Nutritional support in patients with COVID19 can influence the mean stay and complications in the patient in Intensive Care Unit (ICU).
AimsTo evaluate the selection of enteral nutritional treatment in the COVID-19 patient admitted to the ICU. To know the development of dysphagia and its treatment. To evaluate the adjustment to the requirements and its relationship with the patient's complications.
Material and MethodsOne-center longitudinal retrospective study in 71 patients admitted to the ICU with COVID19 infection and complete enteral nutrition between March and April 2020. Clinical variables were collected: length of stay in ICU, mean stay and rate of complications; and estimated anthropometric variables.
ResultsThe mean age was 61.84 (13.68) years. Among the patients analyzed, 33 (46.5%) died. The median stay in the ICU was 20 (15.75−32) days and the mean stay was 37 (26.75−63) days.
The type of formula most prescribed was normoprotein 24 (35.3%) and diabetes-specific 23 (33.8%) depending on the prescribed formula. There was no difference in mean stay (p = 0.39) or death rate (p = 0.35). The percentage of achievement of the estimated protein requirements was 50 (34.38−68.76).
At discharge, 8 (21%) of the patients had dysphagia. A relationship was observed between the mean ICU stay and the probability of developing dysphagia (OR: 1.035 (1.004−1.07); p = 0.02).
ConclusionsIn the patient with COVID19 disease admitted to the ICU, only half of the necessary protein requirements were reached. The presence of dysphagia at discharge was related to the length of time the patient was in the ICU.
El soporte nutricional en el enfermo COVID19 ingresado en Unidad de Cuidados Intensivos (UCI) puede influir en la evolución durante la hospitalización y al alta.
ObjetivosEvaluar la selección del tratamiento nutricional enteral en el paciente con infección COVID-19 ingresado en UCI. Conocer el desarrollo de disfagia y su tratamiento. Evaluar el ajuste a los requerimientos y su relación con las complicaciones del paciente.
Material y MétodosEstudio retrospectivo longitudinal unicéntrico en 71 pacientes ingresados en UCI con infección COVID que recibieron nutrición enteral total entre marzo y abril de 2020. Se recogieron datos de estancia en UCI, estancia media y tasa de complicaciones; variables antropométricas estimadas y diagnóstico de disfagia.
ResultadosLa edad media fue de 61,84(13,68) años. Entre los pacientes analizados fallecieron 33(46,5%). La mediana de estancia en UCI fue de 20(15,75−32) días y la estancia media fue de 37(26,75−63) días.
El tipo de fórmula más prescritas fue normoproteica 24(35,3%) y específica de diabetes 23(33,8%). No hubo diferencia en la estancia media (p = 0,39) o en la tasa de éxitus (p = 0,35) en función de la fórmula prescrita. El porcentaje de consecución de los requerimientos proteicos estimados fue de 50(34,38–68,76) %.
Al alta, 8(21%) de los pacientes tenían disfagia. Se observó un aumento en el riesgo de disfagia en función de la estancia media en UCI (OR:1.035(1.004−1.07);p = 0.02).
ConclusionesEn el paciente con infección COVID19 en UCI únicamente se alcanzaron la mitad de los requerimientos proteicos necesarios en una situación de estrés. La presencia de disfagia al alta se relacionó con el tiempo que el paciente estuvo en la UCI.
The novel SARS-CoV-2 coronavirus triggered a pandemic that modern medicine had never seen before, given that the last event of this scale took place more than 100 years ago. There have been more than 195 million confirmed cases of this disease worldwide and more than four million deaths. Data from Spain are also quite alarming, with 4.13 million confirmed cases and 81,000 deaths1.
No population group is immune to this disease. However, elderly patients or those with multiple comorbidities tend to be most severely affected. In addition, certain metabolic diseases such as obesity and diabetes mellitus are risk factors for the onset of severe disease. They could explain the disparity observed in the different age groups2,3.
It is known that the severity of SARS-CoV-2 infection is directly related to the patient's nutritional status. Firstly, it is a disease that disproportionately affects elderly patients and those with multiple comorbidities with an unfavourable nutritional status. In contrast, another nutritional disease like obesity seems to cause more severe symptoms.
