Short stature is the most frequent reason for Pediatric Endocrinology consultations and sometimes requires treatment with growth hormone.
ObjectiveThe possible correlation of a good response to any early response factor with a better final response was studied, and also whether there was a difference in response to treatment according to the type of deficit.
Patients and methodsThis was a longitudinal, retrospective and observational study of 139 patients treated for idiopathic growth hormone deficiency up to adult height. There were good response criteria in the first year of treatment: a) an increase in growth rate ≥3 cm/year, b) a growth rate ≥1 standard deviation (SD), c) an increase in height ≥0.5 SD, d) an increase in height ≥0.3 SD. Study of the Index of Responsiveness to treatment in the first and second year. Final response variables: adult height with respect to target height, adult height with respect to initial growth prediction and adult height with respect to initial height at the start of treatment. The possible correlation of a good response to any of the early response factors with a better final response to treatment was studied, and also whether there was a difference in the response to treatment according to the type of deficit.
ResultsThe treatment produced a gain in adult height with respect to genetic height of 0.06 ± 0.7 SD. Patients considered good responders in the first year of treatment presented a better final response (growth rate ≥3 cm: p = 0.000, growth rate ≥1 SD: p = 0.008, height gain ≥0.5 SD: p = 0.007, height gain ≥0.3 SD: p = 0.006), as well as patients with a severe deficit (p = 0.04). The index of responsiveness to treatment during the first year was associated with a better final response (r = 0.249, p = 0.003), with this correlation being maintained in the second year (r = 0.294, p = 0.01).
ConclusionsGrowth hormone treatment increased height in the genetic target. The percentage of good responders varied depending on the criteria used. The response in the first year of treatment and a severe deficit were determining factors for achieving a good long-term response.
La talla baja es el principal motivo de consulta en endocrinología pediátrica, precisando en ocasiones tratamiento con hormona de crecimiento.
ObjetivoEvaluar la relación de los factores de buena respuesta al tratamiento con hormona de crecimiento durante el primer año con la respuesta final y valorar si existen diferencias según el tipo de déficit.
Pacientes y métodosEstudio longitudinal, retrospectivo y observacional en 139 pacientes tratados por déficit de hormona de crecimiento idiopático (severo o parcial) hasta talla adulta. Criterios de buena respuesta en el primer año de tratamiento: a) incremento velocidad de crecimiento ≥3 cm/año, b) velocidad de crecimiento ≥ 1 desviación estándar (DE), c) incremento talla ≥ 0,5 DE, d) incremento talla ≥ 0,3 DE. Estudio del índice de repuesta al tratamiento al primer y segundo año. Variables de respuesta final: talla adulta, talla adulta respecto a talla genética, talla adulta respecto al pronóstico de crecimiento inicial y talla adulta respecto a talla al inicio del tratamiento.
ResultadosEl tratamiento produce una ganancia de talla adulta con respecto a la talla genética de 0,06 ± 0,7 DE. Los pacientes buenos respondedores el primer año de tratamiento presentaron una mejor respuesta final (velocidad de crecimiento ≥ 3 cm, p = 0,000; velocidad de crecimiento ≥ 1 DE, p = 0,008; ganancia talla ≥ 0,5 DE, p = 0,007; ganancia talla ≥ 0,3 DE, p = 0,006), así como los pacientes con déficit severo (p = 0,04). El índice de respuesta al tratamiento al primer año asocia una mejor respuesta final (r = 0,249; p = 0,003), manteniéndose el segundo año (r = 0,294; p = 0,01).
ConclusionesEl tratamiento con hormona de crecimiento alcanza la talla genética. El porcentaje de buenos respondedores varía según el criterio. La respuesta al primer año de tratamiento y un déficit severo son factores determinantes para conseguir una buena respuesta final.
