Hospitalized COVID-19 patients may present acute malnutrition which could influence morbidity and mortality. In the first wave of the pandemic severe weight loss was observed in many hospitalized patients. This pilot study evaluates the usefulness of an electronic automatized alarm for the early quantification of a low food intake as a predictor of the risk of malnutrition using COVID-19 disease as a model of severe illness.
MethodsObservational prospective nutritional screening with a daily automatized warning message to the Endocrinology and Nutrition Service provided by the Information Systems. All adult patients admitted for COVID-19 from November 2020 to February 2021 were included. When diet intake was <50% during consecutive 48h, an automated message was generated identifying the patient as “at nutritional risk (NR)” and additional specialist nutritional evaluation and therapy was performed within the next 24h.
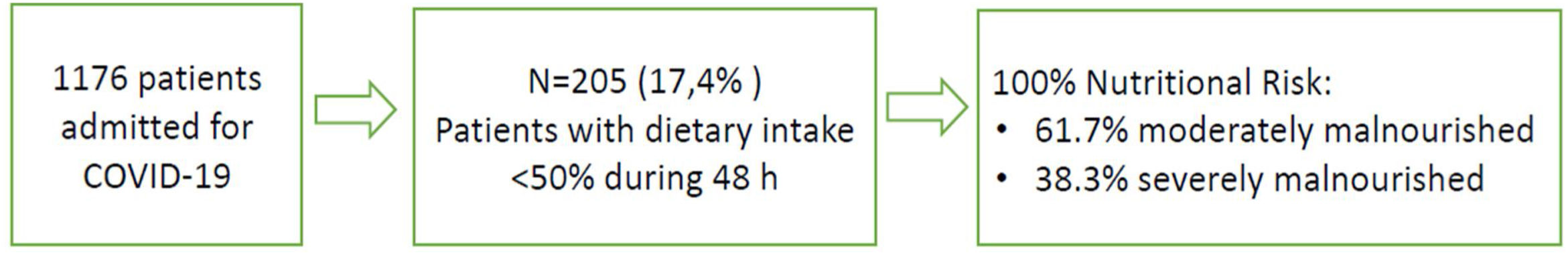
Results205 patients out of 1176 (17.4%) were detected by automatized alarm and were considered as presenting high NR; 100% were concordant by the validated nutritional screening SNAQ. Nutritional support after detection was: 77.6% dietary adaptation+oral supplements; 9.3% enteral nutrition (EN); 1.5% parenteral nutrition (PN); 1% EN+PN and 10.7% no intervention is performed due to an end-of-life situation. Median weight loss during admission was 2.5kg (p25 0.25–p75: 6kg). Global mortality was 6.7% while in those detected by automatized alarm was 31.5%.
ConclusionsThe implementation of an electronic NR screening tool was feasible and allowed the early nutritional assessment and intervention in COVID-19 hospitalized patients and can be useful in patients hospitalized for other pathologies.
Los pacientes hospitalizados por COVID-19 pueden presentar desnutrición aguda, lo que podría influir en la morbimortalidad. En la primera ola de la pandemia se observó una pérdida de peso grave en muchos pacientes hospitalizados. Este estudio piloto evalúa la utilidad de una alarma electrónica automatizada para la cuantificación temprana de una baja ingesta de alimentos como predictor del riesgo de desnutrición, utilizando la enfermedad COVID-19 como modelo de enfermedad grave.
MétodosCribado nutricional prospectivo observacional con un mensaje diario de alerta automatizado al Servicio de Endocrinología y Nutrición proporcionado por Sistemas de Información. Se incluyeron todos los pacientes adultos ingresados por COVID-19 desde noviembre de 2020 hasta febrero de 2021. Cuando la ingesta de la dieta era <50% durante 48horas consecutivas, se generó un mensaje automático identificando al paciente como «en riesgo nutricional» y se realizó una evaluación y terapia nutricional especializada adicional dentro de las siguientes 24 horas.
ResultadosFueron detectados por alarma automatizada 205 pacientes de 1.176 (17,4%), y considerados en riesgo nutricional, 100% resultaron concordantes mediante el cribado nutricional validado «SNAQ». El soporte nutricional tras la detección fue de 77,6% adaptación dietética+suplementos orales; 9,3% nutrición enteral (NE); 1,5% nutrición parenteral (NP); 1% EN+PN y en 10,7% no se realiza ninguna intervención por situación de final de vida. La mediana de pérdida de peso durante el ingreso fue de 2,5kg (p25 0,25-p75: 6kg). La mortalidad global fue de 6,7% mientras que en los detectados por alarma automatizada fue de 31,5%.
