectopic posterior lobe or isolated hypoplastic stalk+hypoplastic anterior lobe on magnetic resonance imaging is highly specific (100% and 79%, respectively), indicating that these abnormalities provide confirmation of diagnosis, all together with biochemical diagnosis.6,18 It is clear that MRI abnormalities are over-represented in those with growth hormone deficiency compared to healthy controls, although data on the true prevalence of structural abnormalities is disputed.
Association of gland hypoplasia with other magnetic resonance abnormalities could suggest the presence of multiple anterior pituitary deficiencies.19
In view of the current limitations on the diagnosis of childhood onset GH deficiency we propose magnetic resonance imaging as one important test in diagnosis.
Furthermore, imaging abnormalities could predict a better response to treatment due to a more likely diagnosis of GH deficiency. It may be observed a poorer response to treatment due to the consequences of the abnormality by itself and the affected tissues. This area warrants further research.
According to that, we suggest a study in order to determine the influence of findings in magnetic resonance imaging during diagnosis, evolution and treatment of isolated growth hormone deficiency in childhood.
Apart from that, our aims would be:
- 1.
To describe the frequency of abnormalities on magnetic resonance imaging in isolated childhood-onset GH deficiency in comparison with healthy children recruited from magnetic resonance protocols in healthy children.
- 2.
To compare the differences between the characteristics at diagnosis of children with isolated GH deficiency according to their findings in magnetic resonance imaging.
- 3.
To compare differences in biochemical characteristics of children with isolated GH deficiency according to their findings in magnetic resonance imaging.
- 4.
To describe total increase in height and final heights of patients with isolated GH deficiency according to their findings in magnetic resonance imaging.
- 5.
To define if persistent GH deficiency in adulthood is related to findings in magnetic resonance imaging.
Longitudinal observational retrospective study in children from 0 to 14 years, of both sexes, diagnosed with isolated GH deficiency with two stimulation tests, and treated with GH to adulthood, with pituitary magnetic resonance imaging assessment, in a tertiary hospital in the last 14 years (2002–2016). In addition, after stopping treatment for reaching final height, reassessment was performed by IGF1 and GH stimulation test with insulin.
Apart from that, we compare findings in magnetic resonance imaging of those cases with isolated GH deficiency and healthy cases published in literature, in order to establish differences.
Inclusion criteria:
Patients diagnosed with isolated GH deficiency in childhood according to the following criteria:
- •
Height less than −2 SD.
- •
Growth velocity less than −1 SD for chronological age in last year or less than −1.5 SD over past 2 years.
- •
Delayed bone maturation in 1 year or more.
- •
Two negative stimulus test for GH with different kind of stimuli in different days each one.
- •
The absence of associated pathology.
- •
At least one year before onset of puberty.
- •
Treated with biosynthetic GH.
- •
Magnetic resonance imaging at diagnosis.
Exclusion criteria:
- -
Children with short stature due to other causes (hypothyroidism, hypocortisolism, chronic systemic diseases, dysmorphic syndromes, skeletal disorders, etc.).
- -
Patients with other associated pathologies such as, more hormone deficiencies, tumor, or hypothalamic disease.
- -
Patients with GH response in stimulation test higher than 7ng/ml.
- -
Pubertal patients.
Pituitary magnetic resonance imaging was performed in a tertiary hospital with high-quality latest generation machinery of magnetic resonance (3Tesla), high definition digitalized images, and assessment for each pituitary imaging according to pituitary structure, shape, size, volume, intensity and localization, by two pituitary neuroradiologists who are sub-specialized in children.
During the study, we collected data from our patient's clinical records such as sex, age of diagnosis, symptoms, initial height, target height, prognosis of height, IGF1 levels, bone age, age of puberty, height at onset of puberty, adult height, increase of height during puberty, total height gain, and GH level in reappraisal, and record them in a database in order to establish if there are differences between our patient cohorts according to magnetic resonance findings.
Target height depicts genetic potential of the patient. It is obtained from the average size of the parents adding 6.5cm if male or subtracting 6.5cm if female (±5).
Prognosis of height is based on the fact that maturation bone can be used to predict the potential final height. The classical method for the prediction of height based on maturation bone of Greulich and Pile, was developed by Bayley and Pinneau, trusting the patient's bone age and height.
Most variables are quantitative except sex, resonance findings, and lack of reassessment, which are qualitative.
We avoided possible bias by collecting confounding variables such as sex and BMI, and by the use of standard deviations (SD) and cut-off values adjusted according to lab method, age and sex.
For measuring weight, we used a Seca manual scale with an accuracy of 0.1kg, and for measuring height a Holtain Stadiometer fixed to the wall, with an accuracy of 0.1cm, which are calibrated daily. For calculating BMI we used the formula: weight/height2.
All measurements were performed by trained and skilled health professionals. To avoid measurement errors, measurements were performed with patients in underwear and without shoes, and were repeated three times.
Moreover, measurements were supervised by our pediatric endocrinology chief, who is a recognized academic member of pediatric endocrinology in our country.
GH stimulation tests included an exercise test, where GH was assessed 40min after moderate exercise such as running or climbing stairs, which results in a hearth rate around 120bpm, and in a different day clonidine test in which we measured GH at 30, 60 and 90min after a dosage of 0.15mg/m2 of clonidine.1,20 Finally, for reassessment at 16–18 years old, we measured after a dosage of 0.1UI/kg of insulin GH values at hypoglycemia and at 30, 45, 60, 90 and 120min21
Cut off values for stimuli tests was 7ng/ml in diagnosis and 5ng/ml in reassessment.4,21
GH and IGF1 values were obtained by IMMULITE 2000 technology in Malaga Children Hospital. This technique is based on the affinity between antigens and antibodies and a marked complex which detects it. On the other hand, this technology have one software which adjusted results of IGF1 by sex and age.