Short stature is the most common reason for consultation at paediatric endocrinology clinics.1 Short stature is considered to be less than −2 standard deviations (SD) from the mean of the reference population for the same age and sex.2 By this definition, short stature affects 2.3% of the population, and is often the cause of significant stress on the growing child and their family.3 The probability that short stature is pathological will depend, among other things, on its severity. It is estimated that in only 1 in 10 patients with height between −2 and −3 SD the cause is organic, while in approximately 58% of patients with height less than −3 SD there is an organic cause.1
Growth hormone (GH) deficiency is a major cause of short stature in childhood. Its diagnosis is based on symptoms, auxological criteria and endocrinological studies. The importance of early diagnosis and treatment of GH deficiency (GHD) has been widely demonstrated due to its influence on adequate neurological, metabolic and auxological development.4
Recombinant human growth hormone (rhGH) has been available as a replacement therapy since 1985, replacing the use of GH derived from the human pituitary due to the risk of contamination with the Creutzfeldt–Jakob prion. The main objective of treatment with rhGH in these patients is to normalise height during childhood and adolescence and to achieve an adult height (AH) within the normal range and in accordance with their target height (TH), minimising the risks of treatment and at the lowest possible cost.5 Like other treatments, rhGH represents a significant expense for the health system that has decreased in recent years. Even so, it would be beneficial to define response predictors to optimise treatment efficacy. This fact justifies the need for long-term studies with rhGH, to provide clinicians with the necessary information to select those patients who could benefit the most from treatment without long-term risks.6
Different clinical trials demonstrate the efficacy of rhGH treatment so that these children can achieve recuperative growth.2 It is thought that an adequate response to treatment in the first year is essential, since it is decisive in achieving an adequate height gain in subsequent years, and is correlated with the result of the final height.7 However, there is significant variability in the response to such treatment, in that not all patients obtain the same degree of benefit.
Therefore, taking as a hypothesis that the response variability may be due to the idiopathic cause of GHD, which probably encompasses other conditions, we set the study objective of assessing efficacy of treatment on AH in GHD, and analysing the relationship with response to it in the first year and with the index of responsiveness in the first and second years, In addition, we aimed to explore whether there is a difference in response to treatment depending on the type of GHD (severe or partial).
