To assess the value of 18F-FDG-PET/CT for detecting recurrent/persistent disease in patients with biochemical incomplete (BIR) or indeterminate response (IR) and to assess the impact of 18F-FDG-PET/CT on the therapeutic management of these patients.
MethodsThe study included patients with BIR, in whom 18F-FDG PET/CT was used within the diagnostic algorithm from our database. Patients with IR referred to our hospital with the 18F-FDG PET/CT already performed were also enrolled. All patients had neck ultrasonography with no structural changes. A change in therapeutic approach was defined as repeat surgery; administration of external beam radiotherapy; and/or the start of systemic therapy.
ResultsSixty patients (85% women) aged 18–86 years were enrolled in this retrospective study. Of these, 75% had BIR and 25% IR. Increased FDG uptake suggesting locoregional lesions was seen in 40% of patients. Sensitivity, specificity, and diagnostic accuracy of 18F-FDG PET/CT to detect local disease were 95%, 87.5% and 90% respectively. The therapeutic approach was modified in 50% of patients with locoregional lesions.
ConclusionsOur study confirmed that 18F-FDG-PET/CT is a useful tool for detecting locoregional recurrence in thyroid cancer patients with BIR or IR with conflicting findings in standard diagnostic procedures. In 50% of patients with locoregional lesions, there was an immediate change in the treatment approach.
Evaluar la utilidad de la tomografía por emisión de positrones con 18F-FDG (18F-FDG PET/TC) para detectar enfermedad recurrente o persistente en pacientes con respuesta bioquímica incompleta (RBI) o respuesta indeterminada (RI), y evaluar el impacto de los resultados del PET/TC en el manejo terapéutico de estos pacientes.
MétodosSe incluyeron pacientes con RBI, en los cuales el PET/TC fue utilizado en el algoritmo diagnóstico durante el seguimiento, y además pacientes con RI referidos a nuestro hospital con el estudio realizado. Todos los pacientes presentaban ecografía de cuello sin evidencia de alteraciones estructurales. Se consideró como cambio en el enfoque terapéutico a: 1) realización de nuevas cirugías, 2) administración de radioterapia externa, y/o 3) inicio de terapia sistémica.
ResultadosSesenta pacientes con edad entre 16 a 86 años fueron incluidos retrospectivamente (85% mujeres), el 75% con RBI y el 25% con RI. En el 40% de los pacientes el PET/TC evidenciaron lesiones locorregionales. La sensibilidad, la especificidad y la precisión diagnóstica del PET/TC para detectar enfermedad locorregional fue del 95, 87,5 y 90%, respectivamente. En el 50% de los pacientes con enfermedad locorregional los resultados del PET/TC determinaron un cambio en la conducta terapéutica.
ConclusionesNuestro estudio demostró que el PET/TC es una herramienta útil en la detección de enfermedad locorregional recurrente o persistente en pacientes con cáncer de tiroides con RBI o RI durante el seguimiento con hallazgos contradictorios en los métodos diagnósticos estándares. En el 50% de los casos con lesiones locorregionales hubo un cambio inmediato en el enfoque terapéutico.
Differentiated thyroid cancer (DTC) is generally characterized by good prognosis and low disease-specific mortality.1,2 The therapeutic approach and follow-up of patients with DTC is currently individualized according to the risk of recurrence (RR). The presence of a structural incomplete response to therapy (SIR) can be observed in 2–75% of patients with DTC3 and it is usually related to the initial risk of recurrence, and to the dynamic risk assessment performed during the long-term follow-up.4–6 The frequency of SIR in patients with an initial indeterminate response (IR) has been reported to be around 13–20% over 10 years of follow-up.4,5 On the other hand, several series have demonstrated that 8–17% of patients with a biochemical incomplete response (BIR) may develop structurally identifiable disease over 5–10 years of follow-up.4,7–9
In these patients, it is important to characterize the presence of structural lesions with morphologic imaging methodology to plan a strategic therapeutic approach. These studies may include neck ultrasonography (US), systemic computed tomography (CT), magnetic resonance imaging (MRI), nuclear imaging procedures, such as whole-body scan after a diagnostic or therapeutic radioiodine dose, and/or the use of positron emission tomography/computed tomography using fluorine-18 fluorodeoxyglucose (18F-FDG-PET/CT scan).