Collected data were adjusted automatically by software AUXOTEC through standard deviation according to the tables of Spanish transverse growth study and formula:22
Finally, we proceeded to the development of descriptive and inferential statistical analysis through bivariate and multivariate formulas, with statistical package SPSS Statistics version 22.
For descriptive analysis, we used percentages, fractions, and measures of central tendency and dispersion such as mean, quartiles and standard deviations.
We used Shapiro–Wilk test for determining normal distribution, Levene test for homogeneity, and ANOVA or Kruskal Wallis tests according to normality for statistical comparisons. Results was considered statistically significant if p was <0.05.
At last, data are shown as mean and standard deviations.
Controls were taken from several studies with a large sample, in which they realized magnetic resonance imaging of the whole brain as part of a scientific magnetic resonance imaging protocol to young healthy volunteers, and to trauma patients who were evaluated for some incidental findings.
It is noteworthy that the participation in this study do not mean any risk for the participants and we do not need any funding to do it, since it was carried out according to routine clinical practice Pediatric Endocrinology department at Malaga children's hospital, based on the recommendations of Andalusian Committee for growth.
Furthermore, we performed a background literature by PUBMED, and MEDLINE with the combined key words: magnetic resonance findings; isolated GH deficiency; healthy children; short stature and diagnosis.