Patients and methodsStudy populationThis was a longitudinal, retrospective and observational study with patients monitored in paediatric endocrinology consultations at a tertiary hospital, who were born between 1989 and 2004 and had been treated for idiopathic GHD.
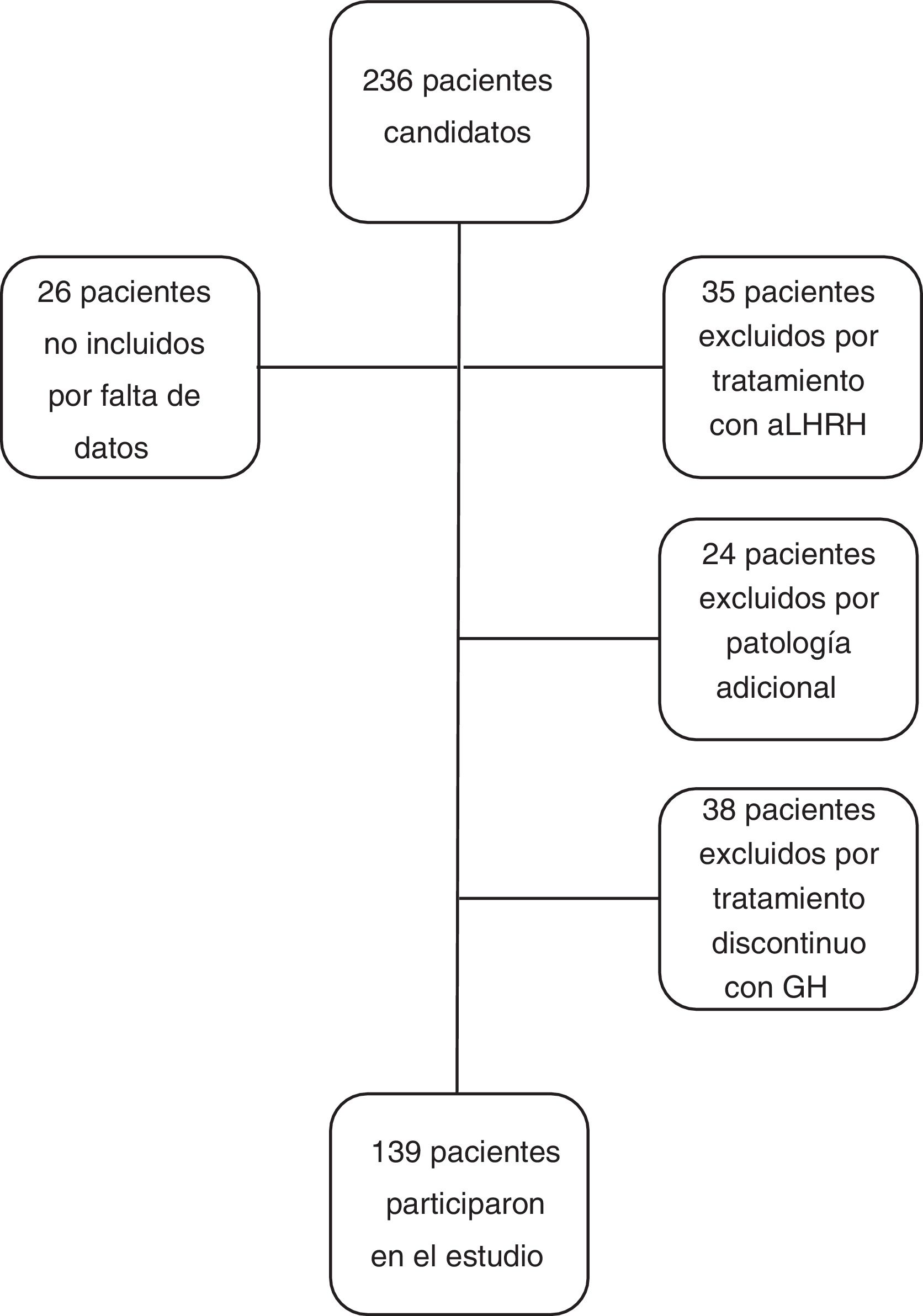
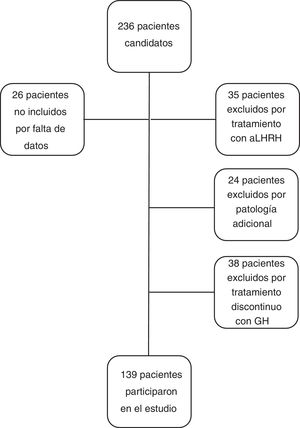
The inclusion criteria for the study were: patients with height less than −2 SD for age and sex at the diagnosis of GHD, with a GH level <10 ng/ml in two GH stimulation tests, who had received treatment with GH at substitute doses for at least one year and who reached near-AH. Near-AH was considered to be when the growth rate is <0.5 cm/year and the bone age is 15 years in females or 17 years in men. This concept has been used in the study when referring to AH since the patients were discharged before reaching the final AH. The exclusion criteria for the study were: patients with GHD of organic aetiology, with additional pathology that could condition growth and height prognosis (cancer, congenital heart disease, infantile cerebral palsy, syndromes or chromosomal diseases), patients who had received concomitant treatment with luteinising hormone-releasing hormone analogues, patients diagnosed with GHD with a single stimulus test, or patients who received treatment with rhGH discontinuously, excluding from the study a total of 97 patients (n = 97), with the final study group being 139 patients (n = 139). No patient was excluded due to poor response or adverse effects to treatment (Fig. 1).
Data collection for studyThe variables studied were the following: 1) genetic background: maternal height, paternal height and TH; 2) perinatal history: type of pregnancy, gestational age at birth, type of delivery, associated perinatal pathology and neonatal anthropometry; 3) anthropometry during follow-up: weight, height, body mass index; 4) variables during follow-up: growth rate (GR), bone age, growth prediction (GP) and AH; 5) lab test results: GH-IGF axis study (maximum peak of GH after stimulation test), insulin-like growth factor type 1, insulin-like growth factor transporter protein 3, 6) treatment: dose of rhGH in mcg/kg/day.
The height, weight and body mass index variables were calculated and expressed in absolute value and in SD according to the Estudios Españoles de Crecimiento 2010 [Spanish Growth Studies].8 The GR variable was expressed in absolute value and in SD according to the Estudios Españoles de Crecimiento 2008.9 To calculate bone age, the Radiographic Atlas of Skeletal Maturation by Greulich and Pyle was used,10 and the AH prediction was calculated based on height and bone age, with the Bayley–Pinneau method.11 All the variables studied were evaluated one year prior to treatment with rhGH, at the beginning of treatment, and annually until reaching near-AH.
