To determine the association and the prognostic value of soluble ST2 (sST2) levels in the development of diabetic retinopathy (DR), diabetic macular oedema (DMO) or diabetic nephropathy (DN), in a cohort of patients with type 1 diabetes (T1D).
MethodsA total of 269 individuals with T1D (154 males and 115 females) were recruited. The overall mean age was 43.2±14.9 years, and the diabetes duration was 17.1±12.1 years. Levels of sST2 in serum were evaluated, and the presence as well as the degree of DR, DMO and DN was recorded. Additionally, other clinical and analytical parameters including demographic variables were recovered from patients’ electronic health record. Ten years later, the presence and stage of DR, DMO and DN were again recorded under the same criteria.
The association between previously mentioned parameters with DR and DN was analysed by univariate and multivariate logistic regression. The variables in the final multivariate models were adjusted from complete models via backward elimination and maintained only when significant.
ResultsAn increase of 10ng/ml in the levels of sST2 was associated with a 1.50 (1.02–2.19) and 1.48 (1.05–2.08) prevalence odds ratio (OR) in DMO and DR, respectively. There was no association between sST2 levels and DN. Meanwhile, sST2 levels did not display a prognostic effect in any of the microangiopathic diabetic complications studied.
ConclusionsLevels of sST2 are associated with the presence of DR and DMO, they do not seem to be predictive for the development or deterioration of DR, DMO or DN.
Determinar si los niveles de sST2 se asocian con, o tienen algún valor pronóstico en, el desarrollo de la retinopatía diabética (RD), el edema macular (EM) o la nefropatía diabética (ND) en una cohorte de pacientes afectos de diabetes tipo 1.
MétodosLa cohorte consta de 269 individuos, 154 varones y 115 mujeres. Su media de edad es de 43,2±14,9 años y la duración de la diabetes, 17,1±12,1 años. Se analizaron sus niveles séricos de sST2 y se evaluó la presencia y grado de RD, EM y ND. Además, de la historia clínica informatizada se extrajeron diversos parámetros clínicos, analíticos y demográficos de interés. Diez años más tarde se estudiaron de nuevo, y con los mismos criterios, la presencia y estadío de RD, EM y ND.
La asociación entre los parámetros previamente mencionados y RD, EM y ND se analizó por regresión logística univariante y multivariante. Las variables en el modelo multivariante final se seleccionaron desde modelos completos mediante regresión logística de eliminación por etapas, manteniéndose sólo aquellas que resultaron significativas.
ResultadosCada incremento de 10 ng/mL en los niveles de sST2, se asocia con una OR (odds ratio, razón de prevalencias) de 1,50 (1,02-2,19) y de 1,48 (1,05-2,08) para el EM y la RD, respectivamente, mientras que no se detecta asociación entre niveles de sST2 y ND. Por otra parte, los niveles de sST2 no muestran valor pronóstico en ninguna de las complicaciones microangiopáticas estudiadas.
ConclusionesLos niveles de sST2 se asocian con la presencia de RD y EM, pero no parecen predecir ni la aparición ni el deterioro de RD, EM o ND.
Cardiovascular diseases (CVD) remain the leading cause of death and disability in patients with diabetes, despite the huge efforts to prevent and treat them precociously.1 Furthermore, diabetes-related long-term microvascular complications cause high economical and personal costs and impact quality of life.2 Indeed, diabetic retinopathy (DR)3 and diabetic macular oedema (DMO)4 are the most important causes of blindness among working adults worldwide, while diabetic nephropathy (DN) remains the most common cause of end stage renal disease and increases it cardiovascular risk for patients with diabetes.5 Age at diagnosis, duration of diabetes, poor glycaemic control, hypertension, dyslipidaemia and smoking are the most frequently described risk factors for both types of microangiopathic complications.5,6 These factors exert their effect through Kinase/Phosphatase changes, oxidative stress, advanced glycation end products, polyol pathway and inflammatory cytokines.7 Regarding the latter, a large body of evidence supports the concept that DR is a manifestation of a persistent low-grade inflammation in which an influx of inflammatory effectors, including cytokines, are responsible for the damage to the retina.3 The inflammatory response mediated by the above-mentioned factors also seems to play an important role in the pathophysiology of DN.8
The suppression of tumorigenicity 2 (ST2L or interleukin 1 receptor-like 1) is a transmembrane receptor expressed mostly on the surface of Th2 lymphocytes and mast cells and belongs to the Toll-like/Interleukin (IL)-1 receptor superfamily.9 The ST2 gene can encode at least two other isoforms in addition to ST2L by alternative splicing, including a secreted soluble ST2 (sST2) form, which can serve as a decoy receptor for IL-33. IL-33 binds to ST2L and activates mitogen-activated protein kinases and several biochemical pathways leading to cardioprotective effects.10 In CVD, sST2 is a promising and important biomarker.11 Circulating levels of sST2 have been associated with increased cardiovascular mortality in subjects with atherosclerotic disease.1 Moreover, and in line with this observation, in patients with type 2 diabetes (T2D), sST2 has been related with poor prognosis of heart failure and all-cause and cardiovascular mortality.12 Regarding microvascular diseases, in Swedish patients diagnosed with T1D or T2D at age 15–34 years, sST2 has been identified as a potential biomarker for the development of DN.2 To the best of our knowledge, the association between sST2 and DR has not been described.
