The use of statins in non-selected type 1 diabetes (T1D) populations is low. We assessed the prevalence and factors associated with statin treatment in patients meeting criteria for this therapy for primary prevention of cardiovascular disease (CVD).
Material and MethodsFrom 2015 to 2018, T1D patients from a tertiary hospital were selected. Inclusion criteria were: ≥40 years-old, diabetic nephropathy, or T1D duration ≥10 years with ≥1 cardiovascular risk factor (CVRF). A standardized cardiovascular risk evaluation protocol was performed. Prevalence of statin treatment was evaluated according to presence of several CVRFs, and multivariable models were constructed to assess independent determinants of statin use.
ResultsWe included 241 patients (50% women, age 48.2±9.9 years, T1D duration 26.6±9.0 years). Diabetic retinopathy and nephropathy, active smoking, and hypertension were present in 38%, 12%, 28%, and 27%, respectively. Overall, 43% of patients were on statins and 27% had LDL-cholesterol <100mg/dl. Statin users were older, and had higher body mass index (BMI), prevalence of kidney dysfunction, and hypertension (p<0.05 for all). However, among both T1D-related and classical CVRFs, only hypertension (odds ratio [OR], 2.96; 95% confidence interval [CI] 1.48-5.91) and BMI (OR, 1.08; CI, 1.01-1.16) were independently associated with statin use in multiple regression analysis.
ConclusionsLess than half of T1D patients from a tertiary hospital who met criteria for statin use were on treatment. Hypertension and BMI emerged as the only CVRFs independently associated with statin therapy. New strategies are needed to better address CVD prevention in this very high-risk population.
El uso de estatinas en población con diabetes tipo 1 (DT1) general es bajo. Estudiamos la prevalencia y factores asociados con su uso en pacientes que cumplían los criterios para esta terapia para la prevención primaria de la enfermedad cardiovascular (ECV).
Material y métodosDel 2015-2018 seleccionamos a pacientes con DT1 de un hospital terciario. Los criterios de inclusión fueron: ≥40 años, nefropatía diabética, o duración de DT1≥10 años con≥1 factor de riesgo cardiovascular (FRCV). Se realizó un protocolo estandarizado de evaluación del riesgo cardiovascular. Finalmente, estudiamos el uso de estatinas en función de diferentes FRCV y los factores independientemente asociados con su uso (modelos multivariantes).
ResultadosIncluimos 241 pacientes (50% mujeres, edad 48,2±9,9 años, duración de diabetes 26,6±9 años). La presencia de retinopatía, nefropatía, tabaquismo e hipertensión fue del 38, 12, 28 y 27%, respectivamente. Un 43% tomaba estatinas y un 27% presentó un colesterol-LDL<100mg/dL. Los usuarios de estatinas tenían mayor edad, índice de masa corporal (IMC), deterioro de la función renal e hipertensión (p<0,05). Entre todos los FRCV clásicos y específicos de la DT1, únicamente la hipertensión (odds ratio [OR], 2,96; intervalo de confianza al 95% [IC], 1,48-5,91) y el IMC (OR, 1,08; IC, 1,01-1,16) fueron los que se asociaron independientemente con su uso.
ConclusionesMenos de la mitad de pacientes con DT1 que cumplen criterios para estatinas de un hospital terciario están en tratamiento. Únicamente la hipertensión y el IMC se asociaron independientemente con su uso. Nuevas estrategias son necesarias para la prevención cardiovascular en esta población de alto riesgo.
Despite the improvements in the management of patients with type 1 diabetes (T1D),1,2 cardiovascular disease (CVD) is still the leading cause of morbidity and mortality in this population.3 A recent study using data from the Swedish National Diabetes Register has shown that the onset of T1D before 10 years of age resulted in a loss of 17.7 life-years for women and 14.2 life-years for men, mainly derived from CVD.3 Although metabolic control has been associated with an improvement in macrovascular complications,4 other risk factors have also been involved in the etiopathogenic process of CVD in T1D.5–8 In type 2 diabetes, multifactorial treatment of the CVRF has shown a dramatic decrease in CVD events (Steno-2 study).13 Similarly, this approach is also recommended by some international societies as part of the standard of care of T1D population.9–12
Lipid-lowering drugs, specially statins, have consistently demonstrated in different populations to decrease the incidence of CVD, quantified in 21% fewer major events per each 1 mmol/L of LDL-cholesterol reduction, even in primary prevention.14 In the setting of T1D, randomized controlled studies assessing the influence of statins on CVD outcomes are lacking, nevertheless, the beneficial effect of these drugs would be similar in this population according to a recent meta-analyses including only T1D patients (n=1,466) from other randomized trials (relative risk 0.79; 95% confidence interval [95%CI], 0.62-1.01).15 Furthermore, population-based studies have also demonstrated that the use of lipid-lowering drugs (97% statins) in daily practice are associated with a reduction in total CVD (hazard ratio 0.77; 95%CI, 0.69-0.87), cardiovascular death (hazard ratio 0.60; 95%CI, 0.50-0.72), as well as all-cause death (hazard ratio 0.56; 95%CI, 0.48-0.64).16
Statin, thus, has become a very important treatment for CVD prevention in T1D, and international guidelines recommend its use in patients over 40 years old, with diabetic nephropathy or with additional CVRF.9–12 Nevertheless, the misperception of T1D as a lower cardiovascular risk entity than type 2 diabetes is maybe one of the main barriers for the widespread use of this cardioprotective drug in this population.17 Indeed, contemporaneous registries have consistently shown a low use of statins in patients with T1D, ranging from 20-21% in younger primary prevention T1D individuals (data from the Swedish National Diabetes Register16 or the T1D Exchange Clinic Registry18) to 40% in older (mixed primary and secondary prevention) T1D patients (German/Austrian DPV Registry19).