The following criteria were analysed, proposed by Bang et al.12 and considered a good response in the first year of treatment:
- a)
Increase in (Δ) GR ≥3 cm/year in the first year.
- b)
GR ≥1 SD in the first year.
- c)
Δ in height ≥0.5 SD in the first year.
- d)
Δ in height ≥0.3 SD in the first year.
The index of responsiveness to treatment (IoR)13 was analysed in the first and second year of treatment. This index of responsiveness is a prediction model initially developed and validated by Ranke et al.,13 in which relevant patient parameters are used in order to define a model that would have practical utility in predicting prepubertal growth during each of the first four years of treatment with rhGH. The IoR was calculated for the first (IoR1) and second (IoR2) year according to the following formulas:
- –
IoR1 = (GR first year−[12.41–0.36*GH starting age + 0.47*birth weight SD + 1.54*(log [3*Initial GH dose (mg/kg/week)])−0.6*[Height SD 1st year–TH SD] + 0.28*weight SD 1st year)/1.72.
- –
IoR2 = GR 2nd−[5.69–0.09*GH starting age + 0.63*(log[3*Initial GH dose (mg/kg/week)]) + 0.24*weight SD 2nd year + 0.31*GR 1st year)/1.19.
The criteria for final response to treatment were:
- a)
AH (SD)
- b)
AH with respect to TH (SD)
- c)
AH with respect to initial GP (SD)
- d)
AH with respect to height at treatment initiation (TI) (SD)
The possible correlation of a good response with any of the early response factors in the first year of treatment, or of IoR1 and IoR2 with a better final response, was studied. It was also analysed whether or not there was a difference in response to treatment depending on the type of GHD (severe or partial). It is defined as severe idiopathic GHD if the maximum GH peak in the stimulus tests is less than 3 ng/ml, and as partial GHD if it is between 3 and 10 ng/ml.
Statistical analysisA normality test was carried out prior to the statistical study and later a study of comparison of means and correlations was performed. The quantitative variables were described by mean, standard deviation and median. The qualitative variables were described as frequency and percentages. To study the relationship between qualitative variables, the Chi-squared test and Fisher’s exact test were used. To study the relationship between quantitative variables, the Pearson correlation coefficient was applied. To study the relationship between quantitative and qualitative variables, Student’s t test was used. For the statistical study, the SPSS v.15 program was used, and statistical significance was considered to be p < 0.05.
This research project was approved by the Comité de Ética de la Investigación de la Comunidad de Aragón (CEICA) [Research Ethics Committee of the Community of Aragon].
ResultsThe sample consisted of 139 patients (96 females and 43 males), of which 71.2% corresponded to severe GHD and 28.2% to partial GHD. The mean GH value found in the stimulus tests at diagnosis was 4.04 ± 2.57 ng/ml (0 ng/ml–9.86 ng/ml). The mean age at the start of treatment was 11.1 ± 2.5 years, with a treatment duration of 4.2 ± 2 years, a mean starting dose of 26.14 ± 2 mcg/kg/day, and an mean age at the end of treatment of 15.3 ± 1.2 years. The TH was −1.3 ± 0.7 SD, the TI was −2.5 ± 0.4 SD and the initial GP was −2.19 ± 0.6 SD; that is, 150.35 ± 3.40 cm in females and 164.9 ± 2.87 cm in males.