The most severe symptoms of this disease may require admission to the intensive care unit (ICU). Critical patients may require several measures or processes that can hinder adequate nutritional intake. On the one hand, it can be complex to adjust the patient's energy-protein intake to their requirements; these patients have increased muscle catabolism, higher energy requirements and exceptionally high protein requirements, especially in the acute phase of the disease4. On the other hand, gastrointestinal intolerance is expected in the acute phase of the disease, causing abdominal distension, diarrhoea and abnormal laboratory parameters. These factors can hinder the administration of nutritional support5.
Severe nutritional deterioration is common among patients following a stay in an ICU, particularly after prolonged admission to an acute ICU; this ICU-acquired weakness is a syndrome related to nutritional deficiency, reduced mobility and the use of certain drugs and therapeutic measures (mechanical ventilation), which can cause severe chronic muscle deterioration6. Another concerning aspect of the ICU is the risk of dysphagia due to the trauma of intubation and the onset of sarcopenic dysphagia associated with the ICU-acquired weakness mentioned above7. Suffering from such dysphagia may prevent the patient from meeting their nutritional requirements during admission, and it can become chronic and still unresolved at discharge4,8.
Malnutrition can affect half of all patients admitted due to COVID-19 infection9. It has been found that in-hospital nutritional intake is below the estimated requirements (up to 39% of patients consumed less than 50% of the prescribed diet)9. As such, artificial nutrition plays an essential role in the proper nutritional support of these patients. Even so, many aspects of this type of treatment still have yet to be clarified due to a lack of evidence owing to the novelty of this disease and its associated care overload.
In summary, this disease is associated with significant nutritional deterioration caused by the gastrointestinal manifestations of the infection (anorexia [26.8%], diarrhoea [12.5%], nausea/vomiting [10.2%] and abdominal pain [9.2%])10, high energy expenditure due to disease-induced inflammation5 and, finally, disease sequelae such as stroke or deterioration associated with admission to critical ICUs11. A study conducted by Li et al. using the Mini Nutritional Assessment (MNA) found that 53% of hospitalised patients over the age of 65 years were malnourished and 27% at risk of malnutrition2.
This study aimed to analyse the nutritional status of patients with COVID-19 infection admitted to the ICU and administered complete enteral nutrition, the artificial nutritional support used during admission and discharge, and the complications associated with malnutrition, such as dysphagia, which these patients can manifest.
Material and methodsStudy designA retrospective, longitudinal, observational study was conducted on patients admitted to the Hospital Clínico Universitario de Valladolid (HCUV) ICU due to COVID-19 during the so-called first wave of the pandemic (March to April 2020).
The inclusion criteria were: aged 18 years or over; patients hospitalised with a diagnosis of COVID-19 disease; and patients who had to be admitted to the ICU at any time during their hospital stay, which required enteral tube feeding during their time in the ICU. The exclusion criteria were: patients with SARS-CoV-2 infection who did not require admission to the ICU; and patients with symptoms consistent with COVID-19 disease but without a confirmatory microbiological diagnosis.
VariablesThe determined variables were categorised into clinical variables (gender, age, Charlson index before admission, length of ICU stay, mean stay, the reason for discharge, readmission); anthropometric variables (estimated weight [kg]; usual weight [kg]; estimated height [m]; BMI [kg/m2]); and treatment-related variables (route of administration, nutrition [pre-admission, admission, discharge]; nutritional support duration, amount of nutritional therapy administered, oral dietary supplements [pre-admission, admission, discharge] and enteral nutrition formula).
Prescribed enteral nutritional support was adjusted to protein requirements, estimating them to be 1.3 g of protein/kg/day based on the ESPEN guideline recommendations for critically ill patients of 1.2−2 g protein/kg/day5. A percentage was obtained using the amount of protein prescribed divided by the amount of protein needed (estimated based on the patient's estimated weight) and multiplying this result by 100.
All patients receive enteral tube feeding (nasogastric tube or gastrostomy). Dysphagia was diagnosed using the Volume-Viscosity Swallow Test (V-VST)12 or by direct laryngoscopy in those patients in whom it was necessary. This test was performed when the patient's consciousness level was sufficient to remove the nasogastric tube safely. Patients whose dysphagia persisted throughout admission were re-assessed for dysphagia at discharge.
Data collection and processingThis study was conducted in accordance with the Declaration of Helsinki, and all procedures were approved by the Hospital Clínico Universitario de Valladolid Medical Research Independent Ethics Committee (mIEC) with the code PI 17-543.