Limitations for our study could be missing data in clinical records, retrospective and single center nature of the study, and interpersonal differences in management of patients. Furthermore, we have not considered classification of patients according to puberty onset (early, normal or late puberty) in order to correlate this situation to height during pubertal ages.
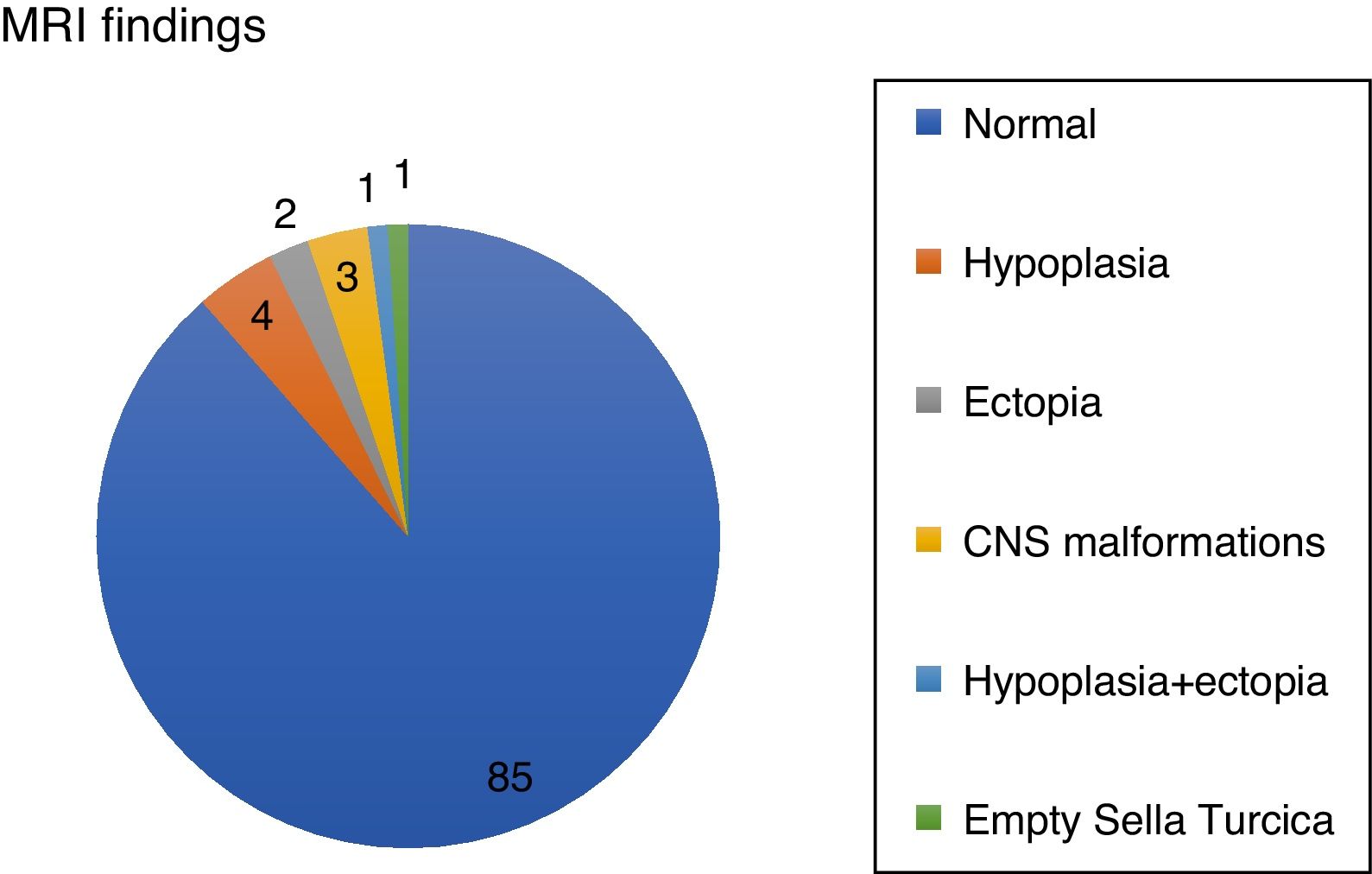
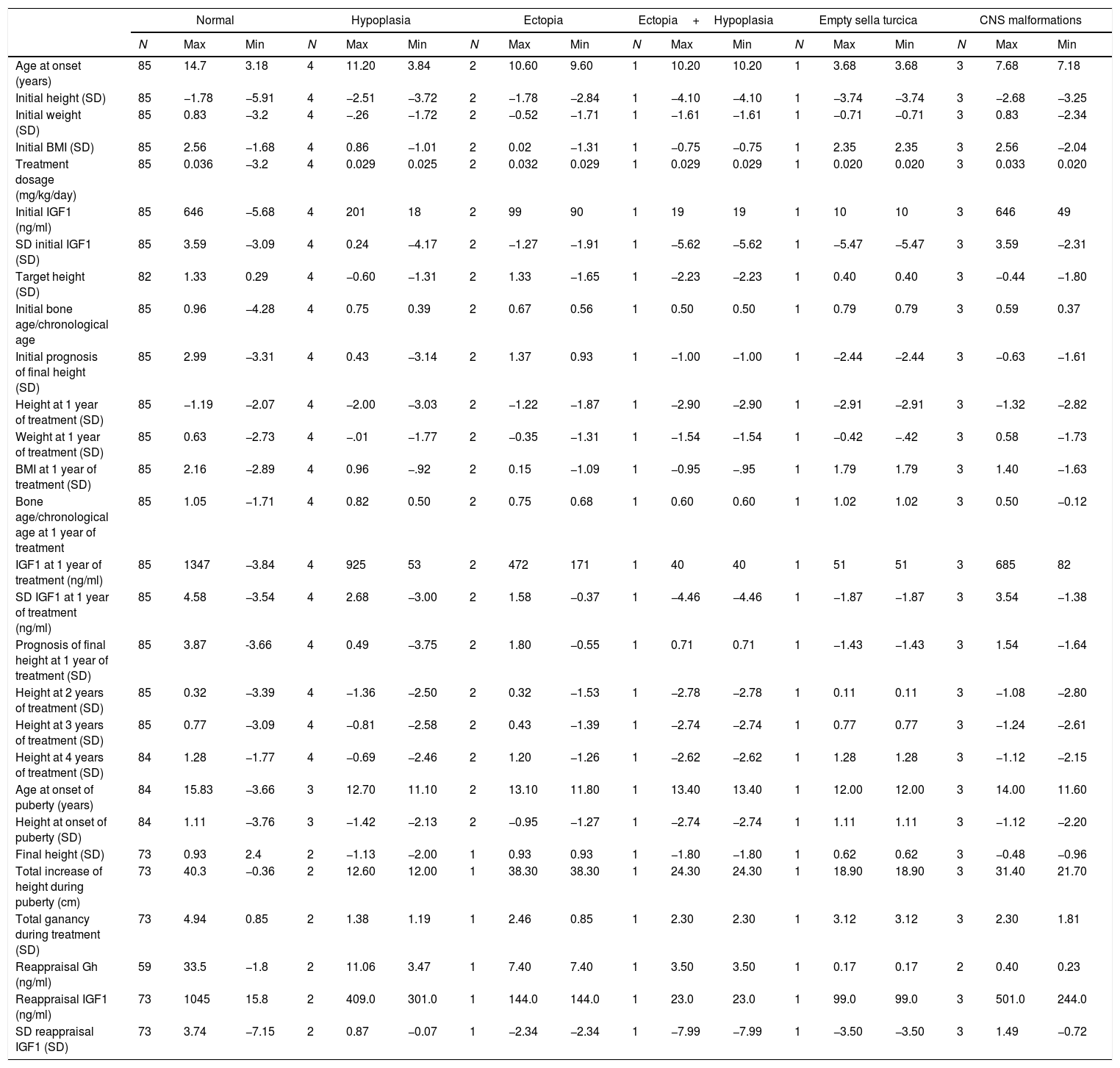
Results96 patients with isolated GH deficiency were studied, of which 66 were boys (68.8%). 81/96 reached final height (84.38%). 11/96 (11.5%) showed abnormalities in brain resonance (4/96 pituitary hypoplasia, 2/96 ectopic neurohypophysis, 1/96 combined pituitary hypoplasia and ectopia, and 1/96 empty sella turcica) (Fig. 1).
Assessment of the structural magnetic resonance imaging by two certified neuroradiologists revealed incidental findings in 16/96 of subjects, most of them normal variants. In 5/96 were pituitary/hypothalamic malformations apart from findings named above, and in 8/96 findings were of potential clinical relevance (most of them vascular findings) and required further diagnostic investigations. None started immediate treatment.
In addition 3/96 were related to central nervous system abnormalities such as Chiari abnormality or arachnoid cyst. On the other hand, tumor pathology was not observed by magnetic resonance imaging in any case, and none had side effects with treatment.
The mean dose of GH used was 0.028mg/kg/day (0.2mg/kg/week), reaching an average increase per year of 0.49 SD.
66 adult patients (68.75% of the sample), were reassessed with stimulation test with insulin, and from them 33/96 maintain GH deficiency.
