We aimed to study the predictive factors for recovery of parathyroid function in hypoparathyroid patients after total thyroidectomy for thyroid cancer.
MethodsWe designed a retrospective, multicentre and nation-wide analysis of patients with total thyroidectomy who were seen in twenty endocrinology departments from January to March 2018. We selected patients with histologically proven thyroid cancer and retrieved information related to surgical procedure and thyroid cancer features. Survival analysis and Cox regression analysis were used to study the relationship between these variables and the recovery of parathyroid function.
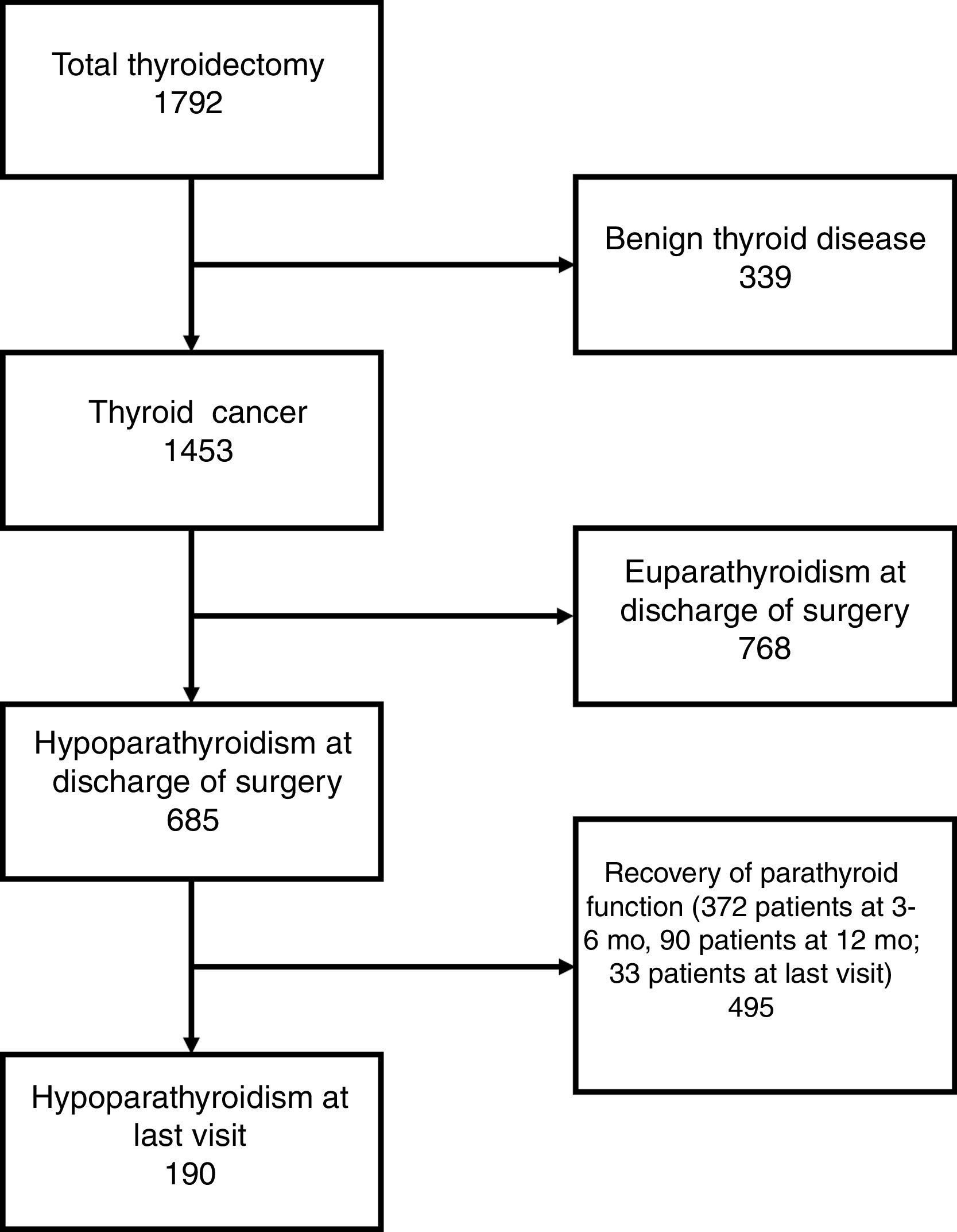
ResultsFrom 685 patients with hypoparathyroidism at discharge of surgery, 495 (72.3%) recovered parathyroid function over time. Kaplan–Meier analysis showed that this recovery was significantly related to the presence of specialized surgical team (P<0.001), identification of parathyroid glands at surgery (P<0.001), papillary histopathology (P=0.040), and higher levels of postoperative calcium (Ca) (P<0.001) and parathyroid hormone (PTH) (P<0.001). Subjects with gross extrathyroidal extension (P=0.040), lymph node metastases (P=0.004), and surgical re-intervention after initial surgery (P=0.024) exhibited a significant risk of persistence of hypoparathyroidism. Multivariate Cox regression analysis showed that the significant and independent factors for recovery of parathyroid function were postoperative concentrations of Ca (P=0.038) and PTH (P=0.049). The presence of lymph node metastases was a negative predictor of recuperation of parathyroid function (P=0.042) in this analysis.