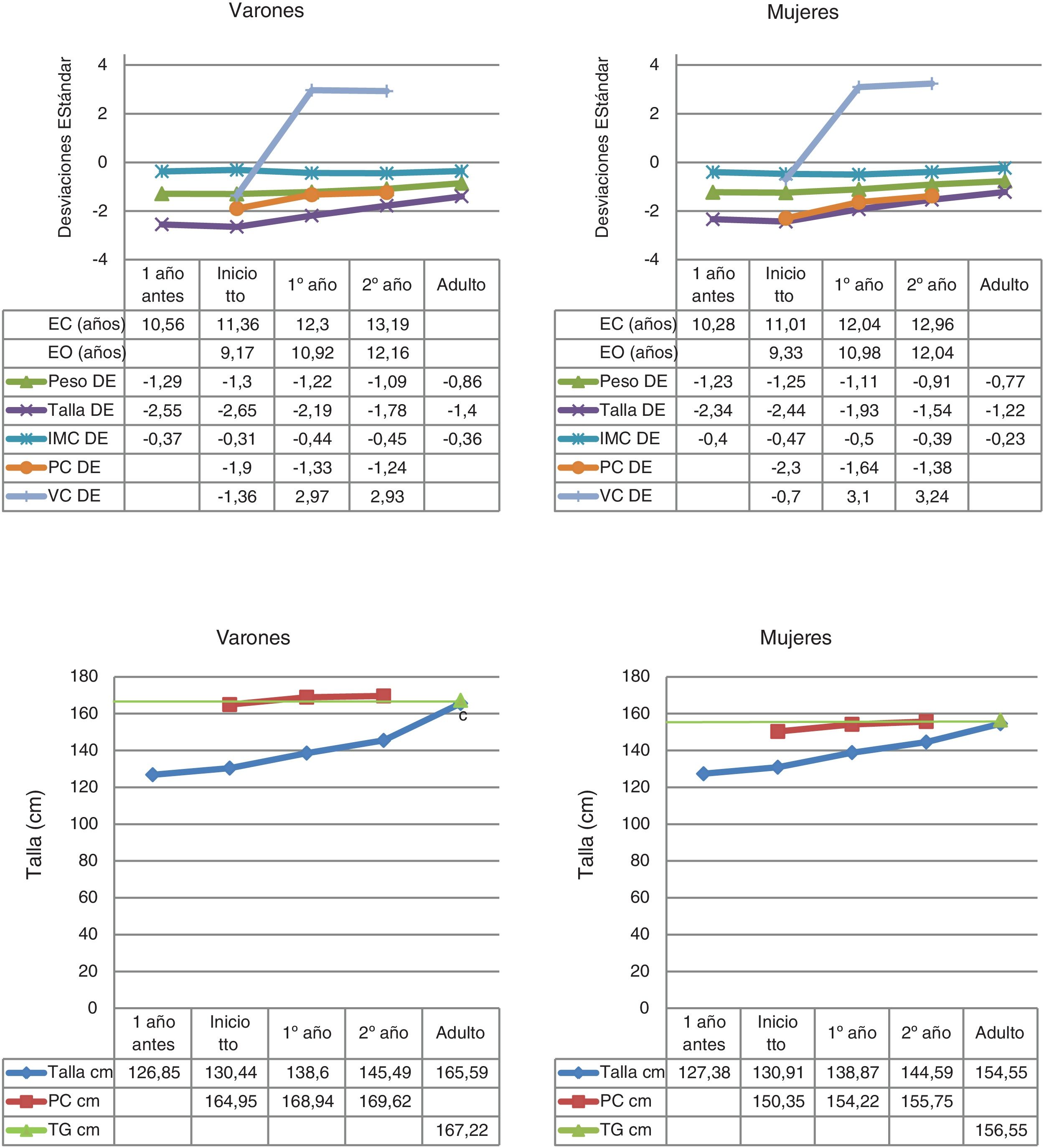
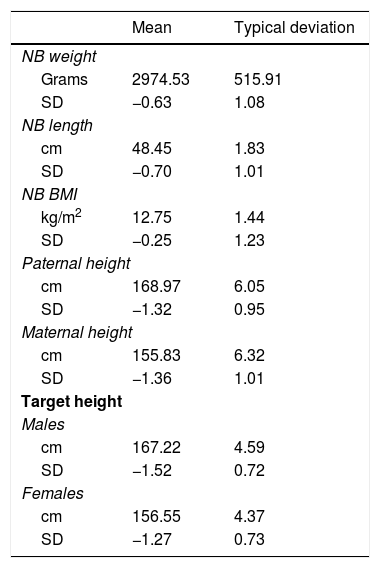
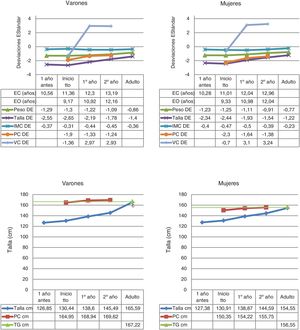
The data on birth, parental height and target height are shown in Table 1. The main descriptive characteristics and their evolution during follow-up are shown in Fig. 2.