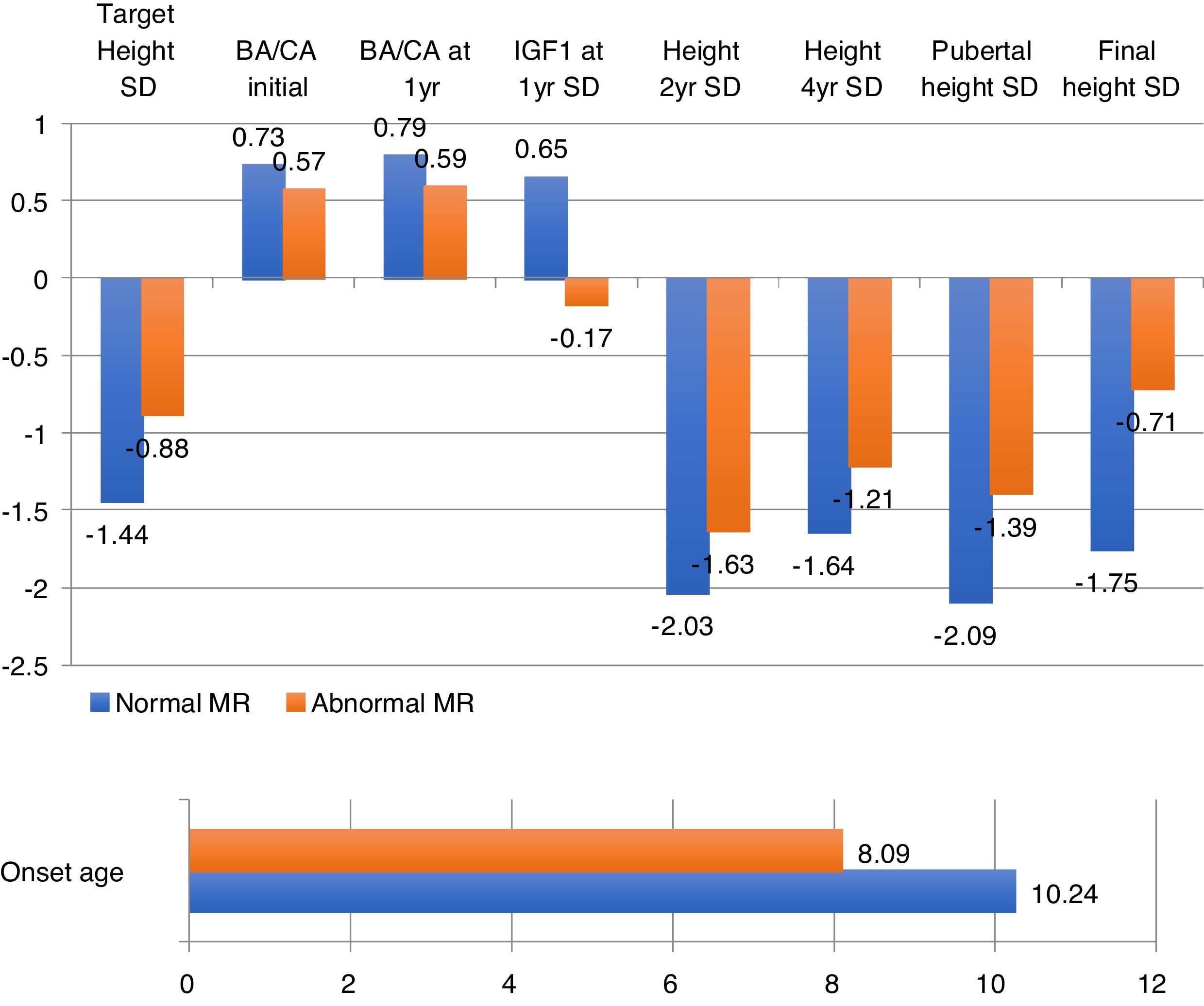
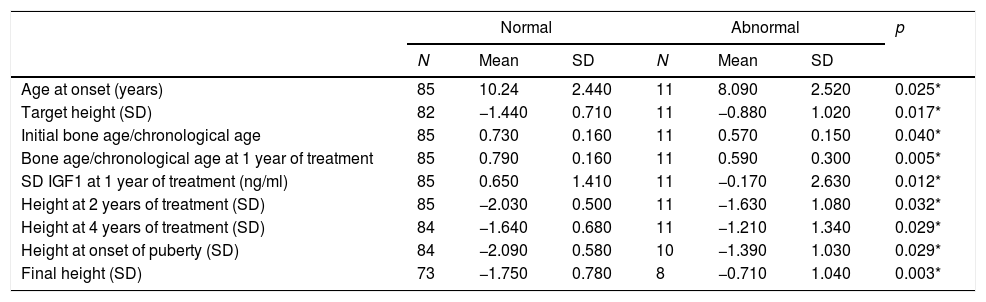
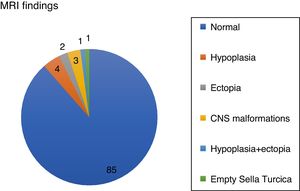
Patients with abnormal imagings showed a mean age at onset of 8 years old, a target height of −0.8SD, and a final height of 1.04SD; while patients with normal magnetic resonance imagings showed an age at onset of 10 years old, a target height of −1.44SD, and a final height of −1.75SD, with statistically significant differences (Table 1).