ConclusionIn patients with thyroid cancer, recovery of parathyroid function after total thyroidectomy was directly related to postoperative Ca and PTH concentrations, and inversely related to lymph node metastases.
Estudiar los factores predictivos de la recuperación de la función paratiroidea en pacientes hipoparatiroideos después de tiroidectomía total por cáncer de tiroides.
MétodosSe diseñó un estudio nacional, retrospectivo y multicéntrico de pacientes con tiroidectomía total, que fueron seguidos en 20 servicios de endocrinología desde enero a marzo de 2018. Se seleccionaron pacientes con demostración histológica de cáncer de tiroides y datos sobre el procedimiento quirúrgico y las características tumorales. Se emplearon el análisis de supervivencia y el análisis de regresión de Cox para analizar las relaciones entre estas variables y la recuperación de la función paratiroidea.
ResultadosDe un total de 685 pacientes con hipoparatiroidismo tras cirugía, 495 (72,3%) recuperaron la función paratiroidea a lo largo del tiempo. El análisis de Kaplan-Meier mostró que esta recuperación estaba significativamente relacionada con la presencia de equipo quirúrgico especializado (p < 0,001), identificación de las glándulas paratiroideas en la cirugía (p < 0,001), histología papilar (p = 0,040) y mayores concentraciones postoperatorias de calcio (Ca) (p < 0,001) y parathormona (p < 0,001). Los pacientes con extensión extratiroidea macroscópica (p = 0,040), metástasis ganglionares (p = 0,004) y reintervención tras cirugía inicial (p = 0,024) mostraron un riesgo significativo de persistencia del hipoparatiroidismo. El análisis de regresión multivariante de Cox mostró que los factores significativos e independientes para la recuperación de la función paratiroidea fueron las concentraciones postoperatorias de Ca (p = 0,038) y parathormona (p = 0,049). La presencia de metástasis ganglionares fue un predictor negativo de la recuperación de la función paratiroidea (p = 0,042) en este análisis.
ConclusiónEn pacientes con cáncer de tiroides, la recuperación de la función paratiroidea tras la tiroidectomía total se relacionó de forma directa con las concentraciones postoperatorias de Ca y parathormona, e inversamente con las metástasis ganglionares linfáticas.
Parathyroid hormone (PTH) deficiency or insufficiency as a result of inadvertent injury, devascularization or removal of the parathyroid glands after cervical surgery (post-surgical hypoparathyroidism) is the most common etiology of chronic hypoparathyroidism and, therefore, of the permanent need for treatment with calcium (Ca) and calcitriol.1 In turn, total thyroidectomy due to benign thyroid disease or thyroid cancer is the most usual cause of post-surgical hypoparathyroidism.2
The prevalence of transient and permanent hypoparathyroidism after cervical surgery has been the subject of diverse surveys by several authors with remarkably variable results.3–10 Concurrently, the analysis of the risk factors for definitive hypoparathyroidism associated with thyroid surgery has also been an issue of intense research in last years.7,9–13 The recent meta-analysis by Edafe et al.10 showed the existence of biochemical (perioperative PTH, preoperative vitamin D, postoperative changes in Ca) and clinical (female sex, Graves’ disease, need for parathyroid autotransplantation, and inadvertent excision of parathyroid glands) predictors for post-thyroidectomy hypocalcemia. Our recent report showed that lymph node dissection, two-stage thyroidectomy, and the presence of parathyroid tissue in the specimen were risk factors for permanent hypoparathyroidism. Postoperative serum Ca levels and the intervention performed by a specialized surgical team appear to be protective factors for hypoparathyroidism. Surprisingly, in this study we found that the presence of thyroid cancer histology was an independent and significant protective factor for the development of chronic hypoparathyroidism.14
Therefore, to delve into this issue, in the present study we set out to investigate which variables related to surgical procedure and also related to the diverse features of thyroid cancer were predictors of the recovery of parathyroid function in patients with histologically proven thyroid carcinoma who have undergone total thyroidectomy and have been diagnosed with hypoparathyroidism at discharge of surgery.
MethodsStudy designThis study was carried out by members of the Thyroid Task Force of the Spanish Society of Endocrinology (SEEN), after receiving the approval of the board of directors of the SEEN and the ethical committee of the Hospital Universitario Ramón y Cajal (Madrid). All the details on patients and methodology have been previously described.14 Briefly, each researcher recruited all patients with total thyroidectomy who attended their outpatient clinic between January 1 and March 31, 2018. Inclusion criteria were: age>14 years at the time of thyroidectomy, availability of surgical and pathological reports, and follow-up in the same hospital for at least one year after thyroidectomy.
In every patient demographic data, details on hospital, details on surgical procedure, histopathological data, analytical data on Ca metabolism, and information on therapy with Ca and calcitriol were retrospectively collected. All investigators retrieved clinical information at various stages, i.e., at discharge from hospital after surgery, 3–6 months after surgery, 12 months after the surgery, and at last visit.