Birth data, parental height and target height, expressed in absolute value and in standard deviations.
| Mean | Typical deviation | |
|---|---|---|
| NB weight | ||
| Grams | 2974.53 | 515.91 |
| SD | −0.63 | 1.08 |
| NB length | ||
| cm | 48.45 | 1.83 |
| SD | −0.70 | 1.01 |
| NB BMI | ||
| kg/m2 | 12.75 | 1.44 |
| SD | −0.25 | 1.23 |
| Paternal height | ||
| cm | 168.97 | 6.05 |
| SD | −1.32 | 0.95 |
| Maternal height | ||
| cm | 155.83 | 6.32 |
| SD | −1.36 | 1.01 |
| Target height | ||
| Males | ||
| cm | 167.22 | 4.59 |
| SD | −1.52 | 0.72 |
| Females | ||
| cm | 156.55 | 4.37 |
| SD | −1.27 | 0.73 |
BMI: body mass index; cm: centimetres; NB: newborn; SD: standard deviation.
Descriptive statistics of the quantitative variables collected during the follow-up to near-adult height of children with GHD.
BA: bone age; BMI: body mass index; CA: chronological age; cm: centimetres; GP: growth prediction; GR: growth rate; SD: standard deviation; TH: target height.
The mean age at pubertal onset was 12.14 ± 1.1 years in the total group, 11.86 ± 0.9 years in females and 12.79 ± 1.2 years in males. In all, 61.9% of patients began treatment with rhGH in the prepubertal stage.
A total of 48.2% of patients were good responders in the first year of treatment according to the criterion for increased GR ≥3 cm/year in the first year, 77% according to the criterion GR ≥1 SD in the first year, 45.3% according to the increase in height ≥0.5 SD in the first year, and 71.9% according to the increase in height ≥0.3 SD in the first year.
The pre-treatment mean GR was 4.73 ± 1.1 cm/year (−0.91 ± 1.8 SD), while the mean GR in the first year of treatment was 7.91 ± 1.5 cm/year (3.06 ± 3 SD), achieving an AH that exceeds the TI by SD with respect to its reference population (AH = −1.28 ± 0.6 SD and TI = −2.5 ± 0.4 SD), with this being 154.5 ± 3.7 cm in females and 165.6 ± 4.1 cm in males. The AH with respect to TH was 0.06 ± 0.7 SD, and the AH with respect to the initial GP was 0.9 ± 0.6 SD. An increase in AH with respect to TI of 1.2 ± 0.6 SD was found, without observing significant differences between sexes.
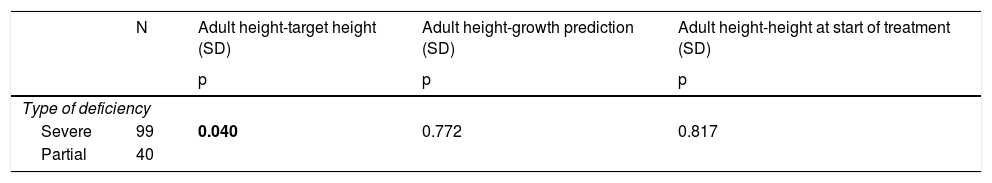
Females presented a better AH with respect to the initial GP (p = 0.00), without finding differences between the sexes in the rest of the final response variables. Those patients with severe GHD present a better AH with respect to their TH (p = 0.04), without finding differences in the rest of the final response variables (Table 2).
Principal relationships between the type of deficiency (severe or partial) and the variables of final response to treatment.
| N | Adult height-target height (SD) | Adult height-growth prediction (SD) | Adult height-height at start of treatment (SD) | |
|---|---|---|---|---|
| p | p | p | ||
| Type of deficiency | ||||
| Severe | 99 | 0.040 | 0.772 | 0.817 |
| Partial | 40 | |||
p: Significance level; SD: standard deviation. Results in bold indicate statistical significance.