Descriptive table for quantitative variables statistically significatives collected during the research from children with normal magnetic resonance imaging and abnormal resonance imaging.
| Normal | Abnormal | p | |||||
|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | ||
| Age at onset (years) | 85 | 10.24 | 2.440 | 11 | 8.090 | 2.520 | 0.025* |
| Target height (SD) | 82 | −1.440 | 0.710 | 11 | −0.880 | 1.020 | 0.017* |
| Initial bone age/chronological age | 85 | 0.730 | 0.160 | 11 | 0.570 | 0.150 | 0.040* |
| Bone age/chronological age at 1 year of treatment | 85 | 0.790 | 0.160 | 11 | 0.590 | 0.300 | 0.005* |
| SD IGF1 at 1 year of treatment (ng/ml) | 85 | 0.650 | 1.410 | 11 | −0.170 | 2.630 | 0.012* |
| Height at 2 years of treatment (SD) | 85 | −2.030 | 0.500 | 11 | −1.630 | 1.080 | 0.032* |
| Height at 4 years of treatment (SD) | 84 | −1.640 | 0.680 | 11 | −1.210 | 1.340 | 0.029* |
| Height at onset of puberty (SD) | 84 | −2.090 | 0.580 | 10 | −1.390 | 1.030 | 0.029* |
| Final height (SD) | 73 | −1.750 | 0.780 | 8 | −0.710 | 1.040 | 0.003* |
NS: no significance; SD: standard deviation; BMI: body mass index; IGF1: insulin growth factor 1.
The obtained data showed statistically significant differences in age at onset of treatment, target height, bone age, heights at second and fourth year of treatment, height at onset of puberty and final height, with a lower age of onset and a better response to treatment (+1.06SD) in patients with abnormal magnetic resonance imaging (Fig. 2).
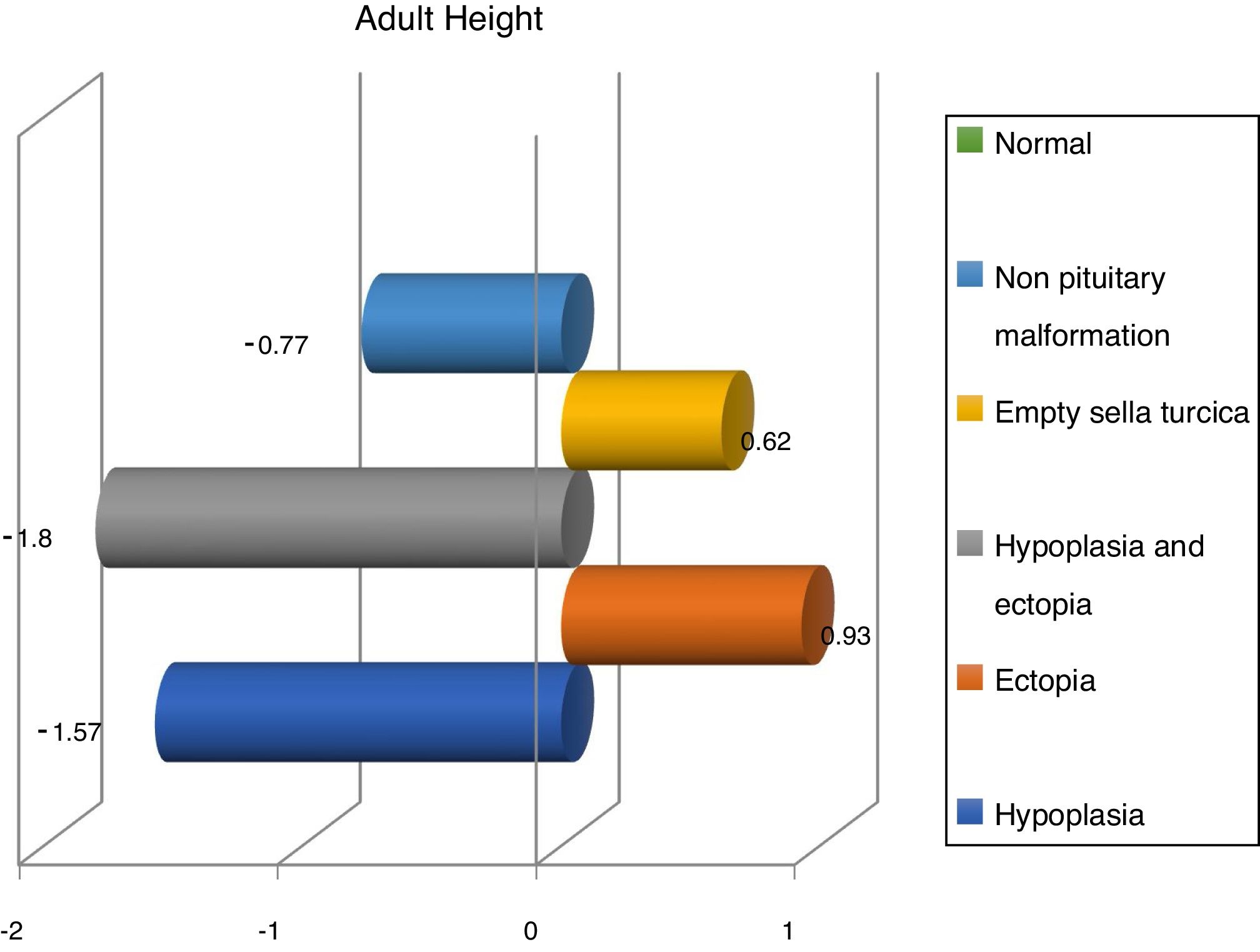
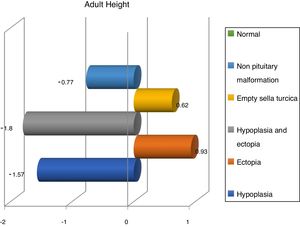
Finally, we have compared variables in different magnetic resonance findings and normal imaging in GH deficient patients. According to that, patients with pituitary disorders such as empty sella or hypoplasia and ectopic hypophysis, were those with lower values in reassessment (Table 2).