The aim of this study was to determine the association with, and the prognostic value of, sST2 levels in the development of DR, DMO or DN in a cohort of patients with T1D.
Materials and methodsPatients and samplesTwo hundred and sixty-nine patients with T1D, diagnosed according to World Health Organization criteria,13 over 18 years of age, were consecutively recruited in their regular check-ups at the outpatient clinic of the Department of Endocrinology and Nutrition of the Hospital Complex of Navarre (Pamplona, Spain) between June 2007 and June 2009.
Medical records of the patients were reviewed in order to extract the information about the following demographic and clinical variables: age, age at diabetes onset, duration of diabetes, sex, obesity (body mass index [BMI] ≥30kg/m2), hypertension (current or previous systolic blood pressure ≥140mmHg, or diastolic blood pressure ≥90mmHg in at least two determinations), smoking status (non-smoker, ex-smoker [stopped smoking more than one year ago] or smoker [currently smoking or stopped smoking within the last year]), hypercholesterolaemia (current or previous LDL-cholesterol >100mg/dl), glycosylated haemoglobin (HbA1c) (%) and history of heart failure and CVD (coronary heart disease [CHD], stroke and peripheral artery disease).
DMO was diagnosed and DR was graded by ophthalmologists, using, the five-degree severity scale based on stereoscopic colour fundus photographs, according to the Early Treatment Diabetic Retinopathy Study.14 Staging of DN was based on the urinary albumin-to-creatinine ratio (UACR) in at least two specimens of UACR collected within a 3- to 6-month period and on the estimated glomerular filtration rate (eGFR), according to the classification in five stages of chronic kidney disease proposed by National Kidney Foundation.15
For the HbA1c %, a score determining the quality of glycaemic control of the patient was used. Briefly, the mean HbA1c values of sequential blood extractions through the years attending our outpatient clinic were categorised
from 0 to 2, with (i) 0 or “good glycaemic control”: HbA1c <7% (53mmol/mol); (ii) 1 or “average glycaemic control”: HbA1c between 7% and 8% (between 53 and 64mmol/mol); and (iii) 2 or “Poor glycaemic control”: HbA1c>8% (>64mmol/mol). HbA1c was determined in all patients with high-performance liquid chromatography (HPLC; Adams A1c HA, Menarini Diagnostics, Florence, Italy; reference range: 4.1–6.2%).
LDL-cholesterol was calculated by the Friedewald equation.
Additionally, sST2 levels were analysed from blood samples of all the patients. Within 30min of venous puncture, serum was obtained by 15min centrifugation at 1000g. Samples were kept at −80°C until analysed. Serum sST2 levels were assayed by ELISA (clinical diagnostics), according to the manufacturer's instructions, with a 1:2 serum dilution.
Ten years after the inclusion in the cohort, between 2017 and 2019, the presence and stage of DR, the presence or absence of DMO, and the presence and stage of DN of 239 patients, were recorded again, with the same methods and under the same criteria, and compared with the initial situation. Twelve patients died (5 due to cancer, 3 due to sepsis, 2 due to CVD, 1 due to pneumonia and 1 due to suicide) and 18 were lost during follow-up, due to living in other autonomous regions (15 of them) or due to having stopped coming to our outpatient clinic (3 of them). However, these 30 subjects had clinical characteristics similar to those included in the last analysis (data not shown).
Informed consent was obtained from each patient at the beginning and at the end of the study, 10 years later. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee (Research Ethics Committee of Navarre, Pyto 2015/67).
Statistical analysisThe comparison between mean values of continuous baseline variables from the patients with and without DR, DMO or DN, was performed using the two-tailed Students t-test. To assess if the distribution of baseline categorical characteristics differed among patients with or without DR, DMO or DN, the Pearson's chi-square test was used.
A univariate non-conditional logistic regression model was used to analyse the association between baseline characteristics and DR, DMO or DN. To test for confounding factors, the model was adjusted for sex, age at onset, and duration of diabetes as well as for all other variables that resulted significantly associated with DR, DMO or DN in the univariate model. Then, a p-value-based backward variable selection was attained maintaining only variables with significant explicative value (meaning statistical significance below p<0.05) in the final models.
The prognostic value of sST2 over microangiopathic deterioration in a 10-year window was also evaluated. DR, DMO and DN deterioration were defined as either the appearance or progression in the stage of any of them. The predictive value of sST2 or tertile of sST2 over deterioration of diabetic microangiopathy was evaluated by binary logistic regression. In a first step, univariate models for deterioration were fitted for each demographic and clinical variable. Then, a full model was fitted with all variables that resulted significant in the univariate analysis and a backward variable selection was carried out. Variables were considered to have a prognostic value when they maintained independent predictive value in the model (i.e.: p<0.05).
All analyses were performed with the R statistical software v3.50.
ResultsFrom the 269 patients enrolled in the study, 154 were males and 115 were females. The overall mean age was 43.2±14.9 years. Serum sST2 levels were significantly higher in men (26.9mg/ml; SD: 12.4) than in women (24.0mg/dl; SD: 9.5) (p=0.04). However, serum sST2 levels did not differ between patients with T1D depending on whether the patient had obesity, hypertension, smoking status, hypercholesterolaemia, glycaemic control, previous macrovascular disease or CHD.