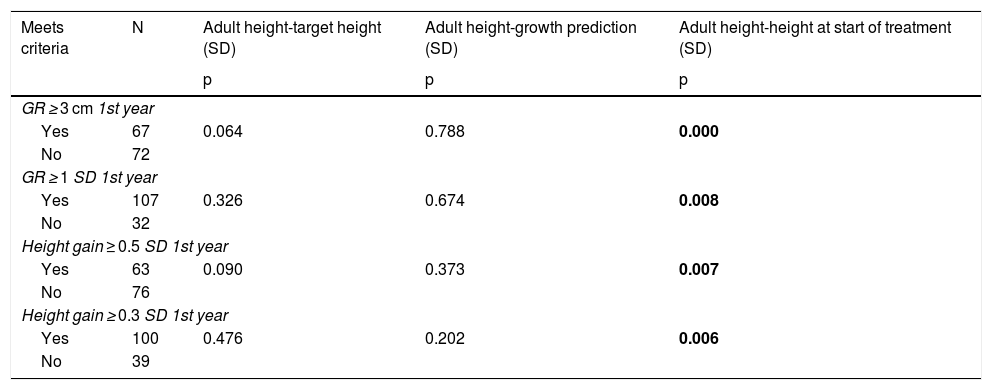
The response in the first year of treatment is a determining factor for achieving a good long-term response, as the patients considered to be good responders in the first year of treatment according to the four criteria presented a better final response according to the AH criterion with respect to the TI. However, there was no correlation with the rest of the final response variables studied (Table 3).
Principal relationships between criteria of good early response and the variables of final response to treatment.
| Meets criteria | N | Adult height-target height (SD) | Adult height-growth prediction (SD) | Adult height-height at start of treatment (SD) |
|---|---|---|---|---|
| p | p | p | ||
| GR ≥ 3 cm 1st year | ||||
| Yes | 67 | 0.064 | 0.788 | 0.000 |
| No | 72 | |||
| GR ≥ 1 SD 1st year | ||||
| Yes | 107 | 0.326 | 0.674 | 0.008 |
| No | 32 | |||
| Height gain ≥ 0.5 SD 1st year | ||||
| Yes | 63 | 0.090 | 0.373 | 0.007 |
| No | 76 | |||
| Height gain ≥ 0.3 SD 1st year | ||||
| Yes | 100 | 0.476 | 0.202 | 0.006 |
| No | 39 | |||
GR: growth rate; p: significance level; SD: standard deviation. Results in bold indicate statistical significance.
The IoR in the first year was associated with a better final response (AH with respect to TH, r = 0.249; p = 0.003), and it was maintained in the second year (AH with respect to TI, r = 0.294; p = 0.001). No correlation of the IoR was observed with the rest of the final response variables.
DiscussionThe study included 139 patients treated with rhGH for at least one year for GHD and who have already reached near-AH. More than half of the patients in this study were females, a percentage similar to that observed in previous studies such as those carried out by Ranke et al.,14,15 Ramchiel et al.16 and Straetemans et al.,7 but it is a higher percentage than that observed in other studies in the literature where there is a predominance of males, such as those carried out by Carrascosa et al.17,18 Most of the patients in the study corresponded to severe GHD, which represents a higher percentage of patients with severe GHD compared to previous studies.7,17,18
Multiple studies support the fact that the initiation of rhGH treatment at an early age favours patients achieving AH according to their TH. Ranke et al. compared 265 children with GHD treated before three years of age with 509 children treated after seven years of age, showing that after the first year of treatment with GH the increase in height was significantly greater per unit dose of rhGH in younger children than older ones.14 Thus, treatment should be started as soon as possible to achieve the maximum growth response.5,19 The mean age at the start of treatment in this study was in accordance with previous studies such as that of Rachmiel et al.,16,20 which is lower than what is observed in older studies,21 but higher than in other studies, where the mean age at treatment initiation is around seven.6,7,17,18 This age difference is due to the fact that the first patients included in the study are old patients, and today treatment with rhGH is started earlier.