ConclusionesLa implementación de una herramienta electrónica automatizada de cribado nutricional fue factible y permitió la evaluación e intervención nutricional temprana en pacientes hospitalizados por COVID-19 y puede ser útil en pacientes hospitalizados por otras patologías.
Malnutrition in hospitalized patients can lead to prolonged recovery times, increased risk of complications, and a higher likelihood of hospital readmission. Patients hospitalized for COVID-19 have been shown to be at high risk of malnutrition1 and may develop disease-related malnutrition (DRM) due to the catabolic situation, the symptoms that interfere with intake and prolonged hospital stay. One of the cardinal signs observed in severe COVID cases is a profound anorexia feeling which interferes with food intake, as we reported in a previous study.2 The European Society for Clinical Nutrition and Metabolism (ESPEN) and other medical societies devised expert statements and practical guidance for the nutritional management of patients with COVID-19 during the pandemic.3–7 These guidelines emphasize the importance of incorporating nutritional intervention and therapy as integral components of the comprehensive care approach for patients. Most expert guidelines recommend earlier assessment and diagnosis of the nutritional status of the patient, as well as the prescription of a medical nutrition therapy adapted to their needs, prioritizing enteral nutrition (EN) versus parenteral nutrition (PN), unless the first is contraindicated.
During the first wave of the pandemic, all COVID-19 patients admitted to our institution were at high nutritional risk (NR) given the acute inflammatory situation with high nutritional requirements. Consequently, we implemented an institutional protocol for nutritional support based on ESPEN recommendations.3 Nevertheless, we found that even the nutritional therapy, patients still presented malnutrition and sarcopenia at hospital discharge, with a very serious loss of weight of more than 10kg in a substantial percentage of them. These findings suggested that these patients required an earlier nutritional assessment after admission for earlier detection of NR or DRM and its prevention and treatment through a prompt initiation and more intensive and prolonged nutritional support and monitoring.2
The future of health care is becoming increasingly performed under digital support strategies. During the COVID-19 pandemic, digital technologies have been harnessed to clinically monitor patients while maintaining social distancing and thus minimizing the risk of virus transmission to hospital health professionals. During the second and third waves, to improve early nutritional risk detection and monitoring, we developed jointly with the hospital Information Systems Department an automatized nutritional screening tool. This alarm was based on the daily quantification of food intake registered in the electronic medical record of every patient by the nurse in charge.
The objective of the present pilot study was to assess the usefulness and effectiveness of the electronic automatized NR screening tool in all non-critical COVID-19 patients admitted to our institution for the detection and treatment of DRM, taking this disease as a model of the catabolic situation that impairs nutritional status in severe illnesses.
Material and methodsPilot observational, prospective study including all adult COVID-19 patients admitted to Germans Trias i Pujol Hospital from November 2020 to February 2021, excluding those in intensive care units (ICU). In all patients admitted, an institutional nutritional support care protocol was applied following previous experience.2 Following hospital protocols, prior to the pandemic, nurses quantified and registered the dietary intake of all patients in their electronic medical history. The risk of malnutrition was assessed by using a simple alarm system based on the quantification of food consumption during the first 48 consecutive hours of admission. In this way, an electronic automatized survey system was set up, allowing all the patients with a dietary intake<50% during these first 48h to be detected by the system and assigned the category of “at nutritional risk” (NR). An alert email was therefore automatically generated and sent to the Endocrinology & Nutrition Service. Confirmation of the NR situation was performed by the administration of the SNAQ questionnaire, a validated NR screening tool.7 Nutritional support was then adjusted by a registered dietitian. Demographic features and comorbidities data were recorded in a specific database; weight was measured at hospital admission on calibrated scales and then every week until discharge. The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethical Research Committee of Germans Trias i Pujol Hospital (protocol code: PI-20-042).