Statistical analysisData were stored in a database of the SPSS 15.0 statistical software (SPSS Inc. II, USA) officially licensed by the Universidad de Valladolid (UVA). The Kolmogorov–Smirnov test was used to analyse the normality of the distribution of the continuous variables.
Continuous variables were expressed as mean (standard deviation), parametric variables were analysed using Student's paired and unpaired t-tests, and non-parametric variables were analysed using the Friedman, Wilcoxon, Kruskal–Wallis and Mann–Whitney U tests. If it is necessary to compare variables in more than two groups, the ANOVA U test (with Bonferroni post-hoc test) will be used. The variables at the different times of the study were analysed using multivariate analysis of variance (MANOVA).
Qualitative variables were expressed in percentage (%) and analysed using the chi-squared test (with Fisher's correction and Yates's correction when necessary).
ResultsIn total, 71 patients were analysed. The mean age was 61.84 (13.68) years; 47 (66.2%) of the patients were men, and 24 (33.8%) were women.
The Charlson index was measured before admission in all patients, recording a median of 3 (2−5).
Thirty-three (33) (46.5%) of the patients analysed died. Of the patients who survived, the median length of ICU stay was 20 (15.75−32) days, and the mean overall hospital stay was 37 (26.75−63) days.
The reason for the end of admission was death in 33 (46.5%) of the patients; discharge home in 30 (42.3%); transfer to the hospital of origin in six patients (8.5%), and move to a long-stay centre in two (2.8%).
Of the patients discharged, four (10.5%) were readmitted.
The general characteristics of our population are shown in Table 1.
General characteristics of the sample.
| Variable | Total |
|---|---|
| Age (years) | 61.84 (13.68) |
| Gender (M/F) (%) | 47 (66.2%)/24 (33.8%) |
| Charlson | 3 (2−5) |
| Death (%) | 33 (46.5%) |
| Enteral nutrition duration (days) | 12 (7−20) |
| Mean stay in ICU (days) | 20 (15.75−32) |
| Total mean stay (days) | 37 (26.75−63) |
| % EN adjustment to requirements | 50% (34.38−68.76) |
EN: enteral nutrition; F: female; ICU: Intensive Care Unit; M: male.
During their stay in the ICU, all patients received complete enteral nutrition. Of these, 66 (93%) received enteral nutrition as their only source of nutrition, and five (7%) received parenteral nutrition at some time during their admission associated with enteral nutrition.
The standard formula with normal calorie and protein content (35%) was the most widely-used enteral formula. The most widely-used specific formula was the diabetes formula (34%). The other formulas used were the high-protein formula (22%) and the adult respiratory distress syndrome formula (9%).
The formula of 44 patients (62%) was changed at some point during their stay in the ICU for the following reasons: hyperglycaemia: 19 (43.2%); diarrhoea: six (13.6%); high protein requirements: 10 (22.7%); acute respiratory distress syndrome (ARDS): three (6.8%); and other causes: six (13.6%).
Protein requirements were assessed based on the estimated weight of discharged patients, with a mean of 116.26 g (19.43) per day. In total, 50% of protein requirements with the enteral nutrition prescribed in the ICU were met (34.38−68.76). In total, 17 patients (50%) met more than 50% of their requirements, while seven (20.6%) met more than 75% of their protein needs. There were no differences between the various formulas used (Fig. 1).
The median enteral nutrition duration was 12 (7−20) days.
There were no significant differences in the outcome variables (death, mean length of ICU and overall stay) between the various enteral tube feeding regimens prescribed, as shown in Fig. 1.