The TH in males in our study was slightly lower than that recorded in previous studies, such as the studies of height gain as a function of GH secretion prior to treatment carried out by Carrascosa et al.,17,18 the work on AH achieved as a function of rhGH doses carried out by Rachmiel et al.,16 or the study by Straetemans et al.7 comparing different criteria for poor response to GH treatment. TI in the present study was similar to that found in the literature with non-prepubertal patients.16 However, previous studies carried out with younger patients show a lower height at the beginning of treatment.6,7,12,15,17,18,22
The most important effect of treatment with rhGH is to normalise GR, but in addition to that, GH has an important influence on the development of subsequent health risks.4 There is wide variability in the response to treatment with rhGH, probably due to compliance problems, severity of the GHD and patient sensitivity to rhGH. Treatment with rhGH must be administered daily subcutaneously, and the FDA-approved dose is 25–100 mcg/kg/day, and 25–35 mcg/kg/day in prepubertal children, which is consistent with the present study.23 The benefits of rhGH treatment in increasing GR are widely demonstrated in the literature, and support the results of this study. Therefore, treatment with rhGH achieves an AH in the target range.
Various authors have been analysing the response to treatment with rhGH during growth, and the publication of retrospective series has demonstrated that this response presents wide margins of variability, regardless of the diagnostic category attributed to the patient. Also, and in view of the response obtained at the end of a certain period of treatment (for example, first or second year of treatment), various authors have calculated how the baseline clinical and biochemical data along with those obtained during the previous period allow for refining the analyses in order to detect the predictive factors and their relative importance in the prediction.24 The definition of criteria of moderate or good response to treatment with rhGH is useful when making the decision to continue or interrupt treatment. Current studies are aimed at adapting rhGH treatment to the growth and response potential of each patient, in order to achieve rational, individualised and optimal use in terms of efficacy, safety and cost.
Different authors have proposed different cut-off points to define an adequate response to treatment with rhGH in these patients.24–27 In this study, the short-term good response criteria proposed by Bang et al. were analysed.12 According to this study, the two criteria with the lowest proportion of good responders were an increase in height ≥0.5 SD and an increase in GR ≥3 cm/year at the first year of treatment. In this study, it was found that, as with Bang et al., the percentage of good responders varies depending on the criteria used. Thus, as was found by Bang et al., the two strictest criteria correspond to an increase in height ≥0.5 SD in the first year and an increase in GR ≥3 cm/year in the first year, followed by an increase in height ≥0.3 SD and the GR ≥1 SD criterion in the first year.
In her study, van Dommelen investigated the effect of adherence to treatment with rhGH on the response to it after two years of treatment in prepubertal children with GHD, and found a strong correlation between high adherence to treatment in the second year and an adequate response to treatment according to the IoR.23 In the present study, it was found that the IoR at the first and second year was strongly associated with a better final response to treatment.
Furthermore, there is evidence that children with organic GHD respond less to rhGH treatment compared to those with idiopathic growth hormone deficiency28, or that patients with permanent GHD derive greater benefit from rhGH treatment, both in the short and long term, compared to patients with transient GHD.29 However, except in the work carried out by Bang et al.12 and later supported by Wit et al.,30 the response depending on the type of deficiency has not been extensively studied by other authors so far, this being corroborated in our study. Thus, this study shows that in patients with idiopathic GHD, those who have a severe deficiency present a better final response to treatment.
In conclusion, the findings of this study show that patients reach their TH, that those patients who responded well to the first year of treatment according to the four criteria analysed presented a better final response, and that IoR1 and IoR2 are associated with a better final response. Furthermore, those patients with severe GHD present a better final response.
Therefore, we can see that a good early response to treatment is a determining factor for achieving a good long-term response, and that those patients with severe GHD will benefit more from treatment. These results will help the clinician to make decisions about whether to continue, modify or even suspend treatment with rhGH.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Sánchez Malo MJ, Hidalgo Sanz J, Hernández Abadía R, Arlabán Carpintero L, Ferrer Lozano M, Labarta Aizpún JI, et al. Déficit de hormona de crecimiento, ¿influye el primer año de tratamiento en la talla final? Endocrinol Diabetes Nutr. 2021;68:534–541.