The 18F-FDG-PET/CT scan has been used with high sensitivity and specificity in the diagnosis of local recurrences and distant metastases when other imaging methods were negative in the presence of a BIR.10,11 Additionally, since the 18F-FDG-PET/CT may reflect disease aggressiveness, it can also provide information about the long-term outcome.12,13 Therefore, the findings provided by this imaging methodology might modify the therapeutic approach in up to 30% of patients.14–16
The aims of this study were: to evaluate the usefulness of 18F-FDG-PET/CT to detect recurrent/persistent disease in patients with BIR or IR at some point during the follow-up; and to evaluate the impact of 18F-FDG-PET/CT on patient's management strategies.
MethodsData source and study populationWe retrospectively reviewed our database containing 790 files records of patients with DTC who were followed-up from January 2001 to February 2018 in the Division of Endocrinology, Hospital de Clínicas-University of Buenos Aires. We included 60 patients submitted to total thyroidectomy and remnant ablation, who had a biochemical incomplete response at some point during the follow-up, in whom the 18F-FDG-PET/CT was used within the diagnostic algorithm to determine the presence of structural disease. We performed 18F-FDG-PET/CT in patients with conflicting results, that is, patients with biochemical incomplete response with increasing serum Tg or TgAb during the follow-up.
We also included in the analysis, those patients with indeterminate response who was referred to our hospital with the 18F-FDG-PET/CT already performed, requested by another physician. To be included, patients had to present a minimum follow-up of 12 months after the evaluation with an 18F-FDG-PET/CT to assess the outcome after the performance of this imaging procedure. All patients with ultrasonographically suspicious lymph nodes were excluded from the study, therefore the total cohort had a cervical ultrasound without findings.
Each patient was stratified using the eighth edition of the American Joint Committee on Cancer/International Union against Cancer (AJCC/UICC) staging system and the risk of recurrence was assessed by using the modified 2015 American Thyroid Association risk stratification system (low, intermediate or high).3,17 The response to therapy was evaluated in the first 12 months and at the end of follow-up, based on serum thyroglobulin determinations, neck ultrasound (US), diagnostic or post radioiodine dose whole body scanning and appropriate additional functional and cross-sectional imaging according to what it is suggested by the ATA guidelines.3 An excellent response to therapy was defined as a suppressed Tg<0.2ng/ml or stimulated Tg<1ng/ml in the absence of anti-thyroglobulin antibodies, with a normal post-operative neck US. The biochemical incomplete response was defined as a suppressed Tg>1ng/ml or stimulated Tg>10ng/ml or increasing TgAb levels in the absence of localizable disease. Indeterminate response was defined as a non-stimulated Tg<1ng/ml or stimulated Tg of 1–10ng/ml or stable or declining TgAb levels with nonspecific structural findings. Patients with persistent or newly identified locoregional or distant metastases with or without abnormal Tg or TgAb were classified as having structural incomplete response.3
Our ablation protocol used fixed radioiodine activities based on the extent of the initial disease. Therapeutic doses of 131I ranged from 3.7 to 7.4GBq (100–200mCi 131I). Even though in our Hospital we began to apply the ATA guidelines from 2008, the group of low risk patients included in this study received radioiodine remnant ablation with high doses in other centers, and then they were referred to our Hospital.
A low iodine diet was prescribed from two weeks before radioiodine administration through two days afterwards. In 92% with thyroid hormonal withdrawal (THW) at least 3 weeks, starting from thyroidectomy, and in only 8% after recombinant human thyrotrophin (rhTSH) administration (exogenous stimulation). The election of one or another methodology for preparation for remnant ablation usually was related to the reimbursement that each patient has in Argentina for rhTSH, according to his or her health insurance. Radioiodine was administered following that interval, in all cases with TSH levels above 50mIU/L. A post-therapy scan (WBS) was performed 5–7 days after therapeutic RAI administration.
After ablation, all patients were kept on a suppressed thyrotropin (TSH) level until January 2008 when all patients received thyroid hormone therapy according to the Latin American Thyroid Society recommendations for each risk of recurrence group (target TSH: <0.1mIU/L for intermediate risk; 0.4–1mIU/L for low risk; and thyroid hormone replacement for very low risk LATS classification.18 From January 2008 until inclusion all patients received hormonal therapy to keep a TSH level according to the risk of recurrence and response to therapy during the follow up according to what was suggested by the American Thyroid Association guidelines.3
A change of the therapeutic approach was defined as: (i) new surgery/surgeries; (ii) administration of external beam radiotherapy; and/or (iii) initiation of systemic therapy determined by the findings detected by the 18F-FDG-PET/CT.