For this study we have considered two groups of variables. On one hand, clinical and surgery related variables, i.e., sex, age, specialized surgical team, lymph node dissection, type of surgery, identification of parathyroid glands by the surgeon, autotransplantation, presence of parathyroid tissue in the specimen, surgical complications and serum concentrations of Ca and PTH. The concept of specialized surgical team was used in a qualitative way, that is, the team of surgeons who usually perform most thyroidectomies in each hospital and with whom the endocrinologist participating in the study usually associates. Serum Ca and PTH levels were quantified according to standard methods used in the clinical laboratories of each participating hospital. On the other hand, we considered variables related to thyroid cancer itself, i.e., incidentally found tumor, histological type, multifocality, tumor size, extrathyroidal extension, lymph node metastases, distant metastases, radioiodine ablation, surgical re-intervention after initial surgery, neck radiotherapy, and treatment with targeted therapy.
Definitions and criteriaWe considered the presence of hypoparathyroidism at discharge in patients who showed, in the immediate postoperative period, serum Ca levels<8.5mg/dl with inappropriate low PTH levels (<15pg/ml), and/or who needed treatment with Ca or calcitriol at discharge from surgery.15,16 Definitive hypoparathyroidism was diagnosed in subjects who needed treatment with Ca or calcitriol at the last visit (always with at least 12 months of follow-up). Recovery of parathyroid function was considered in patients who initially exhibited hypoparathyroidism at discharge of surgery and lastly demonstrated absence of need for Ca and calcitriol therapy at a subsequent visit.12,16
Since this is a retrospective study, analytical data (preoperative Ca, postoperative Ca and PTH) were only available in a fraction of patients. In cases where this information was available, the corrected Ca (mg/dl) was calculated as Ca (mg/dl)+0.8 (4-albumin [g/dl]), or Ca/[(total proteins (g/dl)/16)+0.55].
Statistical analysisFor quantitative variables, results are expressed as mean±SD for normally distributed data and as median (interquartile range, IQR) for nonparametric data. Adjustment to normal distribution was tested by the Kolmogorov test. Categorical variables are described as ratios or percentages. For comparisons of means between two groups of patients, the Student's t test was used for normally distributed data, and the Mann–Whitney test was used for nonparametric data. For ratio comparisons, the chi-square test or Fisher's exact test was used.
To assess the influence of putative predictive factors for the recovery of parathyroid function over time, we performed a survival analysis using the Kaplan–Meier method, with the log-rank test used to compare arms. In the survival analysis some quantitative variables were transformed into categorical of two categories dividing the patients by the median value. Univariate and stepwise multivariate Cox regression models were used to assess the independent effect of several quantitative and qualitative variables on the likelihood of development of euparathyroidism. Two-sided tests were used, and differences were considered significant when P<0.05.
ResultsStudied patientsFrom a cohort of 1792 patients with total thyroidectomy and follow-up longer than 12 months, we selected 1453 subject with histologically proven thyroid cancer. Mean (±SD) age of these patients was 47.9±15.0 yr, and 1131 (77.8%) were women. Histologic subtypes in these subjects were: papillary thyroid cancer in 1213 patients (83.5%), follicular or Hürthle cell carcinoma in 162 (11.1%), poorly differentiated thyroid cancer in 12 (0.8%), medullary thyroid cancer in 65 (4.5%), and other type in 1 (0.1%) patient. Median (IQR) tumor size was 1.6 (1.0–2.9) cm, lymph node metastases were present at diagnosis in 485 patients (33.4%), and distant metastases were found in 53 patients (3.7%). Median follow-up in the endocrinology clinic was 59 (27–108) months.
From this cohort of thyroid cancer patients, 768 subjects presented euparathyroidism and 685 hypoparathyroidism at discharge of surgery (Fig. 1). In 233 of these 685 patients (34.2%) the diagnostic criteria was the need for treatment with Ca or calcitriol at discharge, with no evidence of low Ca or PTH levels. Patients with hypoparathyroidism at discharge exhibited higher percentages of lymph node dissection, identification of parathyroid glands at surgery, autotransplantation, presence of parathyroid tissue in the specimen, surgical complications, papillary histopathology, multifocality of the tumor, extrathyroidal extension, lymph node metastases, and distant metastases, and lower percentages of two-stage thyroidectomy and incidentally found tumor. Serum concentrations of preoperative Ca, postoperative corrected Ca, and postoperative PTH were significantly lower in patients who developed hypoparathyroidism at discharge (Table 1).