Data regarding the use of lipid-lowering drugs in Spanish population with T1D meeting international recommendations are scarce. Based on the foregoing, we aimed to assess the proportion of patients with T1D who attained international guidelines for the use of statins for primary CVD prevention, and to study clinical and laboratory determinants associated with statin therapy in a specialized diabetes unit from a tertiary hospital.
Materials and methodsPatient selectionAll the patients included in the study were from a single center (Hospital Clínic i Universitari of Barcelona; with a reference population of 500,000 people approximately) and were followed regularly by the Diabetes Unit of the department of Endocrinology and Nutrition of this institution. From the total number of patients with the definite diagnosis of T1D (roughly 1,850), we selected adult individuals (≥18 years) with no previous history of CVD, which should receive statin treatment according to several scientific societies.9–12 The inclusion criteria were as follows (as a combination of the recommendations of previous societies9–12: a) Age ≥40 years; b) T1D of any age, but with any stage of diabetic nephropathy; and c) T1D of any age, with disease duration of at least 10 years and one additional risk factor (diabetic retinopathy, impaired hypoglycemia awareness or previous episode of severe hypoglycemia in the previous two years, history of premature CVD in first-degree relatives, active smoking habit, previous diagnosis of hypertension, HDL-cholesterol <40/45mg/dL [men/women], triglycerides >150mg/dL, LDL-cholesterol >160mg/dL, already on statin therapy, or previous episode of preeclampsia/eclampsia). The study protocol was conducted according to the principles of the Declaration of Helsinki and approved by the Hospital Clínic Research Ethics Committee.
Clinical and laboratory determinationsFrom June 2015 to July 2018, all the patients attended a single visit to ascertain their cardiovascular risk profile. Age, sex, smoking habit (current, former or never smoker), history of premature CVD in first-degree relatives (defined as <55 years in men and <65 years in women11) and pharmacological treatment participants were taking (specially focused in cardioprotective drugs like lipid-lowering, antihypertensive and antiplatelet drugs) were recorded. T1D duration was extracted from medical records. Anthropometric data were obtained as follows: patients were weighted in light clothing and without shoes to the nearest 0.1kg by calibrated scales. Height was measured to the nearest half centimetre. Body mass index (BMI) was calculated as weight in kilograms divided by height in squared metres. Waist circumference was measured to the nearest 0.5cm using an anthropometric tape midway between the lowest rib and at the iliac crest at minimal respiration. Blood pressure was registered using a blood pressure monitor (Omron HEM-7223-E; Hoofddorp, The Netherlands) after a few minutes of rest.
The diagnosis of diabetic retinopathy was obtained from medical records, an always was ascertained by an ophthalmologist. Diabetic nephropathy was defined as persistent abnormally increased creatinine-to-albumin ratio (≥30mg/g) or being treated with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for this reason. Hypertension was defined as taking antihypertensive drugs or repeated clinical systolic blood pressure ≥140mmHg or diastolic blood pressure ≥90mmHg. Severe hypoglycaemia was defined as any episode of documented hypoglycaemia that required third-party assistance for its resolution,20 and impaired hypoglycaemia awareness was assessed with the validated Spanish version of the Clarke test (with a cut-off >3 points).21 High-intensity statin therapy was defined according the American College of Cardiology / American Heart Association criteria.22
As part of the usual clinical practice follow-up, all the patients underwent periodic blood and spot first morning urine analyses, performed in a single laboratory (Biomedical Diagnostic Center, Hospital Clinic, Barcelona). HbA1c (National Glycohemoglobin Standardization Program [NGSP] DCCT Tosoh G8 Automated HPLC; Tosoh Bioscience Inc., South San Francisco, CA, USA; normal range 4.0-6.0%) was used to evaluate metabolic control. Total cholesterol, triglycerides, and HDL-cholesterol were analysed by molecular absorption spectrometry adapted to Siemens ADVIA 2400. LDL-cholesterol was assessed by the Friedewald formula. Creatinine was analysed by Jaffe method adapted to a Siemens ADVIA 2400 analyser, and albumin was assessed by immunoturbidimetric assay adapted to a Siemens ADVIA 2400 analyser. Estimated glomerular filtration rate (eGFR) was obtained with the Chronic Kidney Disease-Epidemiology Collaboration equation (CKD-EPI). The most recent laboratory data before the visit to ascertain cardiovascular risk was used in the analysis.