Descriptive table for quantitative variables collected during the research from children classified according to resonance findings.
| Normal | Hypoplasia | Ectopia | Ectopia+Hypoplasia | Empty sella turcica | CNS malformations | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Max | Min | N | Max | Min | N | Max | Min | N | Max | Min | N | Max | Min | N | Max | Min | |
| Age at onset (years) | 85 | 14.7 | 3.18 | 4 | 11.20 | 3.84 | 2 | 10.60 | 9.60 | 1 | 10.20 | 10.20 | 1 | 3.68 | 3.68 | 3 | 7.68 | 7.18 |
| Initial height (SD) | 85 | −1.78 | −5.91 | 4 | −2.51 | −3.72 | 2 | −1.78 | −2.84 | 1 | −4.10 | −4.10 | 1 | −3.74 | −3.74 | 3 | −2.68 | −3.25 |
| Initial weight (SD) | 85 | 0.83 | −3.2 | 4 | −.26 | −1.72 | 2 | −0.52 | −1.71 | 1 | −1.61 | −1.61 | 1 | −0.71 | −0.71 | 3 | 0.83 | −2.34 |
| Initial BMI (SD) | 85 | 2.56 | −1.68 | 4 | 0.86 | −1.01 | 2 | 0.02 | −1.31 | 1 | −0.75 | −0.75 | 1 | 2.35 | 2.35 | 3 | 2.56 | −2.04 |
| Treatment dosage (mg/kg/day) | 85 | 0.036 | −3.2 | 4 | 0.029 | 0.025 | 2 | 0.032 | 0.029 | 1 | 0.029 | 0.029 | 1 | 0.020 | 0.020 | 3 | 0.033 | 0.020 |
| Initial IGF1 (ng/ml) | 85 | 646 | −5.68 | 4 | 201 | 18 | 2 | 99 | 90 | 1 | 19 | 19 | 1 | 10 | 10 | 3 | 646 | 49 |
| SD initial IGF1 (SD) | 85 | 3.59 | −3.09 | 4 | 0.24 | −4.17 | 2 | −1.27 | −1.91 | 1 | −5.62 | −5.62 | 1 | −5.47 | −5.47 | 3 | 3.59 | −2.31 |
| Target height (SD) | 82 | 1.33 | 0.29 | 4 | −0.60 | −1.31 | 2 | 1.33 | −1.65 | 1 | −2.23 | −2.23 | 1 | 0.40 | 0.40 | 3 | −0.44 | −1.80 |
| Initial bone age/chronological age | 85 | 0.96 | −4.28 | 4 | 0.75 | 0.39 | 2 | 0.67 | 0.56 | 1 | 0.50 | 0.50 | 1 | 0.79 | 0.79 | 3 | 0.59 | 0.37 |
| Initial prognosis of final height (SD) | 85 | 2.99 | −3.31 | 4 | 0.43 | −3.14 | 2 | 1.37 | 0.93 | 1 | −1.00 | −1.00 | 1 | −2.44 | −2.44 | 3 | −0.63 | −1.61 |
| Height at 1 year of treatment (SD) | 85 | −1.19 | −2.07 | 4 | −2.00 | −3.03 | 2 | −1.22 | −1.87 | 1 | −2.90 | −2.90 | 1 | −2.91 | −2.91 | 3 | −1.32 | −2.82 |
| Weight at 1 year of treatment (SD) | 85 | 0.63 | −2.73 | 4 | −.01 | −1.77 | 2 | −0.35 | −1.31 | 1 | −1.54 | −1.54 | 1 | −0.42 | −.42 | 3 | 0.58 | −1.73 |
| BMI at 1 year of treatment (SD) | 85 | 2.16 | −2.89 | 4 | 0.96 | −.92 | 2 | 0.15 | −1.09 | 1 | −0.95 | −.95 | 1 | 1.79 | 1.79 | 3 | 1.40 | −1.63 |
| Bone age/chronological age at 1 year of treatment | 85 | 1.05 | −1.71 | 4 | 0.82 | 0.50 | 2 | 0.75 | 0.68 | 1 | 0.60 | 0.60 | 1 | 1.02 | 1.02 | 3 | 0.50 | −0.12 |
| IGF1 at 1 year of treatment (ng/ml) | 85 | 1347 | −3.84 | 4 | 925 | 53 | 2 | 472 | 171 | 1 | 40 | 40 | 1 | 51 | 51 | 3 | 685 | 82 |
| SD IGF1 at 1 year of treatment (ng/ml) | 85 | 4.58 | −3.54 | 4 | 2.68 | −3.00 | 2 | 1.58 | −0.37 | 1 | −4.46 | −4.46 | 1 | −1.87 | −1.87 | 3 | 3.54 | −1.38 |
| Prognosis of final height at 1 year of treatment (SD) | 85 | 3.87 | -3.66 | 4 | 0.49 | −3.75 | 2 | 1.80 | −0.55 | 1 | 0.71 | 0.71 | 1 | −1.43 | −1.43 | 3 | 1.54 | −1.64 |
| Height at 2 years of treatment (SD) | 85 | 0.32 | −3.39 | 4 | −1.36 | −2.50 | 2 | 0.32 | −1.53 | 1 | −2.78 | −2.78 | 1 | 0.11 | 0.11 | 3 | −1.08 | −2.80 |
| Height at 3 years of treatment (SD) | 85 | 0.77 | −3.09 | 4 | −0.81 | −2.58 | 2 | 0.43 | −1.39 | 1 | −2.74 | −2.74 | 1 | 0.77 | 0.77 | 3 | −1.24 | −2.61 |