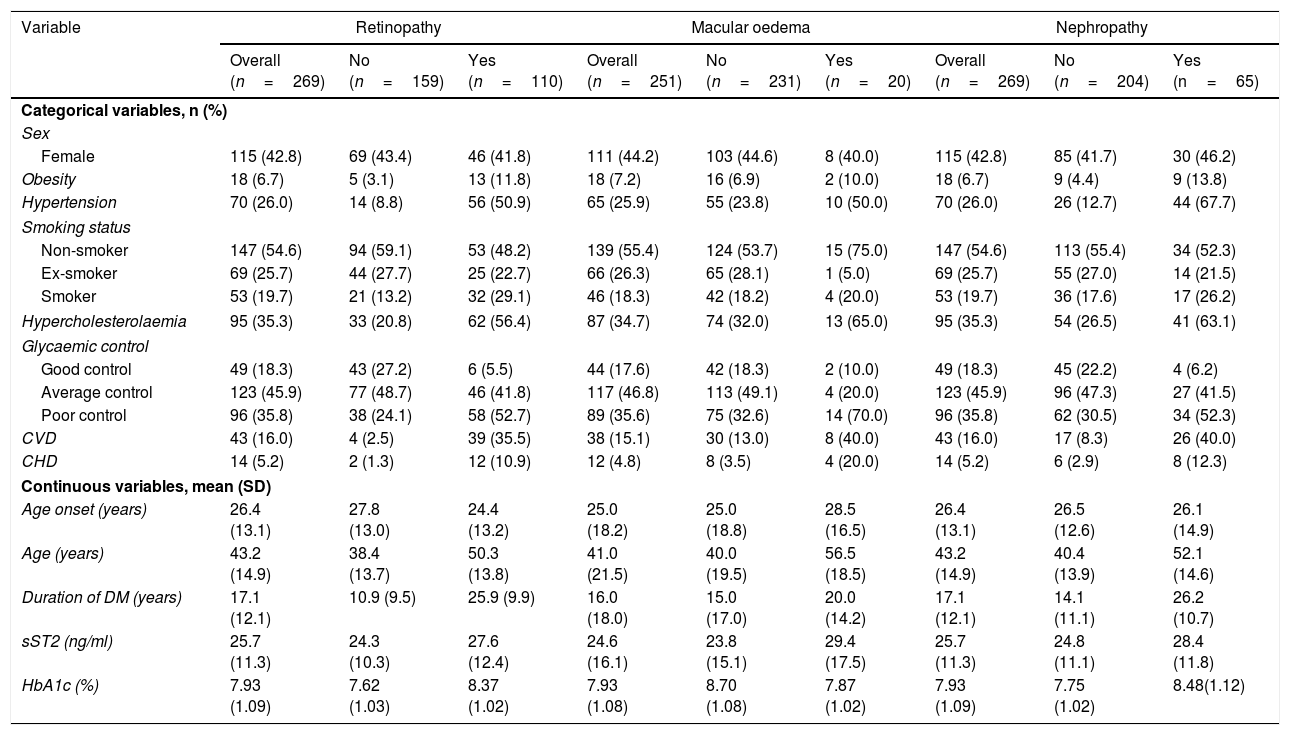
In the descriptive analysis, patients with DR were younger at diabetes onset and they presented higher rates of obesity, hypertension, smoking habit, hypercholesterolaemia, poor glycaemic control, CVD, CHD and higher duration of diabetes. None of the patients who completed the study had been diagnosed with heart failure at the beginning or at the end of the study. Serum sST2 levels were 13.75% higher in patients with DR (Table 1).
Demographic and clinical characteristics of patients by diabetic retinopathy, diabetic macular oedema and diabetic nephropathy.
| Variable | Retinopathy | Macular oedema | Nephropathy | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (n=269) | No (n=159) | Yes (n=110) | Overall (n=251) | No (n=231) | Yes (n=20) | Overall (n=269) | No (n=204) | Yes (n=65) | |
| Categorical variables, n (%) | |||||||||
| Sex | |||||||||
| Female | 115 (42.8) | 69 (43.4) | 46 (41.8) | 111 (44.2) | 103 (44.6) | 8 (40.0) | 115 (42.8) | 85 (41.7) | 30 (46.2) |
| Obesity | 18 (6.7) | 5 (3.1) | 13 (11.8) | 18 (7.2) | 16 (6.9) | 2 (10.0) | 18 (6.7) | 9 (4.4) | 9 (13.8) |
| Hypertension | 70 (26.0) | 14 (8.8) | 56 (50.9) | 65 (25.9) | 55 (23.8) | 10 (50.0) | 70 (26.0) | 26 (12.7) | 44 (67.7) |
| Smoking status | |||||||||
| Non-smoker | 147 (54.6) | 94 (59.1) | 53 (48.2) | 139 (55.4) | 124 (53.7) | 15 (75.0) | 147 (54.6) | 113 (55.4) | 34 (52.3) |
| Ex-smoker | 69 (25.7) | 44 (27.7) | 25 (22.7) | 66 (26.3) | 65 (28.1) | 1 (5.0) | 69 (25.7) | 55 (27.0) | 14 (21.5) |
| Smoker | 53 (19.7) | 21 (13.2) | 32 (29.1) | 46 (18.3) | 42 (18.2) | 4 (20.0) | 53 (19.7) | 36 (17.6) | 17 (26.2) |
| Hypercholesterolaemia | 95 (35.3) | 33 (20.8) | 62 (56.4) | 87 (34.7) | 74 (32.0) | 13 (65.0) | 95 (35.3) | 54 (26.5) | 41 (63.1) |
| Glycaemic control | |||||||||
| Good control | 49 (18.3) | 43 (27.2) | 6 (5.5) | 44 (17.6) | 42 (18.3) | 2 (10.0) | 49 (18.3) | 45 (22.2) | 4 (6.2) |
| Average control | 123 (45.9) | 77 (48.7) | 46 (41.8) | 117 (46.8) | 113 (49.1) | 4 (20.0) | 123 (45.9) | 96 (47.3) | 27 (41.5) |
| Poor control | 96 (35.8) | 38 (24.1) | 58 (52.7) | 89 (35.6) | 75 (32.6) | 14 (70.0) | 96 (35.8) | 62 (30.5) | 34 (52.3) |
| CVD | 43 (16.0) | 4 (2.5) | 39 (35.5) | 38 (15.1) | 30 (13.0) | 8 (40.0) | 43 (16.0) | 17 (8.3) | 26 (40.0) |
| CHD | 14 (5.2) | 2 (1.3) | 12 (10.9) | 12 (4.8) | 8 (3.5) | 4 (20.0) | 14 (5.2) | 6 (2.9) | 8 (12.3) |
| Continuous variables, mean (SD) | |||||||||
| Age onset (years) | 26.4 (13.1) | 27.8 (13.0) | 24.4 (13.2) | 25.0 (18.2) | 25.0 (18.8) | 28.5 (16.5) | 26.4 (13.1) | 26.5 (12.6) | 26.1 (14.9) |