Statistical analysesData are expressed as median and 25th and 75th percentiles, mean ± standard deviation (SD), and n (percentage, %). Normal distribution of continuous variables was evaluated with the Kolmogorov-Smirnov test. The clinical, anthropometric and laboratory differences between patients with or without statin treatment and LDL-cholesterol < or ≥100mg/dL were assessed using the Student's t test for normally continuous distributed variables or the Mann-Whitney U test for the non-normally distributed variables. Proportions were compared using a chi-squared or Fisher exact test as appropriate. Logistic regression analyses including general clinical (age, sex, premature CVD in first-degree relatives, smoking habit, hypertension and BMI), T1D-related (disease duration, impaired hypoglycemia awareness and chronic diabetic complications) and laboratory variables (HbA1c and eGFR) were constructed to evaluate the determinants of statin treatment or LDL-cholesterol <100mg/dL (dependents variables). A p value <0.05 was considered statistically significant. Analyses were performed with IBM Statistical Package for Social Science (SPSS) version 22.0 software (SPSS Inc.; Chicago, IL, USA).
ResultsWe included 241 consecutive patients with T1D (50% women, mean age 48.2±9.9 years), with disease duration of 26.6±9.0 years and HbA1c of 7.6±1.0%. One out of three patients was on continuous subcutaneous insulin infusion (CSII) therapy. Prevalence of chronic complications was 31.5% (n=76) for isolated diabetic retinopathy, 5% (n=13) for isolated diabetic nephropathy and 6% (n=15) for both complications together. Regarding CVRF, hypertension was observed in 27% (n=65), active and former smoking in 28% (n=68) and 22% (n=52), respectively, obesity (BMI ≥ 30kg/m2) in 17% (n=42) and family history of premature CVD in 15% (n=36). Overall, only 1 out of 4 individuals had no comorbidity (including diabetes related and classical risk factors) and 40% of them had two or more of these high-risk conditions.
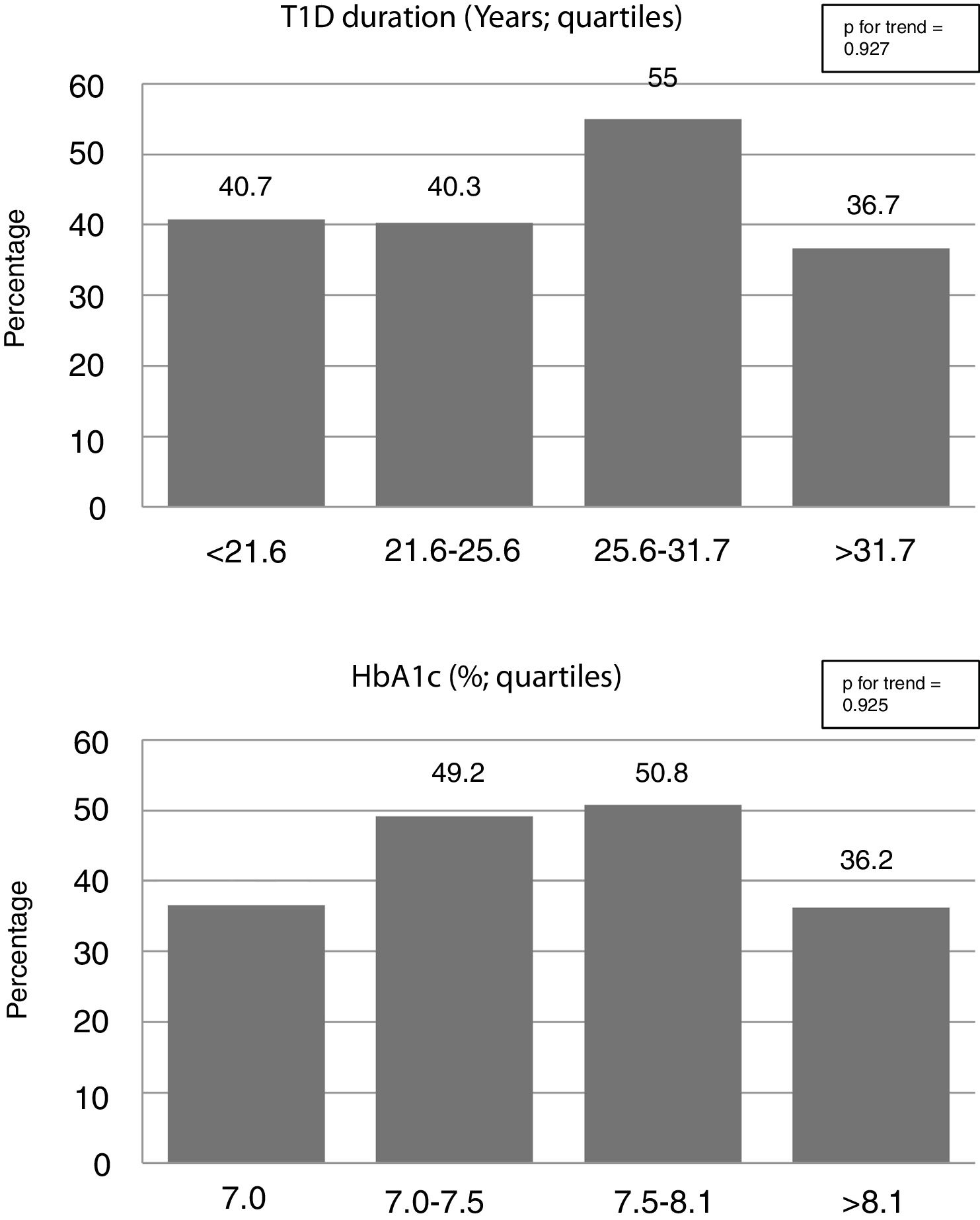
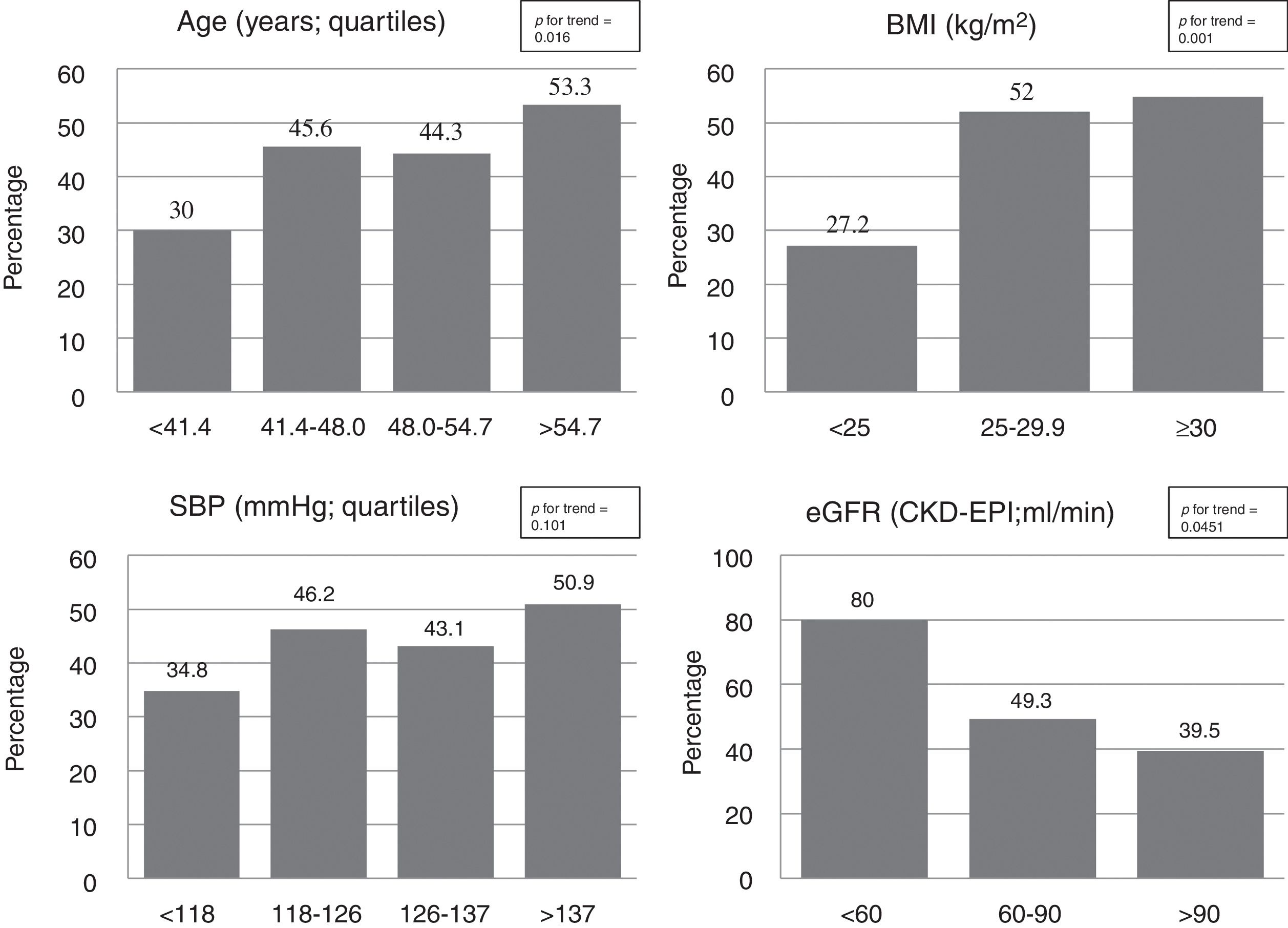