Clinical and histopathological features of 1453 patients with thyroid cancer classified according to the presence of hypoparathyroidism at discharge of surgery.
| Euparathyroid patients at discharge (n=768) | Patients with hypoparathyroidism at discharge (n=685) | ||||
|---|---|---|---|---|---|
| n | Value | n | Value | P | |
| Sex, female | 768 | 579 (75.4) | 685 | 552 (80.6) | 0.019 |
| Age, yr | 758 | 48.7±15.0 | 685 | 47.0±14.9 | 0.034 |
| Specialized surgical team | 768 | 663 (86.3) | 685 | 602 (87.9) | 0.390 |
| Lymph node dissection | 767 | 297 (38.7) | 680 | 435 (64.0) | <0.001 |
| 2-Stage thyroidectomy | 768 | 162 (21.1) | 685 | 69 (10.1) | <0.001 |
| Identification of parathyroid glands | 768 | 545 (71.0) | 685 | 536 (78.2) | 0.002 |
| Autotransplantation | 767 | 42 (5.5) | 685 | 82 (12.0) | <0.001 |
| Parathyroid tissue at histology | 767 | 141 (18.4) | 684 | 206 (30.1) | <0.001 |
| Surgical complications | 759 | 59 (7.8) | 681 | 80 (11.7) | 0.012 |
| Incidental | 753 | 198 (26.3) | 674 | 97 (14.4) | <0.001 |
| Papillary histology | 768 | 617 (80.3) | 685 | 596 (87.0) | 0.001 |
| Multifocal | 766 | 242 (31.6) | 682 | 246 (36.1) | 0.075 |
| Tumor size, cm | 753 | 1.6 (0.9–2.9) | 670 | 1.7 (1.0–3.0) | |
| Extrathyroidal extension | 758 | 667 | <0.001 | ||
| None | 618 (81.5) | 464 (69.6) | |||
| Microscopic | 112 (14.8) | 159 (23.8) | |||
| Macroscopic | 28 (3.7) | 44 (6.6) | |||
| Lymph node metastasis | 767 | 683 | <0.001 | ||
| Nx | 235 (30.6) | 126 (18.4) | |||
| N0 | 341 (44.5) | 263 (38.5) | |||
| N1 | 191 (24.9) | 294 (43.0) | |||
| Distant metastasis | 763 | 21 (2.8) | 683 | 32 (4.7) | 0.067 |
| Radioiodine ablation | 765 | 630 (82.4) | 685 | 579 (84.5) | 0.289 |
| Preoperative Ca (mg/dl) | 509 | 9.55±0.56 | 438 | 9.46±0.51 | 0.007 |
| Postoperative corrected Ca (mg/dl) | 474 | 8.92 | 440 | 7.87±0.68 | <0.001 |
| Postoperative PTH (pg/ml) | 304 | 37 (27–51) | 309 | 8 (3–16) | <0.001 |
Data are the number of patients (percentage), mean±SD for normally distributed data and median (interquartile range) for nonparametric data. The total number of patients used to calculate the values of means, medians and percentages in each group is indicated by n.
Abbreviations: Ca, calcium; PTH, parathyroid hormone.
The time course of recovery of parathyroid function and the number of patients with hypoparathyroidism at different stages of follow-up is summarized in Table 2. As it is shown, 495 out of 685 patients (72.3%) recovered parathyroid function throughout the time of follow-up, and 190 (27.7%) were diagnosed with permanent hypoparathyroidism. We analyzed clinical and surgery related variables in these patients and found that patients who recovered parathyroid function had higher percentages of specialized surgical team, and identification of parathyroid glands, and lower percentage of surgical complication (Table 3A). Six hundred and two patients were operated on by a specialized surgical team. The percentage of parathyroid function recovery in these patients was 75.2% (453/602), while this percentage only reached 50.6% (42/83) in patients who underwent surgery by non-specialized surgeons (P<0.001). Furthermore, postoperative corrected Ca and PTH were significantly higher in subjects who recovered parathyroid function (Table 3A).
Number and percentage of patients with recovery of parathyroid function and with hypoparathyroidism at the different stages considered in the study.
Comparison of patients who recover parathyroid function with those who persist with hypoparathyroidism at the end of the study: A, Clinical and surgery related variables; B, variables related to thyroid cancer.