| Age (years) | 43.2 (14.9) | 38.4 (13.7) | 50.3 (13.8) | 41.0 (21.5) | 40.0 (19.5) | 56.5 (18.5) | 43.2 (14.9) | 40.4 (13.9) | 52.1 (14.6) |
| Duration of DM (years) | 17.1 (12.1) | 10.9 (9.5) | 25.9 (9.9) | 16.0 (18.0) | 15.0 (17.0) | 20.0 (14.2) | 17.1 (12.1) | 14.1 (11.1) | 26.2 (10.7) |
| sST2 (ng/ml) | 25.7 (11.3) | 24.3 (10.3) | 27.6 (12.4) | 24.6 (16.1) | 23.8 (15.1) | 29.4 (17.5) | 25.7 (11.3) | 24.8 (11.1) | 28.4 (11.8) |
| HbA1c (%) | 7.93 (1.09) | 7.62 (1.03) | 8.37 (1.02) | 7.93 (1.08) | 8.70 (1.08) | 7.87 (1.02) | 7.93 (1.09) | 7.75 (1.02) | 8.48(1.12) |
CVD: cardiovascular disease; CHD: coronary heart disease; sST2: soluble ST2.
DMO status at the beginning of the study was gathered for 251 out of the 269 patients (140 males and 111 females). The patients with DMO displayed the same factors that had been seen for DR, except for obesity, smoking habit and age at diabetes onset, which were similar between groups with or without DMO. Finally, the factors that were initially associated with the presence of DN were also encountered in DR, except for the smoking status and the age at which the diabetes was diagnosed. (Table 1)
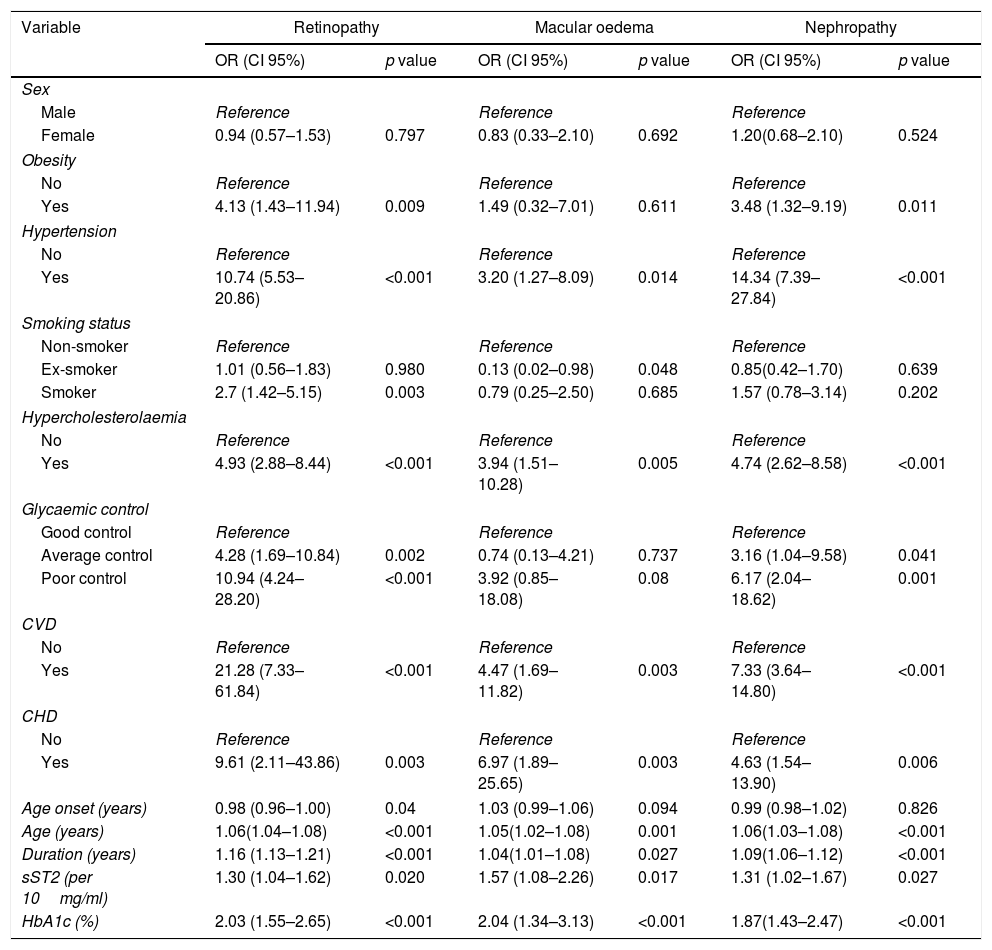
These factors were then analysed by univariate logistic regression as a first step for independent factor multivariate association. Higher sST2 levels were associated with an increased risk of DR [odds ratio (OR) 1.30, 95% confidence interval (CI)=1.04–1.62], DMO [OR: 1.57, CI:1.08–2.26], and DN [OR: 1.31, CI:1.02–1.67]. Additionally, smoking status was associated with DR, but only for smokers [OR: 2.7, CI:1.42–5.15)], while no differences were found between ex-smokers and non-smokers. Good glycaemic control seemed protective in this analysis, whereas, in comparison, average and poor control were both associated with an increased risk for DR, with an OR of 4.28, CI: (1.69–10.84) and 10.94, CI: (4.24–28.20) respectively. Obesity [OR: 4.13, CI: (1.43–11.94)], hypertension [OR: 10.74, CI: (5.53–20.86)], hypercholesterolaemia [OR: 4.93, CI: (2.88–8.44)], CVD [OR: 21.28, CI: (7.33–61.84)], CHD [OR: 9.61, CI: (2.11–43.86)], age at onset [OR: 0,98, CI: (0.96–1.00) and duration of diabetes [OR: 1.16, CI: (1.13–1.21)] were also associated with DR risk. When evaluating DMO, the risk additionally increased in association with the presence of hypertension [OR: 3.20, CI: (1.27–8.09)], hypercholesterolaemia [OR: 3.94, CI: (1.51–10.28)], poor glycaemic control [OR: 3.92, CI: (0.85–18.08)], CVD [OR: 6.97, CI: (1.89–25.65)] and CHD [OR: 6.97, CI: (1.89–25.65)]. Ex-smoker status [OR: 0.13, CI: (0.02–0.98)] and duration of T1D [OR: 1.04, CI: (1.01–1.08)] were also associated. Finally, the risk of DN was associated with the same factors as DR except for age at diagnosis and smoking status, in which no association was observed (Table 2).