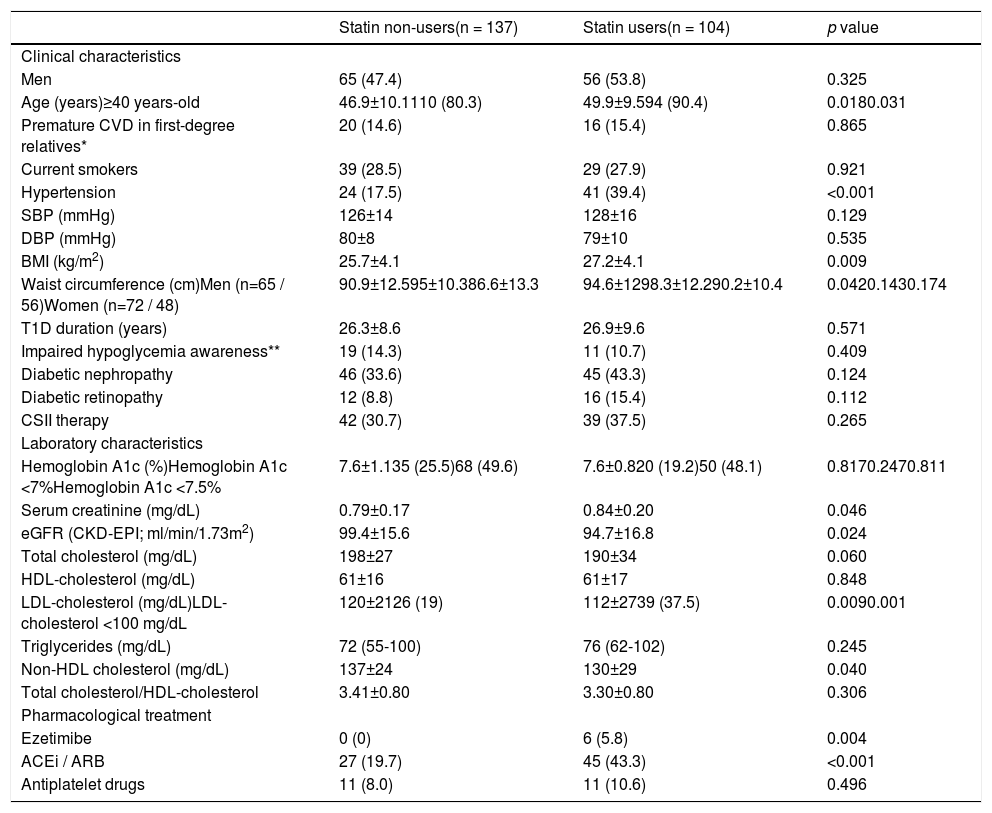
The use of cardioprotective treatment in this pre-specified high-risk group was low overall, being the prevalence of patients on statins, ezetimibe, enzyme angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and antiplatelet drugs 43% (n=104), 2.5% (n=6), 30% (n=72), and 9% (n=22), respectively. Only 15 patients (6%) were under high-intensity statin treatment. Participant characteristics’ according to background statin use are listed in Table 1. Neither general clinical characteristics, such as sex or smoking habit, nor specific T1D-related variables, such as disease duration, metabolic control (Figure 1), or chronic complications, were associated with the use of this drug (p>0.100 for all comparisons). Individuals with a previous diagnosis of hypertension, however, had approximately a two-fold increase in statin use (63.1% vs. 35.8%; p<0.001). Likewise, a stepped increase in statin treatment was also observed in relation with increasing age and BMI, and deterioration of eGFR (p<0.05 for all; Figure 2).