Serum thyroglobulin and anti-thyroglobulin antibodies measurementSerum Tg and TgAb were assessed in one of two reference laboratories using either of two commercial immunometric assays and the same assay was used throughout a patient's follow-up. Serum Tg level was measured by Elecsys Tg Electrochemiluminescence Immunoassay (Roche Diagnostics GmbH, Mannheim, Germany), and the Immulite 2000 Tg Chemiluminiscence Assay (Siemens Corp., Los Angeles, CA, USA). The functional sensitivities of both methods were 0.1ng/ml and 0.3ng/ml, respectively.
TgAb assays comprised the Elecsys Anti-Tg Electrochemiluminescence Immunoassay (RSR Ltd., Pentwyn, Cardiff, UK), or the Immulite 2000 Anti-TG Ab chemiluminescent immunometric assay method (Siemens). The serum TgAb level was considered positive when it was 20IU/ml or greater, in accord with the manufacturer's recommendations.
Neck ultrasonographyThyroid ultrasonography was performed by experienced sonographers in the outpatient clinic using a 13-MHz linear transducer. Ultrasonographically suspicious nodes >10mm in diameter underwent fine-needle aspiration biopsy (FNAB) for cytology with Tg measurement in the needle washout fluid.
Additional conventional imaging proceduresAdditional conventional imaging procedures performed for suspicious lesions regarding metastasis or recurrence included: high resolution computed tomography (n=12, 20%), and bone scintigraphy (n=6, 10%) without positive findings. Twenty-five percent of patients with negative CT had positive findings on 18F-FDG-PET/CT. Only one patient had a negative diagnostic whole-body scan (5mCi 131I).
18F-FDG-PET/CT techniquePositron emission tomography combined with computed tomography were obtained with a Gemini Philips scanner with 16 rows of detectors. Each patient fasted for at least 6h before an intravenous administration of fluorine-18 fluorodeoxyglucose (0.11mCi/kg). Blood glucose levels lower than 200mg/dL. Whole-body PET and CT images were performed 60min after marker injection. PET images were obtained from the base of the skull to half of the thighs, with an acquisition time of 3min per bed position. The reconstruction was made in 3 basic planes after correction for attenuation based on CT examination. CT scan was acquired with a Survey of 90kVp and 20mAs. A full-body acquisition with 120kVp, 250mAs and a high-resolution thorax acquisition of 120kVp, 200mAs with images reconstructed every 5 and 1mm, respectively.
Maximum standardized uptake value (SUVmax) was the semi-quantitative PET/CT parameter used in the study. It was calculated according to a standard protocol on a dedicated workstation. Maximum standardized uptake value corrected for body weight was computed by standard methods from the activity at the most intense voxel in three-dimensional tumor regions from the transaxial whole-body slices on attenuation-corrected PET/CT images. Transaxial, sagittal, and coronal images were shown on a computer display monitor. A visually abnormal focus of 18F-FDG accumulation was defined as a focal uptake relatively higher than that of the surrounding tissue with no similar activity seen in the contralateral side of the body indicative of metastasis/recurrence reinforced by the related CT findings.
Positron emission tomography and neck CT findings were compared with the histopathological examination results if the lesions were surgically removed or biopsied. If those lesions detected by 18F-FDG PET/CT were confirmed by histopathology by surgery or biopsy, the findings provided by the 18F-FDG-PET/CT were classified as true-positive. If recurrence was excluded by histopathological examination in patients with positive lesions on 18F-FDG PET/CT and neck CT, the imaging findings were classified as false-positive results. In the case that recurrence was confirmed by histopathological examinations in patients with negative 18F-FDG PET/CT, the classification of these findings was defined as false-negative results. Finally, negative 18F-FDG PET/CT in patients with negative histopathological examination was classified as true-negative results.
In the last 10 years, we performed the 18F-FDG-PET/CT under suppressed TSH, based on evidence of an uncertain clinical benefit of performing 18F-FDG PET/CT under stimulated TSH. Twenty-seven percent (n=16) of 18F-FDG-PET/CT was done under TSH stimulation, in 44% after recombinant human thyrotrophin (rhTSH) administration (exogenous stimulation), whereas in the remaining 56% after THW. Of these 16 patients in whom 18F-FDG PET/CT was performed under stimulated TSH, 5 with an IR referred to our center, and the study was performed before the new methodological approach in 11 patients with a BIR.