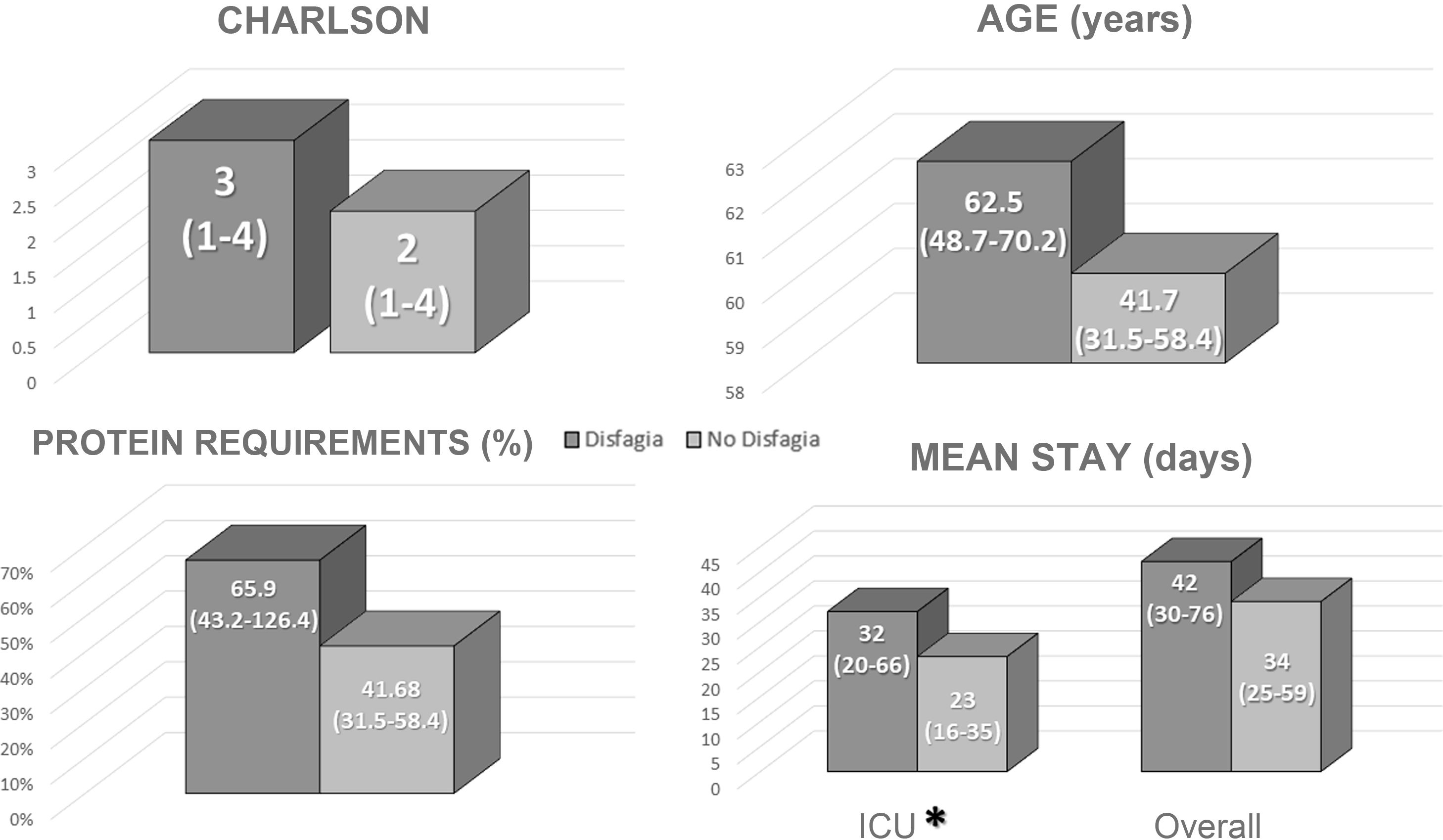
Clinical course of dysphagiaNone of the patients had dysphagia on admission. At discharge, eight patients (11.3% of the total; 21% of survivors) had some degree of dysphagia and had to be educated and prescribed a specific diet.
A direct correlation was found between patients with dysphagia at discharge and length of stay in the ICU (Fig. 2). No differences were identified in mean total length of stay, age, Charlson index or in adjusting to protein requirements between the different types of enteral nutrition administered in the ICU (Fig. 2).
The univariate analysis revealed that a more extended stay in the ICU was associated with an increased risk of manifesting dysphagia at discharge (Table 2). However, the multivariate analysis failed to identify any differences in probability (Table 3).
Univariate analysis of the outcome variables proposed for the onset of dysphagia.
| Dysphagia | OR | 95%CI | p-value |
|---|---|---|---|
| Age (years) | 1.005 | 0.95−1.07 | 0.86 |
| Mean ICU stay (days) | 1.035 | 1.004−1.07 | 0.02 |
| Mean stay (days) | 1.013 | 0.99−1.04 | 0.29 |
| % ICU EN adjust to prot req | 1.03 | 0.997−1.06 | 0.08 |
| Charlson | 0.9 | 0.6−1.27 | 0.45 |
% ICU EN adjust to prot req: percentage adjustment to protein requirements of enteral nutrition prescribed in the ICU; ICU: Intensive Care Unit.