Univariate analysis of diabetic retinopathy, diabetic macular oedema and diabetic nephropathy.
| Variable | Retinopathy | Macular oedema | Nephropathy | |||
|---|---|---|---|---|---|---|
| OR (CI 95%) | p value | OR (CI 95%) | p value | OR (CI 95%) | p value | |
| Sex | ||||||
| Male | Reference | Reference | Reference | |||
| Female | 0.94 (0.57–1.53) | 0.797 | 0.83 (0.33–2.10) | 0.692 | 1.20(0.68–2.10) | 0.524 |
| Obesity | ||||||
| No | Reference | Reference | Reference | |||
| Yes | 4.13 (1.43–11.94) | 0.009 | 1.49 (0.32–7.01) | 0.611 | 3.48 (1.32–9.19) | 0.011 |
| Hypertension | ||||||
| No | Reference | Reference | Reference | |||
| Yes | 10.74 (5.53–20.86) | <0.001 | 3.20 (1.27–8.09) | 0.014 | 14.34 (7.39–27.84) | <0.001 |
| Smoking status | ||||||
| Non-smoker | Reference | Reference | Reference | |||
| Ex-smoker | 1.01 (0.56–1.83) | 0.980 | 0.13 (0.02–0.98) | 0.048 | 0.85(0.42–1.70) | 0.639 |
| Smoker | 2.7 (1.42–5.15) | 0.003 | 0.79 (0.25–2.50) | 0.685 | 1.57 (0.78–3.14) | 0.202 |
| Hypercholesterolaemia | ||||||
| No | Reference | Reference | Reference | |||
| Yes | 4.93 (2.88–8.44) | <0.001 | 3.94 (1.51–10.28) | 0.005 | 4.74 (2.62–8.58) | <0.001 |
| Glycaemic control | ||||||
| Good control | Reference | Reference | Reference | |||
| Average control | 4.28 (1.69–10.84) | 0.002 | 0.74 (0.13–4.21) | 0.737 | 3.16 (1.04–9.58) | 0.041 |
| Poor control | 10.94 (4.24–28.20) | <0.001 | 3.92 (0.85–18.08) | 0.08 | 6.17 (2.04–18.62) | 0.001 |
| CVD | ||||||
| No | Reference | Reference | Reference | |||
| Yes | 21.28 (7.33–61.84) | <0.001 | 4.47 (1.69–11.82) | 0.003 | 7.33 (3.64–14.80) | <0.001 |
| CHD | ||||||
| No | Reference | Reference | Reference | |||
| Yes | 9.61 (2.11–43.86) | 0.003 | 6.97 (1.89–25.65) | 0.003 | 4.63 (1.54–13.90) | 0.006 |
| Age onset (years) | 0.98 (0.96–1.00) | 0.04 | 1.03 (0.99–1.06) | 0.094 | 0.99 (0.98–1.02) | 0.826 |
| Age (years) | 1.06(1.04–1.08) | <0.001 | 1.05(1.02–1.08) | 0.001 | 1.06(1.03–1.08) | <0.001 |
| Duration (years) | 1.16 (1.13–1.21) | <0.001 | 1.04(1.01–1.08) | 0.027 | 1.09(1.06–1.12) | <0.001 |
| sST2 (per 10mg/ml) | 1.30 (1.04–1.62) | 0.020 | 1.57 (1.08–2.26) | 0.017 | 1.31 (1.02–1.67) | 0.027 |
| HbA1c (%) | 2.03 (1.55–2.65) | <0.001 | 2.04 (1.34–3.13) | <0.001 | 1.87(1.43–2.47) | <0.001 |
CVD: cardiovascular disease; CHD: coronary heart disease; sST2: soluble ST2.