Statistical analysisEpidemiological data are presented as the mean±SEM, with median and range when appropriate. For categorical variables, the number and percentage of patients and/or scans was calculated within each category. The diagnostic capacity of the 18F-FDG-PET/CT to identify (regional and distant) metastasis was estimated. The crude estimations were adjusted by TSH value (with and without stimulation) and thyroglobulin levels (≥ and <10ng/ml). The categorical variables were compared by Pearson Chi-square and Fisher exact tests. P<0.05 was used to statistical significance. For each instance, the likelihood ratio (ratio between sensitivity and false positive) and their respective 95% confidence intervals were estimated, checking the statistical significance with the overlapping range width. Receiver operating characteristic (ROC) curve analysis was used to define the SUVmax as a predictor of structural disease. The area under the curve (AUC) was mentioned with the best cut-off estimated.
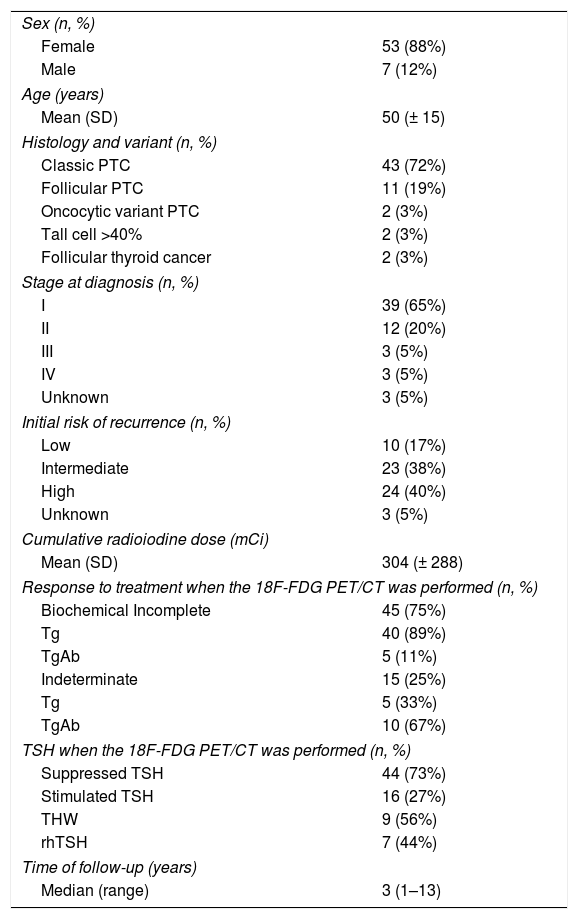
ResultsSixty patients (85% women) aged 18–86 years (median 50 years), were enrolled in this retrospective study. Based on the risk of recurrence classification, 17%, 38%, and 40% were low risk, intermediate risk, and high risk, respectively.3 Seventy-five percent of included patients had a BIR and 25% an IR when the 18F-FDG-PET/CT was performed.
Baseline characteristics of included patients can be observed in Table 1.
Baseline characteristics (n=60).
| Sex (n, %) | |
| Female | 53 (88%) |
| Male | 7 (12%) |
| Age (years) | |
| Mean (SD) | 50 (± 15) |
| Histology and variant (n, %) | |
| Classic PTC | 43 (72%) |
| Follicular PTC | 11 (19%) |
| Oncocytic variant PTC | 2 (3%) |
| Tall cell >40% | 2 (3%) |
| Follicular thyroid cancer | 2 (3%) |
| Stage at diagnosis (n, %) | |
| I | 39 (65%) |
| II | 12 (20%) |
| III | 3 (5%) |
| IV | 3 (5%) |
| Unknown | 3 (5%) |
| Initial risk of recurrence (n, %) | |
| Low | 10 (17%) |
| Intermediate | 23 (38%) |
| High | 24 (40%) |
| Unknown | 3 (5%) |
| Cumulative radioiodine dose (mCi) | |
| Mean (SD) | 304 (± 288) |
| Response to treatment when the 18F-FDG PET/CT was performed (n, %) | |
| Biochemical Incomplete | 45 (75%) |
| Tg | 40 (89%) |
| TgAb | 5 (11%) |
| Indeterminate | 15 (25%) |
| Tg | 5 (33%) |
| TgAb | 10 (67%) |
| TSH when the 18F-FDG PET/CT was performed (n, %) | |
| Suppressed TSH | 44 (73%) |
| Stimulated TSH | 16 (27%) |
| THW | 9 (56%) |
| rhTSH | 7 (44%) |
| Time of follow-up (years) | |
| Median (range) | 3 (1–13) |
SD: standard deviation; DTC: differentiated thyroid cancer; Tg: thyroglobulin; TgAb: anti-thyroglobulin antibodies; TSH: thyrotropin; THW: thyroid hormone withdrawal; rhTSH: recombinant human thyrotropin.