Characteristics of study subjects according to statin use.
| Statin non-users(n = 137) | Statin users(n = 104) | p value | |
|---|---|---|---|
| Clinical characteristics | |||
| Men | 65 (47.4) | 56 (53.8) | 0.325 |
| Age (years)≥40 years-old | 46.9±10.1110 (80.3) | 49.9±9.594 (90.4) | 0.0180.031 |
| Premature CVD in first-degree relatives* | 20 (14.6) | 16 (15.4) | 0.865 |
| Current smokers | 39 (28.5) | 29 (27.9) | 0.921 |
| Hypertension | 24 (17.5) | 41 (39.4) | <0.001 |
| SBP (mmHg) | 126±14 | 128±16 | 0.129 |
| DBP (mmHg) | 80±8 | 79±10 | 0.535 |
| BMI (kg/m2) | 25.7±4.1 | 27.2±4.1 | 0.009 |
| Waist circumference (cm)Men (n=65 / 56)Women (n=72 / 48) | 90.9±12.595±10.386.6±13.3 | 94.6±1298.3±12.290.2±10.4 | 0.0420.1430.174 |
| T1D duration (years) | 26.3±8.6 | 26.9±9.6 | 0.571 |
| Impaired hypoglycemia awareness** | 19 (14.3) | 11 (10.7) | 0.409 |
| Diabetic nephropathy | 46 (33.6) | 45 (43.3) | 0.124 |
| Diabetic retinopathy | 12 (8.8) | 16 (15.4) | 0.112 |
| CSII therapy | 42 (30.7) | 39 (37.5) | 0.265 |
| Laboratory characteristics | |||
| Hemoglobin A1c (%)Hemoglobin A1c <7%Hemoglobin A1c <7.5% | 7.6±1.135 (25.5)68 (49.6) | 7.6±0.820 (19.2)50 (48.1) | 0.8170.2470.811 |
| Serum creatinine (mg/dL) | 0.79±0.17 | 0.84±0.20 | 0.046 |
| eGFR (CKD-EPI; ml/min/1.73m2) | 99.4±15.6 | 94.7±16.8 | 0.024 |
| Total cholesterol (mg/dL) | 198±27 | 190±34 | 0.060 |
| HDL-cholesterol (mg/dL) | 61±16 | 61±17 | 0.848 |
| LDL-cholesterol (mg/dL)LDL-cholesterol <100 mg/dL | 120±2126 (19) | 112±2739 (37.5) | 0.0090.001 |
| Triglycerides (mg/dL) | 72 (55-100) | 76 (62-102) | 0.245 |
| Non-HDL cholesterol (mg/dL) | 137±24 | 130±29 | 0.040 |
| Total cholesterol/HDL-cholesterol | 3.41±0.80 | 3.30±0.80 | 0.306 |
| Pharmacological treatment | |||
| Ezetimibe | 0 (0) | 6 (5.8) | 0.004 |
| ACEi / ARB | 27 (19.7) | 45 (43.3) | <0.001 |
| Antiplatelet drugs | 11 (8.0) | 11 (10.6) | 0.496 |
Data are shown as n (percentage), median (Q1-Q3), or mean ± standard deviation
p values for group comparisons are reported
ARB: angiotensin receptor blocker; BMI: Body Mass Index; CVD: cardiovascular disease; CSII: continuous subcutaneous insulin infusion; DBP: diastolic blood pressure; ACEi: angiotensin-converting enzyme inhibitor; eGFR: estimate glomerular filtration rate; HDL: high density lipoprotein; LDL: low density lipoprotein; SBP: systolic blood pressure; T1D: type 1 diabetes
In the total group, the proportion of patients with LDL-cholesterol meeting primary prevention goals (<100mg/dL) was 27% (n=65). As expected, statins users had lower levels of LDL-cholesterol than non-users (p<0.01), with a higher proportion of patients achieving LDL-cholesterol objectives (37.5% vs. 19%; p=0.001). In addition to statin treatment, the only variable associated with LDL-cholesterol <100mg/dL was being ≥40 years-old (p=0.015, Supplemental Table 1). Individuals with LDL-cholesterol <70mg/dL were all allocated in the statin treated group (3% vs. 0%; p=0.046). No differences in other lipids parameters (triglycerides or HDL-cholesterol) were found between groups (Table 1).
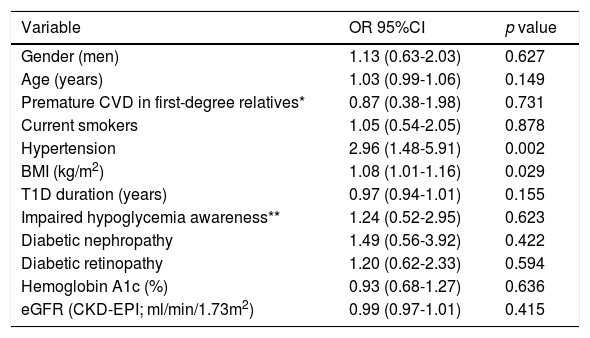
In order to assess independent determinants of statin treatment, a logistic regression analysis was performed (Table 2). In this analysis, eGFR and age were no longer associated with statin use, however, both previous diagnosis of hypertension (odds ratio [OR] 2.96; 95%CI, 1.48-5.91) and BMI (OR 1.08; 95%CI, 1.01-1.16), remained significantly associated with this therapy. T1D-related variables remained non associated with the use of this cardioprotective drug. Similarly, the variables independently associated with a LDL-cholesterol <100mg/dL were statin treatment (OR 2.36; 95%CI, 1.24-4.51) and a trend for being ≥40 years-old (OR 2.97; 95%CI, 0.94-9.37; p=0.064) (Supplemental Table 2).