Statistical analysisAll statistical analyses were performed using the statistical program SPSS V22. We performed descriptive statistics using frequencies to summarize categorical variables (percentage) and expressing quantitative variables as mean±standard deviation (SD). Normal distribution of quantitative data was assessed. The univariate tests used were Chi-square for categorical variables and Student's test for quantitative variables. A p-value of <0.05 was set as statistically significant.
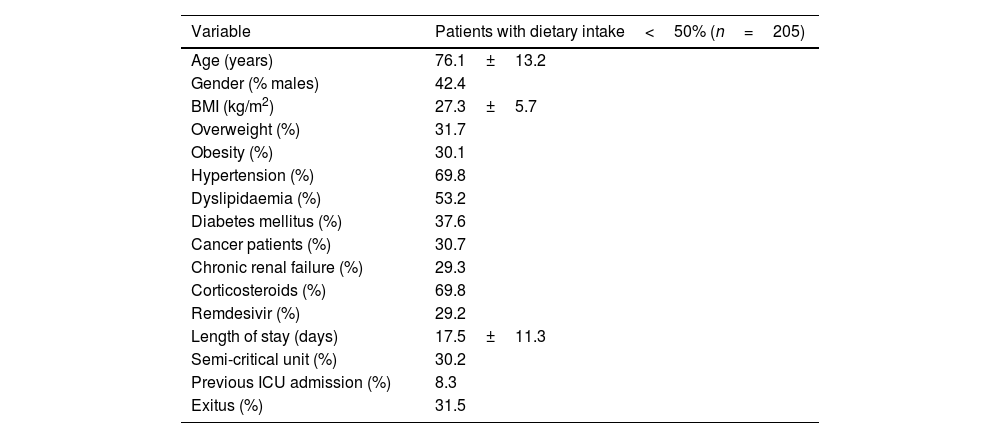
ResultsFrom November 2020 to February 2021, 1176 patients were admitted in non-critical units with the diagnosis of COVID-19. Of those, 205 patients (17.4%) were detected by the electronic automatized alarm. Table 1 shows baseline demographic and clinical data for the total cohort studied. The average age was 76.1±13.2 years, 62.8% were obese or overweight, 8.3% had previously been admitted to the ICU and 30.2% were in a semi-critical unit.
Baseline demographic and clinical data of COVID-19 inpatient.
| Variable | Patients with dietary intake<50% (n=205) |
|---|---|
| Age (years) | 76.1±13.2 |
| Gender (% males) | 42.4 |
| BMI (kg/m2) | 27.3±5.7 |
| Overweight (%) | 31.7 |
| Obesity (%) | 30.1 |
| Hypertension (%) | 69.8 |
| Dyslipidaemia (%) | 53.2 |
| Diabetes mellitus (%) | 37.6 |
| Cancer patients (%) | 30.7 |
| Chronic renal failure (%) | 29.3 |
| Corticosteroids (%) | 69.8 |
| Remdesivir (%) | 29.2 |
| Length of stay (days) | 17.5±11.3 |
| Semi-critical unit (%) | 30.2 |
| Previous ICU admission (%) | 8.3 |
| Exitus (%) | 31.5 |
The 100% of patients detected by the electronic automatized alarm presented NR by SNAQ (61.7% moderately malnourished; 38.3% severely malnourished), represented in Fig. 1.