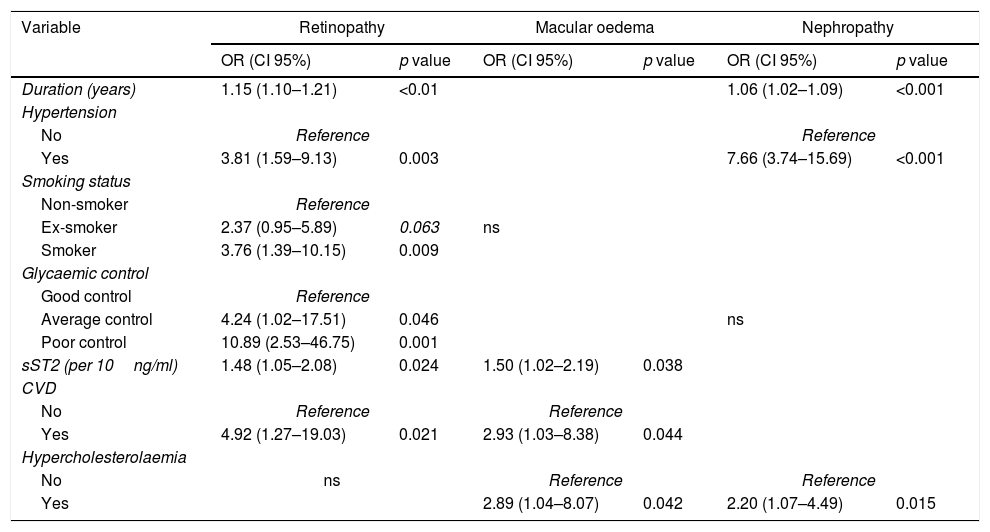
The independent association of risk factors with DR was analysed through multivariate logistic regression. The duration of diabetes, hypertension, current smoking at the beginning of the study, and history of CVD along with average and poor glycaemic control, were associated with an increased risk of DR. Additionally, each 10ng/ml increase in the sST2 levels was associated with a 48% increased prevalence of DR. In the case of DMO, the multivariate analysis showed that hypercholesterolaemia and CVD were independently associated with DMO, despite the statistical power loss for the lesser number of events. Moreover, each 10ng/ml increase in the sST2 levels was associated with a 50% increased prevalence of DMO. Regarding DN, multivariate analysis showed that the duration of diabetes, hypercholesterolaemia and hypertension were associated with an increase of DN risk (Table 3).
Multivariate analysis of diabetic retinopathy, diabetic macular oedema and diabetic nephropathy.
| Variable | Retinopathy | Macular oedema | Nephropathy | |||
|---|---|---|---|---|---|---|
| OR (CI 95%) | p value | OR (CI 95%) | p value | OR (CI 95%) | p value | |
| Duration (years) | 1.15 (1.10–1.21) | <0.01 | 1.06 (1.02–1.09) | <0.001 | ||
| Hypertension | ||||||
| No | Reference | Reference | ||||
| Yes | 3.81 (1.59–9.13) | 0.003 | 7.66 (3.74–15.69) | <0.001 | ||
| Smoking status | ||||||
| Non-smoker | Reference | |||||
| Ex-smoker | 2.37 (0.95–5.89) | 0.063 | ns | |||
| Smoker | 3.76 (1.39–10.15) | 0.009 | ||||
| Glycaemic control | ||||||
| Good control | Reference | |||||
| Average control | 4.24 (1.02–17.51) | 0.046 | ns | |||
| Poor control | 10.89 (2.53–46.75) | 0.001 | ||||
| sST2 (per 10ng/ml) | 1.48 (1.05–2.08) | 0.024 | 1.50 (1.02–2.19) | 0.038 | ||
| CVD | ||||||
| No | Reference | Reference | ||||
| Yes | 4.92 (1.27–19.03) | 0.021 | 2.93 (1.03–8.38) | 0.044 | ||
| Hypercholesterolaemia | ||||||
| No | ns | Reference | Reference | |||
| Yes | 2.89 (1.04–8.07) | 0.042 | 2.20 (1.07–4.49) | 0.015 | ||
CVD: cardiovascular disease; sST2: soluble ST2; ns: not significant.