Increased 18FDG uptake suspicious for locoregional lesions was observed in 24 (40%) of the patients, while in the remaining 36 patients had no pathological 18FDG uptake. The distribution of the lesions in the former 24 patients was as follows: cervical and mediastinal lymphatic nodes in 18 patients, thyroid bed in 4 patients and, thyroid bed together with cervical lymph nodes in 2 cases. The median size of the diagnosed lymph nodes was 13mm (range 7–23mm) with a mean SUVmax of 7.18 (± 4.94). In 14 of these 24 patients with positive 18F-FDG-PET/CT results, the final diagnosis was made by histopathological examination and in 5 patients, the diagnosis of locoregional recurrence was determined during clinical follow-up. The remaining 5 patients were considered as false positive (three patients had negative fine-needle aspiration biopsy of cervical lymph nodes and in two patients the cervical lymph nodes disappeared during the follow-up). The therapeutic approach was modified based on 18F-FDG-PET/CT findings in half of the patients with locoregional lesions (in 46% surgical intervention and 4% the use of external beam radiotherapy was indicated).
Initially, distant metastases detected by the 18F-FDG-PET/CT were in lungs (n=5) and in bone (n=1). Of the 5 patients with lung metastases, 1 patient had positive 18FDG uptake (SUV max 4.5) and 4 patients had small pulmonary nodules (<1cm) detected by the CT scan procedure without any 18FDG uptake. One patient developed progressive disease, and sorafenib 800mg/day was then prescribed. We inferred that this patient did not have FDG uptake due to the size of the pulmonary nodules which were below the resolution of 18F-FDG-PET/CT. In the remaining 3 patients, the pulmonary nodules disappeared during the follow-up, and they were considered as a false positive result. One patient had a bone uptake, only one focus (L1 vertebrae, SUVmax 3.4), with negative additional images (MRI and bone scintigraphy). Therefore, it was also considered as a false positive result. This patient had had a spinal trauma 5 years before.
Finally, the prevalence of distant metastases detected by 18F-FDG-PET/CT was 3.3% (n=2, lung localization). Those two patients had also locoregional lesions.
The only variable that was statistically significant when comparing positive and negative 18F-FDG-PET/CT was age at diagnosis of DTC, patients with negative 18F-FDG-PET/CT were younger than those patients with positive (p=0.004). There were no statistically significant differences considering gender (p=0.92), initial lymph node dissection (p=0.574), histology (p=0.05) or PCT histological variant (p 0.694), multicentricity (p=0.141), bilateral disease (p=0.179), presence of thyroiditis (p=0.35), capsular invasion (p=0.189), tumor diameter (p 0.2883), extrathyroidal invasion (p=0.109), BIR and IR (p=0.086).
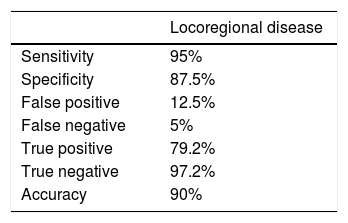
The diagnostic values (sensitivity, specificity, and diagnostic accuracy) of the 18F-FDG-PET/CT to detect locoregional disease were calculated as follows: 95%, 87.5%. and 90%, respectively (Table 2).
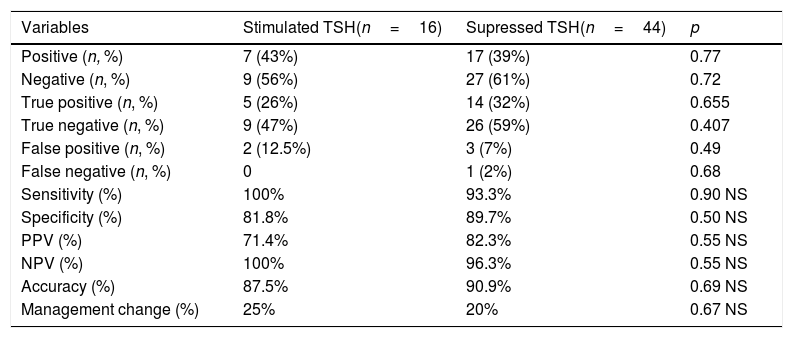
The sensitivity of 18F-FDG-PET/CT carried out under TSH suppression was 93.3%, while when the examination was performed after TSH stimulation, it was 100% (p=0.90). When the 18F-FDG-PET/CT was done after TSH stimulation, its sensitivity did not differ significantly considering the way TSH stimulation was performed (endogenous or exogenous). The specificity was similar independently of the serum TSH levels at the time of the performance of the 18F-FDG-PET/CT. We found no significant differences in the diagnostic performance of 18F-FDG-PET/CT when done with or without TSH stimulation (Table 3).