Clinical determinants of statin use: multiple regression analysis.
| Variable | OR 95%CI | p value |
|---|---|---|
| Gender (men) | 1.13 (0.63-2.03) | 0.627 |
| Age (years) | 1.03 (0.99-1.06) | 0.149 |
| Premature CVD in first-degree relatives* | 0.87 (0.38-1.98) | 0.731 |
| Current smokers | 1.05 (0.54-2.05) | 0.878 |
| Hypertension | 2.96 (1.48-5.91) | 0.002 |
| BMI (kg/m2) | 1.08 (1.01-1.16) | 0.029 |
| T1D duration (years) | 0.97 (0.94-1.01) | 0.155 |
| Impaired hypoglycemia awareness** | 1.24 (0.52-2.95) | 0.623 |
| Diabetic nephropathy | 1.49 (0.56-3.92) | 0.422 |
| Diabetic retinopathy | 1.20 (0.62-2.33) | 0.594 |
| Hemoglobin A1c (%) | 0.93 (0.68-1.27) | 0.636 |
| eGFR (CKD-EPI; ml/min/1.73m2) | 0.99 (0.97-1.01) | 0.415 |
Logistic regression analysis for statin treatment (odds ratio and 95% confidence interval are shown) including all the variables in the rows.
BMI: Body Mass Index; CVD: cardiovascular disease; eGFR: estimate glomerular filtration rate; T1D: type 1 diabetes.
In our sample of patients with T1D followed in a diabetes unit from a tertiary hospital and meeting international criteria for statin treatment, only 43% of them were on this cardioprotective drug. Despite the high prevalence of classical and/or diabetes specifically-related CVRF (50% active or former smokers, 43% chronic diabetes complications and 27% hypertension), the prevalence of patients meeting LDL-cholesterol goals (i.e. <100mg/dL) was 27%. Although T1D-related variables have been strongly associated with CVD risk in previous studies,3,4 only non-specific CVRF such as previous diagnosis of hypertension and BMI emerged as the variables independently associated with statin therapy in this group. Our findings highlight the need to pursue well-established CVD prevention measures in T1D individuals besides glycemic control in this high-risk population. Precisely, these strategies are frequently overlooked due to the challenging of glycemic control in these patients.
Although T1D is a condition associated with an increased cardiovascular risk,3 cardioprotective drugs, namely statins, are scarcely used in this specific population. The young age of most T1D patients and their reluctance to start a lifelong therapy, might contribute to explain this apparent paradox. Thus, in patients younger than 40 years old from both the T1D Exchange Clinic Registry18 and the Swedish National Diabetes Register,3 the use of statins was low overall. However, an stepped increase with age, ranging from 1.5% in patients of 10-18 years to 21% in patients of 25-40 years in the former,18 and from 4% in patients <20 years to 12% in those with 26-30 years in the last registry3 was observed. Similarly, in our cohort the use of lipid-lowering drugs was lower in younger individuals (30% vs. 53% in the lowest vs. the highest quartile, p=0.016; Figure 2). The higher age of our sample (48.2±9.9 years) compared with the previous ones (20±7 years and 28.9±9.4 years, respectively) could explain the different proportion of statin use observed.3,18 In other large registries dealing with primary prevention including T1D individuals of similar age, the use of statin was higher, but, again, far from the recommendations of international guidelines. In a recent publication from the Swedish National Diabetes Register (mean age 39.4±12.5 years; mean diabetes duration 23.9±12.5 years), the use of statins only reach 21%,16 and in the Australian National Diabetes Audit (mean age 40.0±16.7 years; mean diabetes duration 19.2±14.4 years), the prevalence of statins users was only 30%.7 The higher prevalence of statin use in our cohort (43%), besides demonstrating a closer adherence to clinical practice guidelines, could be explained by inclusion criteria used in this study as compared with a non-selected approach in the previous ones.