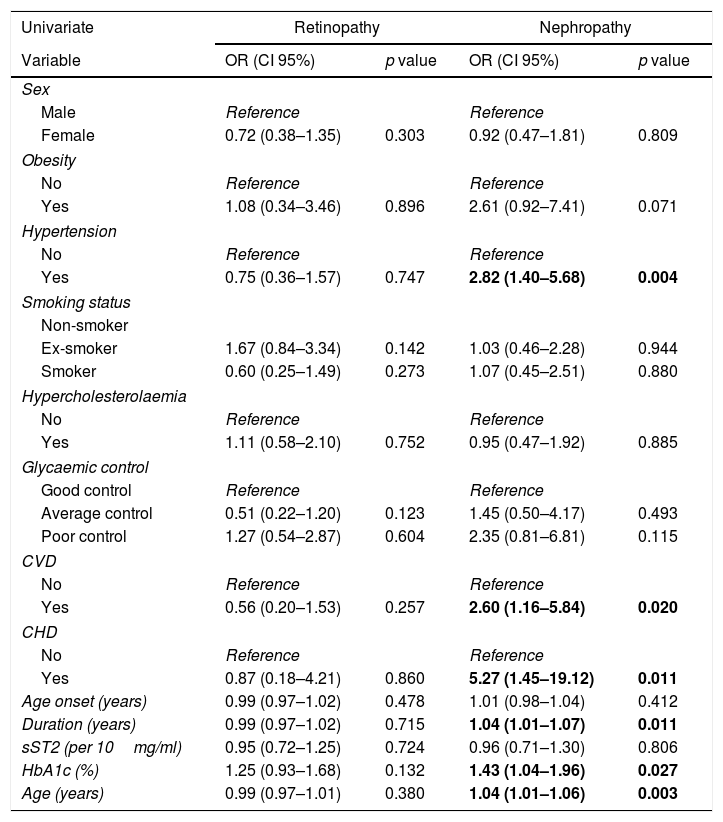
The study for the microangiopathy deterioration over 10 years showed that 53 patients were impaired by DR and 41 by DN. DMO did not change in all 20 patients. With the univariate logistic regression analysis, no factor was found to be associated with the DR evolution. However, age, diabetes duration, HbA1c value, hypertension, CVD and CHD were associated with DN. Levels of sST2 did not display any prognostic effect on DR or DN (Table 4). With the multivariate logistic regression analysis, none of these associations persisted when adjusted for baseline nephropathy.
Univariate analysis of diabetic retinopathy and diabetic nephropathy deterioration, 10 years after inclusion.
| Univariate | Retinopathy | Nephropathy | ||
|---|---|---|---|---|
| Variable | OR (CI 95%) | p value | OR (CI 95%) | p value |
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 0.72 (0.38–1.35) | 0.303 | 0.92 (0.47–1.81) | 0.809 |
| Obesity | ||||
| No | Reference | Reference | ||
| Yes | 1.08 (0.34–3.46) | 0.896 | 2.61 (0.92–7.41) | 0.071 |
| Hypertension | ||||
| No | Reference | Reference | ||
| Yes | 0.75 (0.36–1.57) | 0.747 | 2.82 (1.40–5.68) | 0.004 |
| Smoking status | ||||
| Non-smoker | ||||
| Ex-smoker | 1.67 (0.84–3.34) | 0.142 | 1.03 (0.46–2.28) | 0.944 |
| Smoker | 0.60 (0.25–1.49) | 0.273 | 1.07 (0.45–2.51) | 0.880 |
| Hypercholesterolaemia | ||||
| No | Reference | Reference | ||
| Yes | 1.11 (0.58–2.10) | 0.752 | 0.95 (0.47–1.92) | 0.885 |
| Glycaemic control | ||||
| Good control | Reference | Reference | ||
| Average control | 0.51 (0.22–1.20) | 0.123 | 1.45 (0.50–4.17) | 0.493 |
| Poor control | 1.27 (0.54–2.87) | 0.604 | 2.35 (0.81–6.81) | 0.115 |
| CVD | ||||
| No | Reference | Reference | ||
| Yes | 0.56 (0.20–1.53) | 0.257 | 2.60 (1.16–5.84) | 0.020 |
| CHD | ||||
| No | Reference | Reference | ||
| Yes | 0.87 (0.18–4.21) | 0.860 | 5.27 (1.45–19.12) | 0.011 |
| Age onset (years) | 0.99 (0.97–1.02) | 0.478 | 1.01 (0.98–1.04) | 0.412 |
| Duration (years) | 0.99 (0.97–1.02) | 0.715 | 1.04 (1.01–1.07) | 0.011 |
| sST2 (per 10mg/ml) | 0.95 (0.72–1.25) | 0.724 | 0.96 (0.71–1.30) | 0.806 |
| HbA1c (%) | 1.25 (0.93–1.68) | 0.132 | 1.43 (1.04–1.96) | 0.027 |
| Age (years) | 0.99 (0.97–1.01) | 0.380 | 1.04 (1.01–1.06) | 0.003 |
CVD: cardiovascular disease; CHD: coronary heart disease; sST2: soluble ST2.
In the present study, we observed that in addition to the traditional risk factors for DR appearance, high levels of sST2 were associated with the presence of DR and DMO. However, sST2 levels were not predictive for the development or deterioration of DR, DMO or DN.