| Patients who recover parathyroid function (n=495) | Non-recovery patients(n=190) | ||||
|---|---|---|---|---|---|
| n | Value | n | Value | P | |
| A: Clinical and surgical related variables | |||||
| Sex, female | 495 | 404 (81.6) | 190 | 148 (77.9) | 0.282 |
| Age, yr | 495 | 47.3±14.9 | 190 | 46.2±14.8 | 0.376 |
| Time of follow-up, mo | |||||
| Specialized surgical team | 495 | 453 (91.5) | 190 | 149 (78.4) | <0.001 |
| Lymph node dissection | 490 | 303 (61.8) | 190 | 132 (69.5) | 0.075 |
| 2-stage thyroidectomy | 495 | 46 (9.3) | 190 | 23 (12.1) | 0.321 |
| Identification of parathyroid glands | 495 | 410 (82.8) | 190 | 126 (66.3) | <0.001 |
| Autotransplantation | 495 | 64 (12.9) | 190 | 18 (9.5) | 0.238 |
| Parathyroid tissue at histology | 494 | 139 (28.1) | 190 | 67 (35.3) | 0.077 |
| Surgical complications | 491 | 50 (10.2) | 190 | 30 (15.8) | 0.047 |
| Postoperative corrected Ca (mg/dl) | 309 | 7.94±0.66 | 131 | 7.69±0.69 | <0.001 |
| Postoperative PTH (pg/ml) | 214 | 10.2 (4.0–20) | 95 | 5.4 (3.0–9.9) | <0.001 |
| B: Variables related to thyroid cancer | |||||
| Incidental | 487 | 72 (14.8) | 187 | 25 (13.4) | 0.714 |
| Papillary histology | 495 | 440 (88.9) | 190 | 156 (82.1) | 0.022 |
| Multifocal | 495 | 178 (36.0) | 187 | 68 (36.4) | 0.929 |
| Tumor size, cm | 488 | 1.6 (1.0–2.8) | 182 | 2.0 (1.1–3.0) | 0.132 |
| Extrathyroidal extension | 481 | 186 | 0.049 | ||
| None | 336 (69.9) | 128 (68.8) | |||
| Microscopic | 120 (5.2) | 39 (21.0) | |||
| Macroscopic | 25 (5.2) | 19 (10.2) | |||
| Lymph node metastasis | 494 | 189 | 0.004 | ||
| Nx | 102 (20.6) | 24 (12.7) | |||
| N0 | 197 (39.9) | 66 (34.9) | |||
| N1 | 195 (39.5) | 99 (52.4) | |||
| Distant metastasis | 494 | 22 (4.5) | 189 | 10 (5.3) | 0.686 |
| Radioiodine ablation | 495 | 417 (84.2) | 190 | 168 (85.3) | 0.814 |
| Surgical re-intervention | 494 | 61 (12.3) | 190 | 37 (19.5) | 0.021 |
| Neck radiotherapy | 494 | 12 (2.4) | 189 | 10 (5.3) | 0.086 |
| Multitargeted kinase inhibitors therapy | 493 | 11 (2.2) | 184 | 9 (4.9) | 0.077 |
Data are the number of patients (percentage), mean±SD for normally distributed data and median (interquartile range) for nonparametric data. The total number of patients used to calculate the values of means, medians and percentages in each group is indicated by n.
Abbreviations: Ca, calcium; PTH, parathyroid hormone.