The recommendation of statin use in T1D differs, slightly, between international societies, specially concerning to disease duration, but there is a consensus in using this drug in patients aged 40 years and older, with diabetic nephropathy or with additional major CVRF like active smoking or hypertension.9–12 The selection of these specific subgroups in our study, although increased the proportion of individuals on statin treatment compared with the whole sample, showed that the adoption of the international guidelines remained low. So, the proportion of patients ≥40 years (n=204), with diabetic nephropathy (n=28), active smokers (n=68) or with concomitant hypertension (n=65) receiving statins was 46%, 57%, 43% and 63%, respectively. These percentages, along with the low proportion of our patients meeting goals of LDL-cholesterol (27%), highlights the need for improving CVD prevention in this specific group besides the achievement of an optimal glycaemic control (depicted by our high use of CSII therapy, 33.6%). In fact, a recent study including 658 T1D patients followed for up to 25 years showed that, while the proportion of patients under intensive insulin therapy and meeting HbA1c goals improved over the years (p<0.0001 for both), blood pressure control and LDL-cholesterol goals did not. Indeed, there was a worsening in the proportion of patients meeting LDL-cholesterol goals as compared with the baseline situation (62.3% vs. 39.7%, p<0.0001).23
Data from DCCT/EDIC study showed that glycaemic control over the time is one of the main determinants, not only for microvascular, but also for macrovascular diabetic complications.24,25 Nevertheless, although some large population-based studies have found a progressive, non-linear, reduction of CVD as HbA1c decreases, even in those with an optimal glycaemic control (defined as HbA1c <7%) CVD mortality was 3-fold greater.4 Thus, other classical, such as age, blood pressure and lipids, and non-classical (for instance BMI), or unknown CVRF are contributing to CVD risk besides glycaemic control in this population.24 In this sense, it is noteworthy that in our cohort the two risk factors independently associated with statin use were previous diagnosis of hypertension and BMI (Table 2). On the other hand, T1D specific risk factors, such as duration of the disease or HbA1c, were surprisingly not associated with the use of this drug (Table 2 and Figure 1). Since large cohort studies have found that CVRF (both general such as blood pressure, LDL-cholesterol or smoking habit; and T1D-related as HbA1c or albuminuria) has an additive effect on CVD risk,8 the deleterious effect of suboptimal glycaemic control on CVD could be partially blunted by strict control of the other CVRF. So, treatment intensification of these risk factors is a good opportunity to improve our daily practice in those patients with worse T1D-related factors (i.e., 4th quartiles of HbA1c and/or T1D duration; Figure 1).
In view of our results, and the low use of lipid-lowering drugs in other T1D populations,7,16,18,19 the need for focusing on CVD prevention in this group of patients is imperative. Other strategies including the assessment of preclinical atherosclerosis by imaging studies26,27 or the use of specific cardiovascular risk scores,28 would be welcomed in order to identify patients at highest risk and to tailor cardioprotective treatment accordingly. The shared goal of both approaches is to tackle CVD, still the main cause of mortality in T1D population.2,29
Several strengths and limitations should be acknowledged in this study. Its main strength is that it strictly includes a selected substantial sample size of patients with T1D from a Mediterranean area requiring treatment with statins according to guideline recommendations, in comparison with previous studies that included non-selected populations from different geographical regions. Furthermore, our cardiovascular risk assessment protocol served to obtain detailed, reliable and standardized information regarding general and T1D-specific CVRF, as well as the use of additional cardioprotective treatments. The study, however, has also some limitations. First, the inclusion of patients from only one tertiary centre with extensive experience, though reduces physician variability in T1D management, could not be representative of other geographic areas or less dedicated centres. Second, the exclusion of patients with previous CVD selects patients with lower risk and, therefore, less prone to be on cardioprotective drugs. Nonetheless, the proportion of patients in ours1,30 and in other geographic areas3,7,19 with established CVD is low overall, making our data representative of T1D population. Finally, due to the cross-sectional design, a causal relationship between CVRF and statin initiation cannot be truly established, as well as the LDL-cholesterol levels prior to prescription of this treatment.
In conclusion, less than half of the high-risk T1D individuals who attended a tertiary diabetic clinic and meet criteria for statin therapy, were taking this treatment. Approximately three quarters had LDL-cholesterol >100mg/dL. Although T1D-related variables have been associated with CVD in several studies,3,24 only non-specific CVRF, such us previous diagnosis of hypertension or BMI, were the only variables independently related with statin treatment. Considering that CVD is still the main cause of mortality in T1D, greater focus on cardiovascular disease protection, including a better risk assessment and lipid profile management, might be warranted. Further prospective studies are needed to ascertain the deleterious and protective factors for CVD in individuals from Mediterranean areas, a population at low overall cardiovascular risk31 underrepresented in studies involving T1D patients.
Financial supportA.J.A received a research grant from the Associació Catalana de Diabetis (ACD), “Ajut per a la recerca en diabetis modalitat clínica 2018”.
Declarations of interestNone.