| Height at 4 years of treatment (SD) | 84 | 1.28 | −1.77 | 4 | −0.69 | −2.46 | 2 | 1.20 | −1.26 | 1 | −2.62 | −2.62 | 1 | 1.28 | 1.28 | 3 | −1.12 | −2.15 |
| Age at onset of puberty (years) | 84 | 15.83 | −3.66 | 3 | 12.70 | 11.10 | 2 | 13.10 | 11.80 | 1 | 13.40 | 13.40 | 1 | 12.00 | 12.00 | 3 | 14.00 | 11.60 |
| Height at onset of puberty (SD) | 84 | 1.11 | −3.76 | 3 | −1.42 | −2.13 | 2 | −0.95 | −1.27 | 1 | −2.74 | −2.74 | 1 | 1.11 | 1.11 | 3 | −1.12 | −2.20 |
| Final height (SD) | 73 | 0.93 | 2.4 | 2 | −1.13 | −2.00 | 1 | 0.93 | 0.93 | 1 | −1.80 | −1.80 | 1 | 0.62 | 0.62 | 3 | −0.48 | −0.96 |
| Total increase of height during puberty (cm) | 73 | 40.3 | −0.36 | 2 | 12.60 | 12.00 | 1 | 38.30 | 38.30 | 1 | 24.30 | 24.30 | 1 | 18.90 | 18.90 | 3 | 31.40 | 21.70 |
| Total ganancy during treatment (SD) | 73 | 4.94 | 0.85 | 2 | 1.38 | 1.19 | 1 | 2.46 | 0.85 | 1 | 2.30 | 2.30 | 1 | 3.12 | 3.12 | 3 | 2.30 | 1.81 |
| Reappraisal Gh (ng/ml) | 59 | 33.5 | −1.8 | 2 | 11.06 | 3.47 | 1 | 7.40 | 7.40 | 1 | 3.50 | 3.50 | 1 | 0.17 | 0.17 | 2 | 0.40 | 0.23 |
| Reappraisal IGF1 (ng/ml) | 73 | 1045 | 15.8 | 2 | 409.0 | 301.0 | 1 | 144.0 | 144.0 | 1 | 23.0 | 23.0 | 1 | 99.0 | 99.0 | 3 | 501.0 | 244.0 |
| SD reappraisal IGF1 (SD) | 73 | 3.74 | −7.15 | 2 | 0.87 | −0.07 | 1 | −2.34 | −2.34 | 1 | −7.99 | −7.99 | 1 | −3.50 | −3.50 | 3 | 1.49 | −0.72 |
CNS: central nervous system; SD: standard deviation; BMI: body mass index; IGF1: insulin growth factor 1; Gh: growth hormone; mg: milligrams; kg: kilograms; ng: nanograms; ml: millilitre; max: maximum; min: minimum.
According to literature incidental findings are very frequent in healthy youngers. Assessment of the structural magnetic resonance imaging in young healthy patients revealed incidental findings in 19% of the subjects (39/206). In approximately half of these subjects (n=21), these findings were arteriovenous malformations, cavernomas and pituitary abnormalities.23 Weber et al. report in 2536 young healthy applicants for military flying duties 1.7% arachnoid cysts, 0.51% vascular abnormalities, and 0.47% intracranial tumors.24
However, intracranial incidental findings on computed tomography (CT) imaging are showed in 1% (30/3000) of head trauma patients.25
According to structural findings in healthy children studies with big samples claim that incidental findings are very frequent in healthy youngers23–25 although, based on our experience, findings are more frequent in isolated GH deficiency. Nevertheless, studies show that hypothalamus and pituitary defects are equally distributed between GH deficient patients and healthy patients from a functional point of view, although they maintain that patients with multiple endocrine defects have the smallest pituitary volume and abnormal stalk,2,18,26 fact that we could not check because we only study isolated GH deficiencies. However, a study of 3000 head trauma patients refer that the most common incidental findings are large cisterna magna and tumors and arachnoid cyst at second,25 whereas other study of 2536 healthy young men affirm that the most common findings are arachnoid cyst and vascular abnormalities,24 which is more similar to our results, because we have not found any tumor or cisterna magna enlargement, but we have seen one arachnoid cyst and several vascular abnormalities.