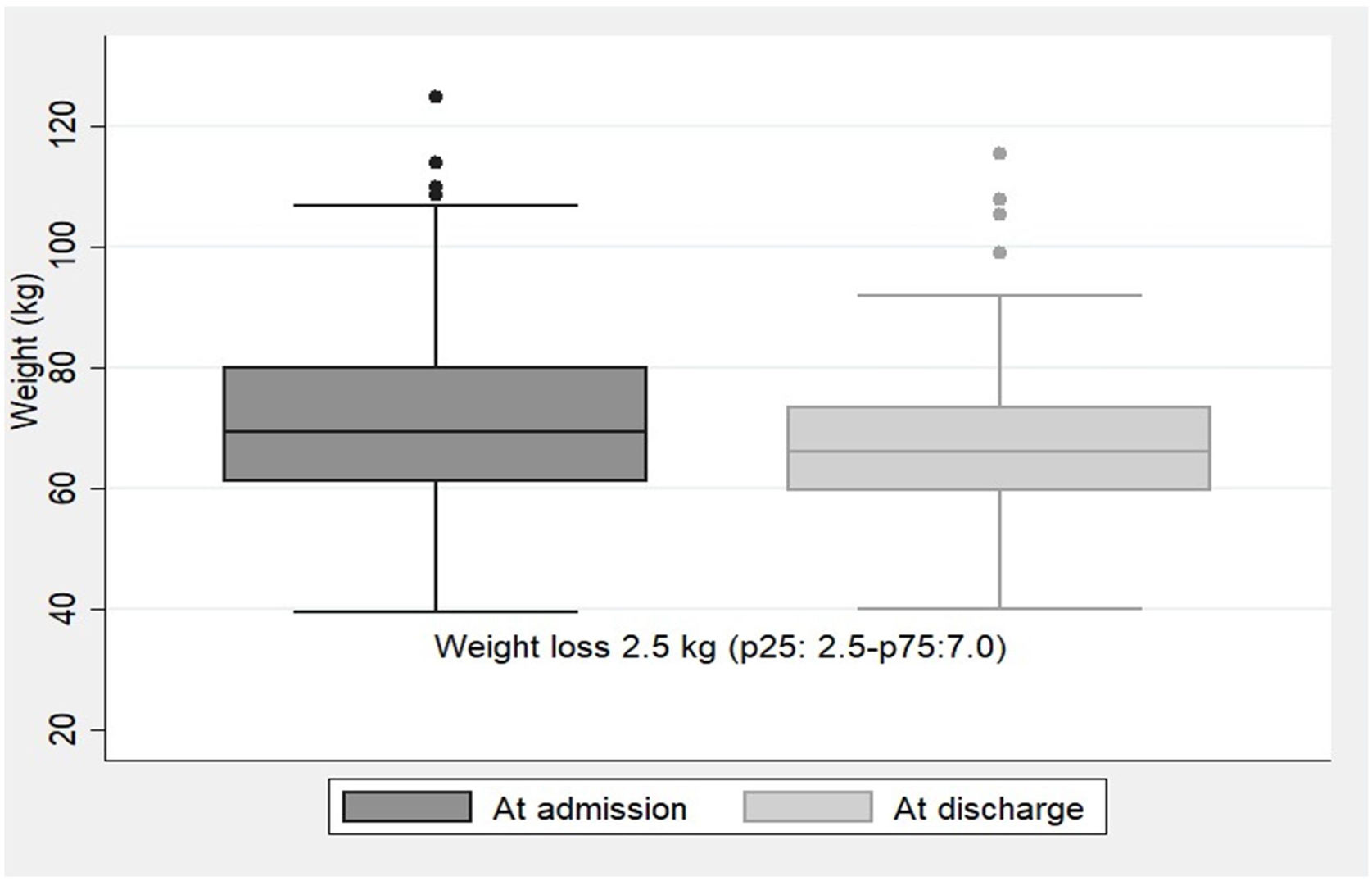
NR was detected at a median of 4 days (p25:2.5–p75:7.0) after hospital admission with a 48-h dietary intake of 22.5±14.6% of the diet supplied. The type of nutritional support performed thereafter was: 77.6% dietary adaptation+oral nutritional supplements; 9.3% EN by nasogastric tube; 1.5% PN; 1% EN+PN; and 10.7% no medical nutritional therapy was performed due to end-of-life situation. During hospitalization, the median weight loss for the whole group of patients was 2.5kg (p25:2.5–p75:7.0). Weight trajectories at admission and at discharge are illustrated in Fig. 2.
In terms of mortality, the prevalence of global mortality of all admitted patients in the second and third wave was 6.7%, whereas in those detected by the electronic automatized alarm was 4 times higher, 31.5%, excluding those patients in end-of-life situations at NR detection.
DiscussionThis pilot observational prospective study shows that all COVID-19 patients detected by the electronic automatized alarm tool presented NR when a validated screening procedure, such as SNAQ, was performed. Therefore, electronic automatized alarm seems a potentially valuable, easy-using marker, and the subsequent validation by the SNAQ tool confirmed it. In this regard, the automatized generated alarm based on low food intake saved time, avoiding the necessity of performing a screening test by the nurse or the physician and the subsequent formal consultation with the nutrition experts. To our knowledge, this is the first study that uses an electronic automatized alarm based on dietary intake as a screening for malnutrition. This simple approach has allowed the implementation of much earlier screening and prescription of nutritional support in hospitalized patients.