Multivariate analysis of the outcome variables proposed for the onset of dysphagia.
| Dysphagia | OR | 95%CI | p-value |
|---|---|---|---|
| Age (years) | 1.02 | 0.89−1.16 | 0.79 |
| Mean ICU stay (days) | 1.08 | 0.95−1.24 | 0.25 |
| Mean stay (days) | 0.95 | 0.86−1.06 | 0.39 |
| % ICU EN adjust to prot req | 1.04 | 0.99−1.09 | 0.16 |
| Charlson | 0.74 | 0.23−2.38 | 0.61 |
% ICU EN adjust to prot req: percentage adjustment to protein requirements of enteral nutrition prescribed in the ICU; ICU: Intensive Care Unit.
Of the survivors discharged from the ICU (38 patients [53.5%]), 21 (55.2%) received nutritional supplementation. The types of supplementation prescribed were as follows: high-calorie, normal protein, two (9.5%); high-calorie, high protein: 13 (61.9%); diarrhoea-specific: two (9.5%); dysphagia-specific: five (23.8%).
Nutritional supplementation was prescribed during admission to elderly patients and those with a more extended ICU stay (Table 3), although only six surviving patients (15.7%) were still prescribed these supplements at discharge. The patients still prescribed nutritional supplementation at discharge had a more prolonged ICU and hospital stay (Table 4).
Comparison of differences in the different variables according to the prescription of oral nutritional supplements during admission and discharge.
| ONS admission | p-value | ONS discharge | p-value | |||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | |||
| Age | 61.81 (13.39) | 55.05 (15.86) | 0.04 | 67.40 (11.55) | 56.50 (15.12) | 0.13 |
| Mean ICU stay (days) | 40.47 (29.10) | 24.57 (17.58) | 0.01 | 65.83 (40.19) | 25.28 (13.59) | <0.01 |
| Mean stay (days) | 63.71 (35.34) | 34.86 (21.71) | 0.18 | 83.50 (49.85) | 41.06 (22.51) | <0.01 |
| Charlson | 2.8 (1.77) | 2.24 (1.81) | 0.28 | 3.33 (1.37) | 2.38 (1.84) | 0.24 |
ICU: Intensive Care Unit; ONS: oral nutritional supplements.
In italics: statistical significance p < 0.05.
The multivariate analysis did not reveal an increased risk of nutritional supplement prescribing based on mean length of stay, age or functional status at admission.
DiscussionThis study was conducted in patients admitted to the ICU for SARS-CoV-2 infection during the first wave of the COVID-19 pandemic who received complete enteral nutrition. During their stay in the ICU, standard formulas were predominantly used (normal calorie and normal protein content) without observing apparent differences in variables such as mortality or mean length of stay between the various formulas used. Moreover, despite increased calorie and protein requirements, nutritional supplementation was prescribed in half of the surviving patients during post-ICU hospitalisation, and only 15% of survivors continued receiving supplements at discharge. 11% of all patients had some degree of dysphagia at discharge.
The mean patient age was 62 years. This is similar to the findings of a prospective study conducted by Ferrando et al., which identified the mean age of patients admitted to the ICU during the first wave of the COVID-19 pandemic to be 64 years13. In contrast, Berenguer et al. report that the mean age of hospitalised patients is 70 years14. In our sample, almost half of the patients analysed died. The mortality rate of other series was lower. The study by Ferrando et al. showed a 30% mortality rate among patients admitted to the ICU due to SARS-CoV-2 infection during the first wave13. In comparison, the European series by Wendel et al. reported a mortality rate of 24%15. This could be explained by the inclusion of patients in the most acute phase of the first wave. A mortality rate of 24% was observed among non-critical hospitalised patients at our hospital during the third wave of the pandemic, similar to other series16. Of those patients who survived, the median length of stay in the ICU was 20 days, somewhat longer than in the aforementioned studies13,15. This could explain why the mortality rate was higher than in other studies. In contrast, the mean length of hospital stay was a little over a month, similar to the findings of other studies14.
According to the latest ESPEN guideline on nutritional support in critically-ill patients, patients admitted to the ICU are recommended to achieve energy requirements of 15−20 kcal/kg and protein requirements of 1.2−2 g protein/kg in the first week of admission, with a consensus of around 1.3 g protein/kg/day on average5,6. In our study, half the patients met more than half their estimated protein requirements, while just seven patients achieved more than 75% of their protein requirements. The use of different empirical treatments (with other metabolic complications) and patient difficulties tolerating specific measures, such as proning, could explain why adequate volumes of enteral nutrition were not achieved. However, there is no evidence of increased complications with the use of enteral nutrition in conjunction with proning or in non-COVID-19 ICU patients17. For COVID-19 patients, the guidelines recommend nutritional support always with measures that prevent possible complications such as vomiting or aspiration18,19.