Diagnostic yield in relation to TSH levels at the time of 18F-FDG-PET/CT.
| Variables | Stimulated TSH(n=16) | Supressed TSH(n=44) | p |
|---|---|---|---|
| Positive (n, %) | 7 (43%) | 17 (39%) | 0.77 |
| Negative (n, %) | 9 (56%) | 27 (61%) | 0.72 |
| True positive (n, %) | 5 (26%) | 14 (32%) | 0.655 |
| True negative (n, %) | 9 (47%) | 26 (59%) | 0.407 |
| False positive (n, %) | 2 (12.5%) | 3 (7%) | 0.49 |
| False negative (n, %) | 0 | 1 (2%) | 0.68 |
| Sensitivity (%) | 100% | 93.3% | 0.90 NS |
| Specificity (%) | 81.8% | 89.7% | 0.50 NS |
| PPV (%) | 71.4% | 82.3% | 0.55 NS |
| NPV (%) | 100% | 96.3% | 0.55 NS |
| Accuracy (%) | 87.5% | 90.9% | 0.69 NS |
| Management change (%) | 25% | 20% | 0.67 NS |
PPV: positive predictive value; NPV: negative predictive value; NS: not significant.
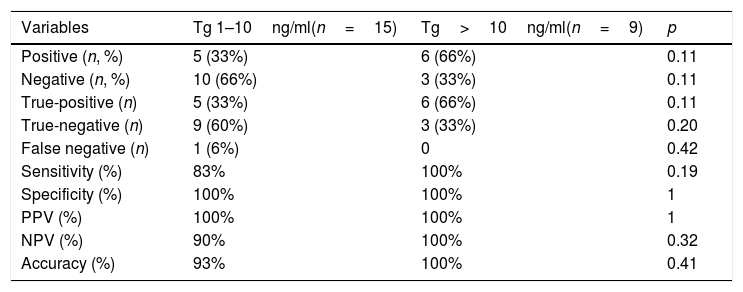
The cut-off value of serum Tg level that carried to the indication of 18F-FDG-PET/CT influenced its sensitivity. We compared the sensitivity, specificity, and accuracy of 18F-FDG-PET/CT for different thyroglobulin levels under hormonal therapy: (i) 1–10ng/ml and (ii) >10ng/ml. The sensitivity was 83% in patients with Tg levels under thyroid hormone therapy between 1 and 10ng/ml and 100% for those with Tg levels >10ng/ml (p=0.19) (Table 4).
Diagnostic performance of 18F-FDG PET/CT for different thyroglobulin levels under hormonal therapy.
| Variables | Tg 1–10ng/ml(n=15) | Tg>10ng/ml(n=9) | p |
|---|---|---|---|
| Positive (n, %) | 5 (33%) | 6 (66%) | 0.11 |
| Negative (n, %) | 10 (66%) | 3 (33%) | 0.11 |
| True-positive (n) | 5 (33%) | 6 (66%) | 0.11 |
| True-negative (n) | 9 (60%) | 3 (33%) | 0.20 |
| False negative (n) | 1 (6%) | 0 | 0.42 |
| Sensitivity (%) | 83% | 100% | 0.19 |
| Specificity (%) | 100% | 100% | 1 |
| PPV (%) | 100% | 100% | 1 |
| NPV (%) | 90% | 100% | 0.32 |
| Accuracy (%) | 93% | 100% | 0.41 |
PPV: positive predictive value; NPV: negative predictive value.
On the other hand, the PET/CT was not useful to identify structural disease in patients with indeterminate response defined by thyroglobulin levels (non-stimulated Tg<1ng/mL or stimulated Tg<10ng/mL). The 18F-FDG-PET/CT was negative in 100% of patients with suppressed thyroglobulin levels <1ng/mL, and in 80% with stimulated thyroglobulin levels between 1 and 10ng/ml.
The median stimulated Tg levels in patients with and without structural disease were 119ng/ml (range 14–288ng/ml) and 10.2ng/ml (2–53ng/ml), respectively (p=0.03). On the other hand, the median suppressed Tg level was 15ng/mL (range 2.5–788ng/mL) in patients with a structural disease, and 4.05ng/ml (range 0.9–231) in patients without a structural disease (p=0.41).
Diagnostic accuracy of 18F-FDG-PET/CT and serum anti-thyroglobulin antibodiesOf the entire cohort, 15 patients had positive anti-thyroglobulin antibodies. Three patients (20%) had positive 18F-FDG-PET/CT findings, with a sensitivity of 100% and a specificity of 91%.