When studying variables related to thyroid cancer (Table 3B), we found that subjects who were diagnosed with definitive hypoparathyroidism had higher percentages of extrathyroidal extension, lymph node metastases, and surgical re-intervention, and a lower proportion of patients with papillary histology.
Survival analysisWe analyzed the clinical, surgery-related, and cancer-related variables that were found to be significant or near significant (P<0.10) in the comparison performed in Table 3A and B.
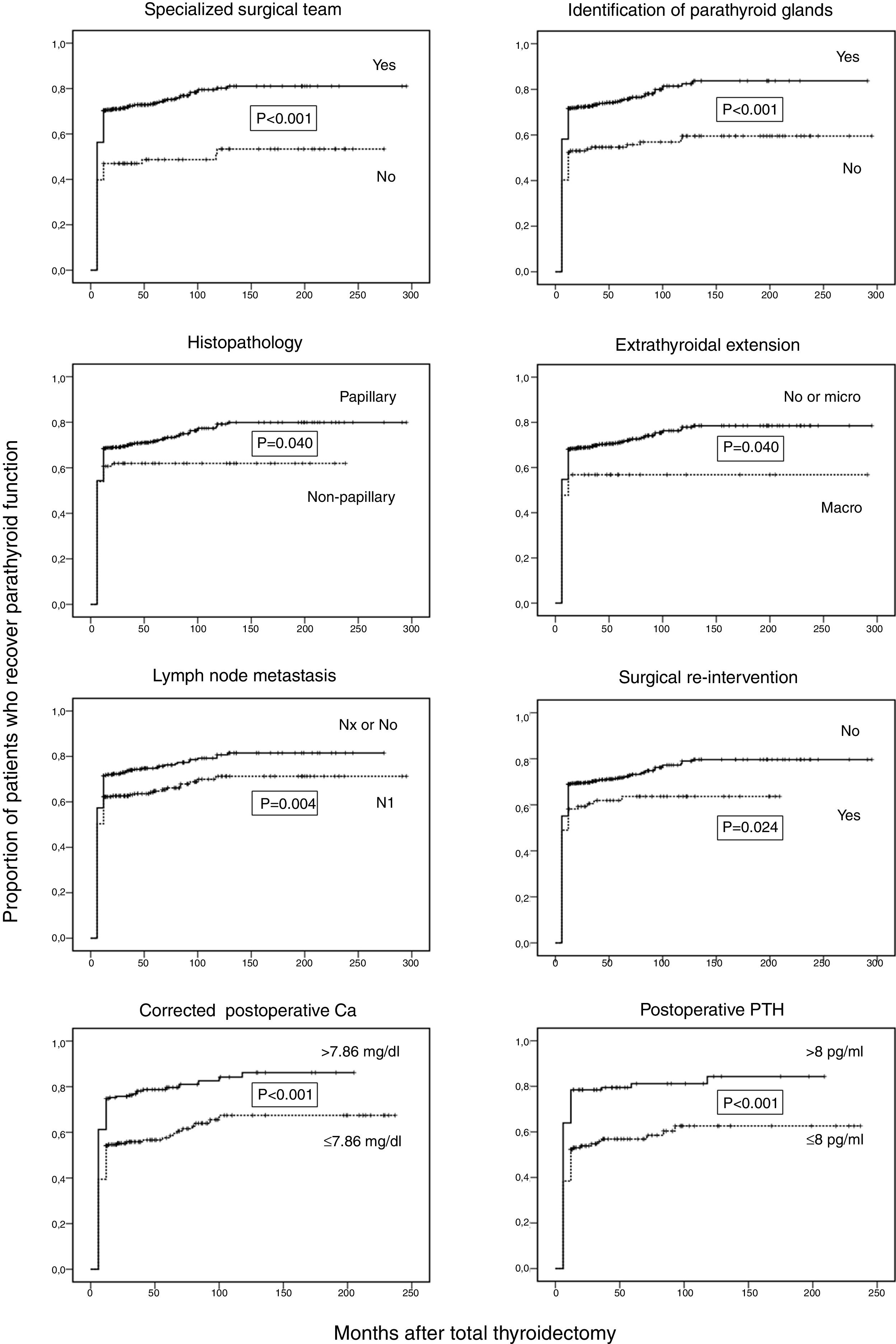
Surgical complications, lymph node dissection, the presence of parathyroid surgery in the specimen, neck radiotherapy, and therapy with kinase inhibitors had no influence on the time to parathyroid function recovery. However, we found that the proportion of patients who recovered parathyroid function was higher in the subgroups of patients with specialized surgical team, identification of parathyroid glands at surgery, papillary histopathology, and serum concentrations of corrected Ca and PTH above the median value (7.86mg/dl and 8.0pg/ml, respectively). On the contrary, patients who had gross extrathyroidal extension of their tumors, lymph node metastases, and those who underwent a subsequent neck surgery after the initial operation exhibited a significant lower time to recovery of parathyroid function (Fig. 2).
Kaplan–Meier curves for time of follow-up up to recuperation of parathyroid function in 685 thyroid cancer patients classified according to the following factors: specialized surgical team, identification of parathyhroid glands at surgery, papillary histopathology, extrathyroidal extension, lymph node metastasis, neck re-intervention after initial surgery, postoperative corrected calcium (n=440), and postoperative PTH concentrations (n=309). Ordinate scale: proportion of patients who recover parathyroid function. Abscissa scale: time of follow-up after thyroidectomy (months).
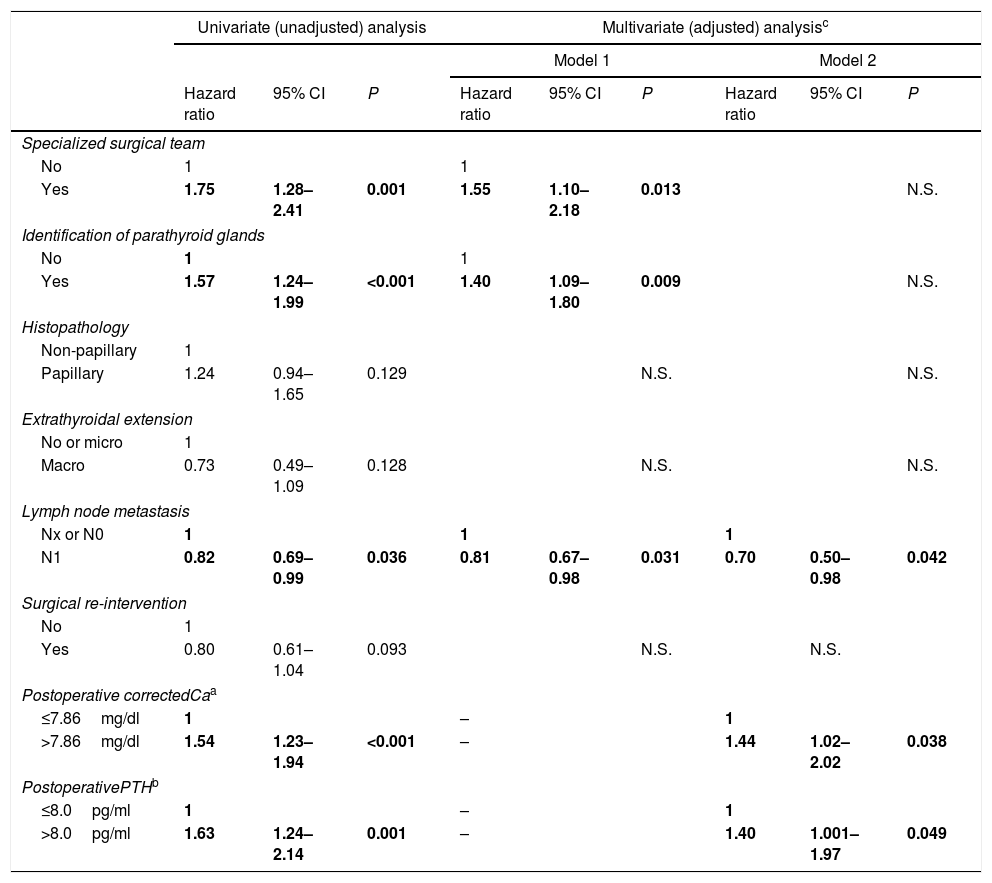
A stepwise Cox proportional hazards regression univariate and multivariate analysis was conducted to evaluate the relative importance of individual factors for the development of recuperation of parathyroid function (Table 4). In the univariate analysis, the probability of recover parathyroid function was significantly greater for patients with specialized surgical team, identification of parathyroid glands, corrected Ca levels>7.86mg/dl, and PTH levels>8.0pg/ml. The presence of lymph node metastases significantly increased the hazard of persisting with hypoparathyroidism.