For complete enteral nutrition, it is recommended to use energy-dense (>1.25 kcal/mL) and high-protein (>20% total energy value) formulas18. There is no consensus on the use of specific diabetes or omega-3 enriched formulas (such as those used in adult respiratory distress syndrome)20. In our sample, formulas with normal calorie and normal protein content were most widely used, probably to minimise tolerance abnormalities to them, as well as the recommendation to use potentially energy-dense nutrition in the hyperacute phase. However, this recommendation is associated with a high-protein component, which was not achieved21. The second most widely used were diabetes-specific formulas (energy-dense and high-protein), probably due to hyperglycaemia associated with the high doses of corticosteroids used by these patients.
Despite recommendations for different types of formula22, there is no evidence that any specific formula (diabetes-specific, ARDS-specific or diarrhoea-specific) has been associated with an improvement in complications. Our study also found no difference in mortality or mean length of stay between the different enteral formulas.
Prolonged ICU stays can be associated with a severe deterioration in functional status related to various factors (underlying disease, immobility, changes to nutritional support, etc.) that hinder patient recovery even several months after admission6. Deterioration in muscle mass and muscle function has been observed in almost 50% of patients admitted to the ICU due to COVID-19 infection23. In this context and in combination with various circumstances associated with ICU admission, such an intubation, mechanical ventilation and sedation, dysphagia can be perpetuated, which may be the cause or consequence of an abnormal nutritional condition in these patients. A recent study by Martín-Martínez et al. found that 51.7% of patients with COVID-19 infection developed dysphagia during admission, which acted as an independent risk factor for malnutrition. Of these patients, 39.8% still manifested some degree of dysphagia at discharge24. In our study, 21% of patients had some degree of dysphagia at discharge. This discrepancy could be due to the type of patient studied, given that this study only analysed those patients admitted to the ICU rather than all hospitalised patients.
The results of the NOURISH study25 have shown that administering nutritional supplementation during admission and at discharge in patients with cardiorespiratory diseases improves morbidity and mortality and is cost-effective. Although this study did not include patients with COVID-19 infection, patient characteristics in stress and convalescence were similar. As a result, the various guidelines recommended nutritional supplementation, preferably energy-dense and high-protein, provided that the patient did not meet their requirements through an oral diet19. In our study, oral nutritional supplements were prescribed during admission to more than half of all surviving patients, although at discharge, only 16% were still receiving these supplements. The main reasons for their prescribing were advanced age and longer stay in the ICU, leading to the inference that those patients with the greatest deterioration received such regimens. However, we could not establish a relationship between the adjustment of dietary intake to requirements and the need for oral supplementation.
Our study has several limitations. Because it is a retrospective study, data collection is conditioned as variables were not requested or collected at the time. Similarly, the explosive nature of the first wave of the pandemic hindered the collection of certain anthropometric parameters and more complex nutritional tests owing to a healthcare system overwhelmed by the number of admissions and staff shortages in the first wave. The small sample size may result in an underestimation of our findings.
The main strength of this study is that it could serve as guidance on key issues for conducting studies to identify patients at risk of malnutrition and dysphagia and which patients require more intensive care. In addition, the description of the nutritional complications of this emerging disease, particularly during the first wave, could help bolster our knowledge of SARS-CoV-2 infection in the different fields of medicine it affects.
ConclusionsIn patients with COVID-19 infection admitted to the ICU, only half the necessary protein requirements were met in a situation of stress. No differences in mean length of stay or mortality rate were observed between the different nutritional formulas administered.
The manifestation of dysphagia at discharge in patients hospitalised for SARS-CoV-2 pneumonia was associated with their length of stay in the ICU.
The prescribing of oral nutritional supplements at discharge to patients admitted for SARS-CoV-2 pneumonia was associated with the mean length of hospital stay and the patient's age.
Conflicts of interestThe authors declare that they have no conflicts of interest.