Silva, et al. in a review, indicate that in COVID-19 adult patients most tools have high sensitivity for identifying nutritional risk, but none is recognized as the best method for nutritional screening in this group of patients.8 Evidently, in accordance with the guidelines, it is crucial to view screening, assessment, and nutritional support as essential components in the comprehensive care of COVID-19 patients, extending their application to all individuals admitted for alternative medical conditions.
One multicentre retrospective study in China that compared early (<48h) versus not-early (>48h) initiation of nutritional therapy in critical COVID-19 patients demonstrated that in non-survivors the time to start nutrition therapy was later than that in survivors.9 With our screening method with automatized alarms, we detected NR at a median of 4 days (p25:2.5–p75:7.0) of hospital admission. Taking into account that our population is non-critical COVID-19 patients, the electronic automatized alarm allowed very early detection of patients with NR as well as an early initiation of nutritional therapy. Probably, the lower weight loss observed in our patients during the second and third waves of COVID-19 is partly a consequence of these earlier screening and nutritional interventions facilitated by the electronic automatized alarm. The decision to use dietary intake as a nutritional marker was based on the particularly high frequency of anorexia associated with COVID-19 infection,10,11 and the cut-off of <50% follows the recently published recommendations of ESPEN guidelines on hospital nutrition.12 It must be noted that only 17.4% of the patients included during the study period (second and third waves) had a low dietary intake (<50% of the diet supplied). It's probable that the use of dexamethasone as a first-line treatment of COVID-19 patients during these waves, in contrast to the first wave, may have reduced anorexia and improved intake.
A recent publication of the “NUTRICOVID study”, an observational cohort study conducted in 16 public hospitals of the Community of Madrid with COVID-19 survivors who were admitted to the ICU during the first outbreak, found these patients experienced an average weight loss of 16.6% (8.3%) during their hospital stay. Upon discharge, 83.5% and 86.9% of the patients were at risk of malnutrition and sarcopenia, respectively, but only 38% received medical nutrition therapy.13 In our cohort, the nutritional risk of patients with low intake (17.4%) detected by automated alarms and confirmed by a valid screening method was 100%, highlighting that we were already in the second and third wave of the pandemic where knowledge and resources, were already better. Furthermore, from the first wave, all patients received nutritional support and those detected by the automated alarms received an early adjustment by the endocrinology and nutrition team.
Pironi et al. reported a one-day audit performed in general and critical care wards during the first wave of the pandemic (April 2020) and showed that hospital diet intake was less than 50% in most of the patients with a higher prevalence of intermediate care unit and ICU cases and median energy and protein intake/kg body weight (BW) in the range of 25kcal and 1.1g respectively.1 Nevertheless, one recent study conducted in 2021 observed that hospitalized patients with COVID-19 had a higher percentage of low appetite compared to those without COVID-19, however, they were also those who had a higher dietary intake (median of 63.9% of the dish served). The authors justify these findings to the fact that patients with COVID-19 are concerned about the deadly consequences of the disease and, consequently, would be more motivated to consume the hospital diet.14
The electronic automatized alarm model may be eventually applicable to all hospitalized patients since low dietary intake is one of the causes that favor the appearance of DRM or worsen it during admission. Furthermore, the electronic automatized alarm not only could serve as a nutritional screening at admission, but also allow us to detect nutritional risk throughout hospitalization. ESPEN guidelines on hospital nutrition12 recommended that food intake measurement should be part of the nutritional assessment and should be monitored over time. Moreover, the guideline highlighted that in nutritionally at-risk patients, insufficient food intake equal to or less than 50% of energy requirements over 3 days during the hospital stay should lead to a nutritional intervention. This recommendation is based on the results of the European multicentre NutritionDay® survey that showed that decreased food intake represents an independent risk factor for hospital mortality in 16,290 adult hospitalized patients worldwide.15 Moreover, one study that evaluated nutrition intake in critically ill patients with coronavirus disease, observed that those who died had lower protein intake and lower caloric intake than those who survived.16
Gomez-Candela et al. made a similar alarm approach with the CONUT system. This is an automatic method based on analytical parameters (albumin, cholesterol, total lymphocytes) and the generation of NR alerts.17 Nevertheless, nutritional assessment methods that are almost exclusively based on biochemical and immunological markers are not the most suitable for hospitalized patients. Indeed, alterations in albumin, lymphocytes, and cholesterol concentrations may be caused by changes in hydration and inflammation states, or they may occur secondary to treatment, such as statins.