Nonadjusted and adjusted hazard ratios (with 95% CI) for the development of recovery of parathyroid function (Cox models) in several clinical, surgery-related, and cancer-related variables.
| Univariate (unadjusted) analysis | Multivariate (adjusted) analysisc | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||||||
| Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P | |
| Specialized surgical team | |||||||||
| No | 1 | 1 | |||||||
| Yes | 1.75 | 1.28–2.41 | 0.001 | 1.55 | 1.10–2.18 | 0.013 | N.S. | ||
| Identification of parathyroid glands | |||||||||
| No | 1 | 1 | |||||||
| Yes | 1.57 | 1.24–1.99 | <0.001 | 1.40 | 1.09–1.80 | 0.009 | N.S. | ||
| Histopathology | |||||||||
| Non-papillary | 1 | ||||||||
| Papillary | 1.24 | 0.94–1.65 | 0.129 | N.S. | N.S. | ||||
| Extrathyroidal extension | |||||||||
| No or micro | 1 | ||||||||
| Macro | 0.73 | 0.49–1.09 | 0.128 | N.S. | N.S. | ||||
| Lymph node metastasis | |||||||||
| Nx or N0 | 1 | 1 | 1 | ||||||
| N1 | 0.82 | 0.69–0.99 | 0.036 | 0.81 | 0.67–0.98 | 0.031 | 0.70 | 0.50–0.98 | 0.042 |
| Surgical re-intervention | |||||||||
| No | 1 | ||||||||
| Yes | 0.80 | 0.61–1.04 | 0.093 | N.S. | N.S. | ||||
| Postoperative correctedCaa | |||||||||
| ≤7.86mg/dl | 1 | – | 1 | ||||||
| >7.86mg/dl | 1.54 | 1.23–1.94 | <0.001 | – | 1.44 | 1.02–2.02 | 0.038 | ||
| PostoperativePTHb | |||||||||
| ≤8.0pg/ml | 1 | – | 1 | ||||||
| >8.0pg/ml | 1.63 | 1.24–2.14 | 0.001 | – | 1.40 | 1.001–1.97 | 0.049 | ||
Abbrteviations: CI, confidence interval; N.S., non significant; Ca, calcium; PTH, parathyroid hormone.
Two models of multivariate analysis have been used: model 1, adjusted for specialized surgical team, identification of parathyroid glands, histopathology, extrathyroidal extension, lymph node metastasis, and surgical re-intervention; model 2, adjusted for the same co-variates, and postoperative corrected Ca and postoperative PTH.
Data are the number of patients (percentage), mean±SD for normally distributed data and median (interquartile range) for nonparametric data. The total number of patients used to calculate the values of means, medians and percentages in each group is indicated by n.
Abbreviations: Ca, calcium; PTH, parathyroid hormone.
Two multivariate models were used. Model 1 did not include the analytical variables and encompassed 6 variables and 664 patients. Model 2 contained 8 variables, but only 228 patients. In model 1, the findings of the univariate analysis were retained (except for the analytical variables). In model 2, serum Ca and PTH were significant factors for recovery of parathyroid function. The presence of lymph node metastasis continued to be a significant factor for the maintenance of chronic hypoparathyroidism.
DiscussionThe present results in patients with thyroid cancer treated by total thyroidectomy show that the recovery of parathyroid function is directly and independently related to the expertise of the surgical team and to the identification of the parathyroid glands at surgery and also, negatively, with the presence of lymph node metastasis. Furthermore, in a group of 228 patients we have found that higher levels of both postoperative Ca and PTH favor functional recovery over time. Our results suggest that patients with postsurgical hypoparathyroidism should be closely monitored because the recovery of parathyroid function can occur at any time during the natural course of the disease.
Although some authors recommend that the diagnosis of definitive hypoparathyroidism is made at least 6 months after surgery,1,17 our results confirm that recovery of parathyroid function in thyroid cancer patients is a dynamic process which may occur long after surgery, as stated by other authors in patients with thyroidectomy because of benign or malignant disease.13,15,18–20 Our values on the prevalence of hypoparathyroidism in the various stages of follow-up are higher than those previously reported by others.3,5–10,22 This might be accounted for by the setting were patients were recruited, that is, the endocrinology clinics, where patients with more complications or who need more intensive follow-up are cared for.