Related to volumetry, current studies claim that the pituitary volume and sella size is lower in those patients with GH deficiency than in those not deficient.9
On the other hand, The Growth Hormone Research Society Workshop and related authors say that structural abnormalities are more frequent in patients with severe deficits,6,27 which is supported by our results, in which more severe deficiency is present in patients with empty sella turcica and combined pituitary ectopia and hypoplasia. Moreover, studies reflect that the group of GH deficiency with more severe deficit were at greater risk of morphological abnormality, which supports our appreciation that those with abnormal resonance findings showed a greater severity.11 The severity of growth hormone deficiency is defined by the fact that patients with abnormal resonance are shorter at diagnosis than those with normal magnetic resonance imaging. Furthermore, those patients with abnormal magnetic resonance imaging had lower GH levels and a better answer to substitutive treatment, which is also showed in our study.14
Our incidence of anatomical findings at resonance in children with short stature is lower than that reported in a retrospective international database study for short stature (11.5% versus 26.8%), probably because we only studied patients with isolated GH deficiency without any associated hormone deficiencies, in which is more frequent to see magnetic resonance abnormalities. On the other hand, we agree that one of the most frequent findings is hypoplasia.19 Furthermore, in international studies, children with earlier onset of GH treatment show a better response to treatment, which is compatible with our results in children with abnormalities in resonance, who also have early onset.12
According to our results we could claim that magnetic resonance imaging is a useful tool in classifying severity of growth hormone deficiency, because patients who showed abnormalities in magnetic resonance imaging were those with earlier diagnosis and shorter height at diagnosis. However, this tool is also a good predictor of response of GH treatment in children with GH deficiency, because those who showed abnormalities in MRI also reached better adult height with treatment.
According to that, a study involving 164 short children also claim that patients with abnormal findings are shorter and younger at the time of diagnosis, which is the same appreciation that we have had in our research.28 Apart from that, it is also interesting to note that in our study those patients with abnormal magnetic resonance findings show statistically significant results in the comparison with normal resonance imaging in terms of target height, bone age/chronological age, pubertal and adult height, which are factors that have not been studied before in published studies, which also support the relation between resonance findings and severity.
In addition, the retrospective international database study for short stature declare that there are lower IGF1 and GH peaks after treatment in patients with abnormal magnetic resonance in comparison with those with normal images, which is supported by our lower peaks in reassessment in those patients with empty sella turcica and combined pituitary hypoplasia and ectopia. Instead of that, they claim that pituitary GH reserve is maintained in small anterior pituitary and empty sella, with low GH response only in pituitary stalk abnormality and multiple pituitary hormonal deficiency because of residual somatotrope cells. Having said that, they propose prolonged GHRH infusion in order to measure the amount of residual GH pituitary tissue.19
Nevertheless, several published studies also mention the permanence of the deficit and a more severe deficit in those with abnormal image as we have said.15,27
Finally, we share with French studies and The Growth Hormone Research Society Workshop the opinion about children with magnetic resonance abnormalities show a better response to treatment with recombinant GH than children with normal imaging.6 Nevertheless, Indian studies refer a better response in patients with hypoplasia,28,29 whereas we have seen a better response in ectopia and empty sella turcica (Fig. 3).
Having said that, the strength of our study is supported by the large sample size, careful methodology and statistical significance confirmed by various statistical tests.
It is remarkable to highlight that in our sample most patients (84.38%) have already reached adult height, so a significant number of patients have been reassessed, which gives scientific strength for establishing their evolution throughout the treatment and after it.
About studies published so far on children with isolated GH deficiency treated with biosynthetic hormone, except for Ranke et al.12 which shows a controlled clinical trial, are all uncontrolled observational studies of between 20 and 2852 children of both sexes, followed for 1 and 9 years, which means a shorter follow-up time with respect to ours. In addition, only 40% of the studies show a greater number of patients than ours, and in some cases for very few patients. As well as in our research, all studies include prepubertal children with male predominance.30 As for the mean dose, the major difference was with french group who show an average dose of 0.42IU/kg/week versus 0.6IU/kg/week, while the rest of the studies present similar doses.31 In published studies there is a heterogeneity of ideas about the onset of puberty, stating in some studies that there is a delay in comparison to others that do not find statistical differences, as it is in our case. In publications establish a total ganancy of height between +0.4SD and +1.5SD,32 which is in line with our gain of +1.15SD. For that, we could justify the disparity of results among studies because of the strong correlation with height at the onset of puberty. In addition, french studies refer a gain of +1.2cm and +0.2SD per year of treatment, compared to our higher gain data (+0.49SD).30
About the gain of height during puberty the National Cooperative Growth Study experience establish a gain of +0.8SD, compared to our results of +0.43SD.32 It may be due to bone age and the onset of puberty not very delayed in our case, associated with resistance or lack of compliance, without being able to confirm it. Having said that, puberty seems to play an important role in the increase of pituitary size in isolated GH patients and multiple pituitary hormone deficiencies.30
Finally, the main limitations of the study are the loss of follow-up, loss of information in retrospective data, and the fact that although most patients have reached adult height, they are not 100% of the sample. In addition, we always have to rely on possible inter-observer errors and possible measurement errors.
Having said that, an abnormal MRI could confirm growth hormone deficiency in patients with deficient growth hormone stimulation tests instead of test's low specificity in this diagnosis, and we would know that they would have a better response, probably because these patients show a more severe GH deficit. On the other hand, we could not use this theory in the other way, because one patient with normal MRI could also have growth hormone deficiency, although we would have less confidence in this diagnosis and in treatment response.
ConclusionsPituitary/hypothalamic magnetic resonance imaging does not show abnormalities in most cases of childhood-onset GH deficiency. However, structural abnormalities in pituitary/hypothalamic magnetic resonance imaging are more frequent in those children with isolated childhood-onset GH deficiency than in healthy controls. Furthermore, there are significant differences in the severity and evolution of GH deficiency among patients with abnormal imaging compared to those with normal imaging of the pituitary/hypotlamic region. So, patients with isolated GH deficiency and abnormal imaging have a more favorable response to GH replacement; and patients with pituitary disorders such as empty sella or hypoplasia and ectopic hypophysis, were those with lower values in reassessment.
Conflicts of interestThere are no conflicts of interest or financing related to this article.