Most expert guidelines recommend that in COVID-19 non-intubated patients not reaching the energy target with an oral diet, oral nutritional supplements (ONS) should be considered first and then enteral nutrition. If there are limitations for the enteral route it could be advised to prescribe parenteral nutrition.3–5 In this way, the nutritional support provided to our patients after NR detection followed these recommendations, as less than a quarter of our patients required EN or PN, although the average dietary intake when detected by electronic automatized alarm was very low (22.5±14.6%). Compared to our recently published study corresponding to the first wave of the COVID-19 pandemic, where even with medical nutrition therapy the medium weight loss was 10kg (p25:7–p75:15),2 with this electronic automatized NR screening tool, less weight loss was observed during admission [2.5kg (p25:0.25–p75:7)], which represents a percentage of weight loss of 5.3%. Another study reports an average weight loss of 5.3% in hospitalized COVID-19 patients, although weight loss>5% was present in 62% of the patients.1 In our study, we only analyzed weight loss in those patients detected by electronic automatized alarm (17.4%), which may probably be those with higher weight loss. However, we cannot confirm this since we do not have anthropometric data at hospital discharge for patients who were not detected by the alarms.
At the hospital, malnutrition is known to be associated with increased mortality, morbidity, length of stay, and costs.18 From our data, NR detected by electronic automatized alarm is also highly informative of poor prognosis, according to the high mortality of 31.5% that this subset of our patients presented, although the medical nutrition therapy applied. Nevertheless, it's probable that mortality would have been higher if no NR screening and nutritional intervention had been performed, as medical nutrition therapy is known to reduce morbidity and mortality in hospitalized patients.19,20
There were limitations in our study. Firstly, the organization of data case recording was influenced by the extreme emergency experienced during the whole pandemic explosion, thus some bias selection may have arisen from this fact. The consideration of centring the NR detection in electronic automatized alarm may be risky because dietary intake during hospitalization depends on many factors, including the patient's diet preferences, periods of fasting due to medical explorations, and the correct record of intake by nurses. Finally, different treatments may have influenced anorexia development, but also, others, like corticosteroids, may have modified hunger sensation, thus, confounders may have played some role in the misinterpretation of the electronic automatized alarm as well as regarding the specific outcomes, mostly in mortality.
ConclusionThe implementation of an electronic automatized NR screening tool was easily applicable in daily clinical practice and allowed early nutritional assessment and intervention in patients hospitalized for COVID-19 and can be useful in patients hospitalized for other pathologies.
Authors’ contributionsConceptualization, A.R., C.J., N.A. and M.P.-D.; methodology, A.R., C.J., N.A. and M.P.-D.; software, A.R., C.J., M.R., M.M., M.C, M.S., E.M., G.S., A.P.M.; M.J.S., J.M.S.-M.; validation, A.R., C.J. and M.P-D.; formal analysis, A.R. and C.J.; investigation, A.R., C.J., M.R., M.M., M.C, M.S., E.M., G.S., A.P.M.; M.J.S., J.M. S-M.; resources, A.R., C.J., N.A. and M.P.-D.; data curation, A.R., C.J., M.R., M.M., M.C., M.S., E.M., G.S., A.P.M.; M.J.S., J.M.S.-M.; writing-original draft preparation, A.R., C.J., and M.P.-D.; writing-review and editing, A.R., C.J., and M.P.-D.; visualization A.R., C.J., M.R., M.M., M.C., M.S., E.M., G.S., A.P.M.; M.J.S., J.M.S.-M., N.A. and M.P.-D.; supervision, A.R., C.J., N.A. and M.P.-D.; project administration, A.R., C.J., N.A. and M.P.-D.; All authors have read and agreed to the published version of the manuscript.
Informed consent statementInformed consent was obtained from all subjects involved in the study.
Institutional review board statementThe study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethical Research Committee of Germans Trias i Pu-jol Hospital (protocol code: PI-20-042).
FundingNo funding or sponsorship was received for this study or publication of this article.
Conflicts of interestThe authors declare that there are no conflicts of interest.