Factors influencing the recovery of parathyroid function have been studied by some investigators.7,9–11,13,20 In a group of 135 thyroid cancer patients with postoperative hypoparathyroidism, Paek et al.9 found that autotransplantation, bilateral central neck dissection, gross extrathyroidal extension and the presence of parathyroid tissue in the specimen were risk factors for transient hypoparathyroidism, but only the presence of parathyroid gland in the specimen and the early period of the surgeon's practice were significant risk factors for permanent hypoparathyroidism. However, to our knowledge, the detailed evaluation of the different features of thyroid cancer and their relationships with the recovery of parathyroid function, using multivariate analysis in a large number of subjects, is absent in the literature.
The expertise of the surgical team is a recognized protective factor for parathyroid function9,11 and our survival analysis and regression analysis (model 1) confirm this in our cohort of thyroid cancer patients. Identification of parathyroid glands at surgery and the number of parathyroid glands remaining in situ have been reported to be protective factor for permanent hypoparathyroidism.10,11,13,21 Data of the present study seem to confirm the predictive value of surgical identification of parathyroid glands (model 1), although we could not quantify the number of glands remaining in situ. It is possible that the loss of statistical significance of the specialized surgical team and identification of parathyroid glands in the model 2 of our regression analysis is due to the decrease in sample size that occurs when introducing the analytical variables in the model. Our survival analysis showed that patients with papillary thyroid cancer and those without gross extrathyroidal extension had better prognosis concerning functional recovery. However, in the stepwise Cox proportional hazards regression analysis these variables were non-significant. We are not aware of data from the literature that support or oppose these results.
Despite not having analytical data in all our thyroid cancer patients, we have been able to demonstrate that patients with postoperative concentrations of Ca and PTH above the median values exhibited a significantly better prognosis than subjects with lower values. Several researchers have shown the relationship between low Ca and PTH levels after thyroidectomy and permanent hypoparathyroidism.7,10,16,20–24 The protective influence of high-normal Ca concentrations during the first weeks after surgery has been accounted for by a parathyroid splinting effect.7 According to this hypothesis, high-normal Ca levels would allow the injured, but viable, glands to rest, which would favor their long-term recovery.26
PTH concentration after thyroidectomy is a predictor of early postoperative hypocalcemia and also has been related to recovery of parathyroid function by several researchers.6,7,11,16,21,23,24 Our results seem to suggest that a cutoff value of 8pg/ml for serum PTH at short term after surgery could be a predictor of recovery of long-term parathyroid function. This value is slightly higher than that described by other authors, ranging between 3.15 and 7pg/ml.16,23,26–28 These differences may be due to the large number of hospitals that participated in our study and the diversity of analytical methods for measuring serum PTH. However, the value of 8pg/ml is above the limit of quantification of most PTH assays currently used in clinical laboratories.29
It is assumed that lymph node dissection implies an increased risk of parathyroid injury. In patients with thyroid cancer, Lee et al.5 reported that the extent of surgery was related with postoperative hypoparathyroidism, but the influence of lymph node metastases was not assessed. Paek et al.9 found that lymph node metastases were not risk factors for permanent hypoparathyroidism. However, in our cohort, the presence of lymph node metastases was significantly related to the functional outcome of the patients.
Different mechanisms have been argued to explain the recovery of parathyroid function. A slow but steady recovery of blood flow via neovascularization occurring over the small surface area of the remaining parathyroid has been proposed.19 Medical therapy may also exert a beneficial effect. Vitamin D and calcium are known to negatively regulate parathyroid function,30 and their supplementation might result in a splinting of the parathyroid glands for a time, thus facilitating their subsequent recovery.25 It is also possible that different episodes of hypocalcemia throughout the natural history of the disease progressively stimulate the numb glands and bring on their final reactivation.
Our study is a multicenter survey of national scope and with a high sample size, which are its main strengths. The design of our analysis allows not only to provide data on the chronology of the recovery of parathyroid function, but also to shed light on the factors, related with surgery and also with cancer itself, that influence this recovery. We acknowledge several limitations such as the retrospective nature of the study, the differences in the volume of thyroid surgery, the use of different protocols for clinical assessment, diagnostic procedures, treatments and laboratory measurements, and the fact that we could not assess several variables (i.e., number of parathyroid glands remaining in situ, vitamin D status, and magnesium concentrations). It is possible that the number of patients with hypoparathyroidism at discharge is slightly overestimated, since some patients lacked Ca and PTH values. Our study cannot offer a quantitative definition of expert surgical team since it is heterogeneous in terms of participation from different hospitals. Another limitation is the loss of sample size when analyzing analytical variables (Ca and PTH), since we could not retrieve these data in all patients.
In conclusion, our study emphasizes the need for thyroid cancer patients to be operated by expert surgeons and allows the identification of profiles of patients in whom parathyroid function recovery is more likely. In some of these patients, the judicious and periodic withdrawal of calcium and vitamin D replacement could prevent the maintenance of unnecessary life-long medication.
Financial supportThe present investigation has not received any financial support from public sector agencies, commercial sector or non-profit entities.
Conflict of interestsThe authors declare that they have no conflict of interests.