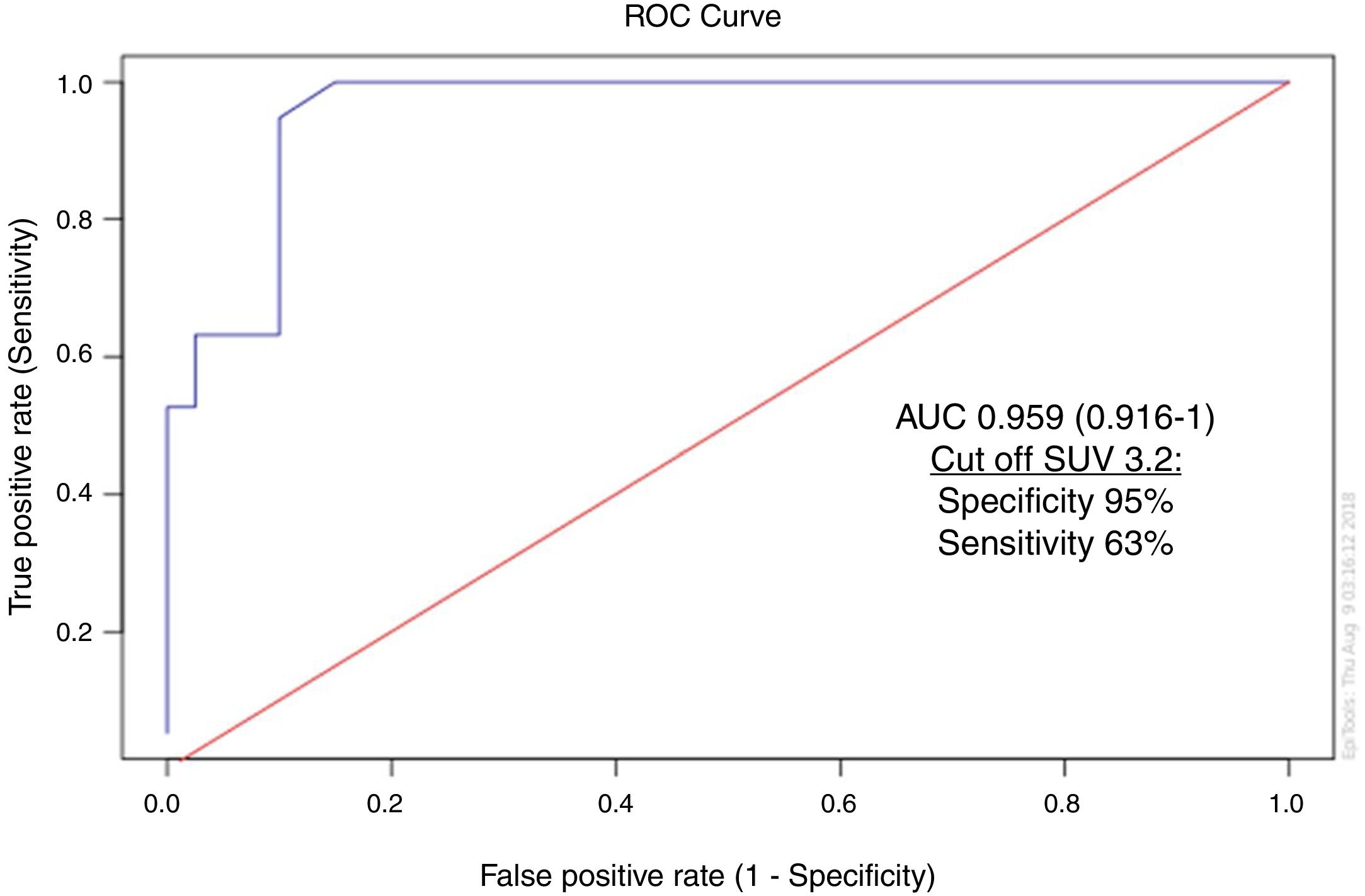
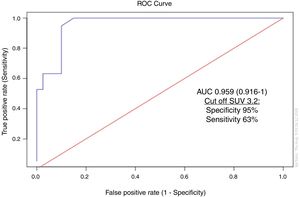
Standardized uptake value (SUV) as a predictor of structural diseaseMean SUVmax value was 7.18 (median: 6.3, range: 2–19). The ROC curve for SUVmax can be observed in Fig. 1. A cutoff value of 3.2 for SUVmax was able to detect metastasis/recurrence locoregional with a sensitivity of 63% and a specificity of 95%.