We found increased levels of circulating sST2 in men. This observation concurs with previous studies that demonstrated a link between sST2 levels and male gender.16,17 A recent study investigated the mechanism behind this gender bias by measuring the association of sST2 levels with androgen and oestrogen levels,18 but did not find an independent association of sST2 with sex hormones in healthy males and females. Therefore, the reason for the sex-specific difference of sST2 concentrations remains uncertain.
The duration of diabetes and poor glycaemic control are the risk factors most closely related with all forms of DR. Other factors, such as hypertension, smoking and dyslipidaemia appear to influence the onset and progression of DR.19 Furthermore, DR is an independent predictor of both microvascular and macrovascular complications.6 Our results in a cohort of patients with T1D are in line with these observations.
Samuelsson et al.2 observed that there was a strong association between higher levels of sST2 at clinical onset of diabetes and development of DN within 10 years. Moreover, sST2 levels increased with the severity of nephropathy. Thus, sST2 levels can be a potential biomarker for the development of DN.
However, to our knowledge, this is the first cohort in which an association of sST2 with DR has so far been described.
Elevated blood glucose levels per se and the metabolic pathways related to hyperglycaemia – such as the polyol and hexosamine pathways, activation of the diacylglycerol-protein kinase C pathway, and accumulation of advanced glycation end products – are involved in the pathophysiology of DR.4 However, the Joslin Diabetes Centre 50-Year Medalist Study of patients surviving more than 50 years with T1D, showed that 30–35% of them were without significant microvascular complications, regardless of their HbA1c levels and other classical risk factors thought to predict diabetic vascular complications.20 Furthermore, the DCCT/EDIC Research Group showed that HbA1c values explained only up to 11% of the risk of DR.21 This suggests that these patients may possess endogenous tissue factors that increase or diminish the adverse microvascular effects of hyperglycaemia.6 In this sense, inflammation, alteration of retinal blood flow autoregulation and haemorheological factors also play an important role in the pathogenesis of DR.4 Circulating cytokines could also increase the vascular leakage, but their contribution to the DR and DMO development remains uncertain.6 The soluble fraction of ST2 binds to the circulating IL-33 avoiding it to bind the membrane receptor ST2. Thus, sST2 may play part in the inflammatory process of diabetes mellitus, although the mechanisms behind this are not yet fully understood.2 The loss of cardioprotective effect derived from the IL-33 binding to the soluble form of sST2 is already known.1 In view of the results of our study, we hypothesise that the blockade of IL-33 signalling by sST2 could facilitate an inflammatory response that contributes to DR and DMO appearance.
Although higher sST2 levels found in patients with DR and DMO could be influenced by the presence of concomitant CVD, on multivariate analysis, levels of sST2 are independently associated with DR and DMO, suggesting a possible pathological role of sST2. Conversely, circulating levels of sST2 are not associated with the presence of DN. In this study, in the multivariate analysis, only hypertension, hypercholesterolaemia and duration of diabetes have shown to be related with DN.
Elevated sST2 levels found in DR patients, but not in DN patients, suggest that the pathogenesis of both diabetic complications could be different. In fact, not all patients with DR have concomitant DN.2 Interestingly, in our cohort of patients, sST2 levels did not correlate with classical risk factors of DR.
Notably, sST2 serum levels did not exert any prognostic effect over deterioration of DR, DMO or DN after a 10-year follow-up period. Our results are not in accordance with data found by Samuelsson and coworkers.2 In their study, sST2 levels are similar in men and in women, and play a prognostic role only in DN development. The discrepancy between our study and Samuelsson's study could be due to the differences between Spanish and Swedish populations. What's more, they included patients aged 15–34 years old with both T1D and T2D. Another difference is that we analysed sST2 in serum, while in the Swedish study, sST2 was measured in plasma. However, since it is a protein not involved in coagulation, there should be no differences.
The strength of this study lies in the fact that it is a well-defined cohort of adult patients with T1D, who were studied initially in the period 2007–2009,22 and again after 10 years of follow-up.
The limitations are: the scarce number of cases that make up the sample; the fact that this is a study done in a single hospital, which limits the extrapolation of the results; the loss of 30 patients during follow-up; and the regression model used, that can eliminate variables with known effect on the outcome to be evaluated.
In summary, our results confirm the influence of traditional risk factors in the development of microvascular complications in patients with T1D. Additionally, for the first time, the relationship between high levels of the soluble fraction of ST2 and DR and DMO has been suggested. Additional larger studies on sST2 levels and the development of DR and DN are needed to better define the possible role in the pathogenesis of these microvascular complications of diabetes mellitus as well as a possible target for potential therapies.
Data availabilityThe data used to support the findings of this study are available from the corresponding author upon request.
FundingThis study has been funded by grants given to this research project by the Instituto de Salud Carlos III [PI10/02715] and the Government of Navarre [53/2008]. The funding sources have not participated in the study in any way.
Conflict of interestThe authors declare that there is no conflict of interest regarding the publication of this article.
The authors acknowledge the rest of the components of the Type 1 Diabetes Study Group of Navarre, cited herein, and their contribution to this work: Ana Iriarte Beroiz, Amaya Sainz de los Terreros Errea, Laura Chinchurreta Díez and Marta Toni García.
The authors also acknowledge Patricia Andrada Álvarez's collaboration in translating the manuscript into English.
Finally, the authors acknowledge the “Instituto de Salud Carlos III”and Government of Navarre his collaboration, funding this study.