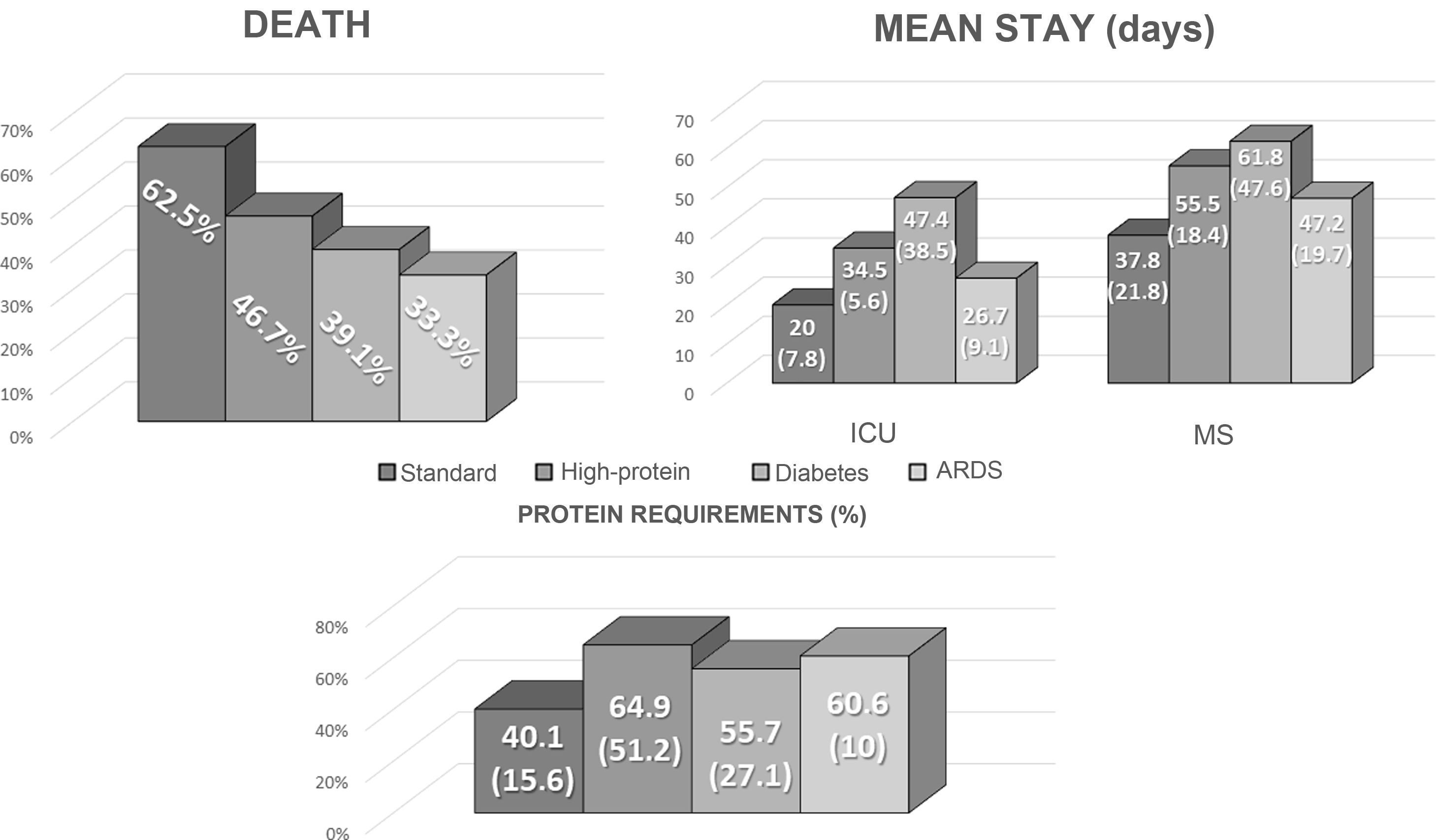
![Comparison of the outcome variables (mean length of stay [ICU and general], death) and adjustment to protein requirements by type of enteral nutrition used in the ICU. ICU: Intensive Care Unit; EN: enteral nutrition; prot req: protein requirements. Comparison of the outcome variables (mean length of stay [ICU and general], death) and adjustment to protein requirements by type of enteral nutrition used in the ICU. ICU: Intensive Care Unit; EN: enteral nutrition; prot req: protein requirements.](https://static.elsevier.es/multimedia/25300180/0000006900000010/v2_202301310826/S2530018022002165/v2_202301310826/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