Discussion18F-fluorodeoxyglucose is a glucose analog that provides unique information about the glucose metabolism of normal or abnormal tissues.19 Due to the increased glucose metabolism of malignant cells, 18FDG uptake is frequently increased. Thus, positron emission tomography performed with 18FDG is a commonly used imaging methodology for various tumors.1918F-FDG-PET/CT could be considered as an additional image procedure in patients with DTC and elevated serum Tg level (>10ng/ml) and negative 131I whole-body scans (WBS).3,18 FDG uptake is a negative predictive factor for therapeutic response to radioactive iodine in metastatic DTC, and it is also an independent prognostic factor for survival.12,13 Lesions with high 18FDG uptake (standardized uptake value) may be more aggressive and should be targeted for therapy or followed-up closely.12,13
In our investigation, 18F-FDG-PET/CT had a sensitivity of 95% and a specificity of 87.5% to detect locoregional disease similar to other reported studies20,21 and changed the therapeutic approach in 50% of our patients. The rate of false positive was 12.5%.
There were controversial reports about the need of performing the 18F-FDG-PET/CT after TSH stimulation. Wang et al. did not report any improvement in the detection of DTC foci after TSH stimulation.22 However, Moog et al. suggested to carry out 18F-FDG-PET/CT after TSH stimulation, proposing that glucose uptake by DTC cells would depend on serum TSH concentration.23 Additionally, Leboulleux et al. found that the detection of more DTC foci was observed after rhTSH stimulation.24 However, the clinical benefit of identifying these additional small foci remains to be proven.24 In our study, TSH stimulation did not improve the sensitivity of 18F-FDG-PET/CT.
On the other side, there is no consensus on the cut-off value of the Tg level that characterizes sufficient diagnostic accuracy of 18F-FDG-PET/CT to detect recurrences or metastases. Some authors recommend that, to obtain a benefit from this image study, the Tg level should be higher than 10ng/ml, measured under TSH suppression,25–26 whereas others among them ATA guidelines recommend that a stimulated Tg level higher than 10ng/ml may be sufficient to determine the indication of an 18F-FDG-PET/CT.3 In patients with a stimulated Tg level<10ng/mL, the sensitivity of 18F-FDG-PET/CT is generally low, ranging from <10 to 30%.3 However, some authors showed higher sensitivity rates with Tg levels<10ng/ml.27–29 Moreover, Giovanella et al. determined that the best Tg threshold for the selection of patients who should undergo 18F-FDG-PET/CT in clinical practice was 4.6ng/ml under thyroid hormone therapy.28 Given these findings, it appears that the cut-off value of the Tg level has not been established yet. We evaluated the cut-off value under TSH suppression, is that in 73% of the cohort the Tg measurement was carried out under hormonal therapy. The sensitivity of 18F-FDG-PET/CT was 83% in patients with non-stimulated Tg levels between 1 and 10ng/ml and 100% with Tg levels higher than 10ng/ml.
Furthermore, in our study, the 18F-FDG-PET/CT was not useful to identify structural disease in patients with indeterminate response defined by thyroglobulin levels (non-stimulated Tg<1ng/mL or stimulated Tg<10ng/mL). As mentioned previously we included these patients who were referred to our hospital with the 18F-FDG-PET/CT already performed, requested by another physician.
On the other hand, in patients with persistent anti-thyroglobulin antibodies, the level of serum Tg cannot be reliably assessed and 18F-FDG-PET/CT might help to localize structural disease. In our study, 18F-FDG-PET/CT detected recurrence/metastasis in 20% of these patients. We found a sensitivity of 100% and a specificity of 91% like other reports.30–33
Additionally, we found that the ideal cut-off value for SUVmax to detect structural disease was of 3.2, with a sensitivity of 63% and a specificity of 95%.
ConclusionOur study confirmed that 18F-FDG-PET/CT is a useful tool in the detection of recurrences in patients with DTC with conflicting results of standard imaging procedures. The sensitivity of 18F-FDG-PET/CT was high for detecting locoregional lesions and it helped to change the immediate therapeutic approach in half of our patients with locoregional lesions and in 20% of all included patients. Despite this, the location of metastatic tissue by itself is an element to be taken into consideration for individualized follow-up and future therapeutic alternatives. However, this method had a low sensitivity for detecting structural disease in patients with an indeterminate response.
Conflict of interestThe authors declare that they have no conflict of interest.
Ethical approvalThe study was approved by the Institutional Review Board.
Informed consentInformed consent was obtained from all individual participants included in the study.