Patients with incomplete response to initial therapy of thyroid cancer can be managed with ongoing observation or potentially additional therapies.
Our aim was to assess the effect of a second radioactive iodine treatment (RAIT) and its relationship with causes and clinical variables.
Material and methodsPatients undergoing a second RAIT for biochemical or structural incomplete response to initial therapy of DTC were retrospectively included (n=120). They were categorised based on the American Thyroid Association (ATA) classification of response to initial therapy.
Patients were reclassified in the following 6–18 months after second RAIT based on imaging findings and measurements of thyroglobulin and antithyroglobulin antibody levels.
The associations of a downgrading of response category and progression-free survival (PFS), and the related variables, were evaluated.
ResultsSixty-six patients (55%) had a downgrading on ATA response category after second RAIT. A significant interdependence of causes for second RAIT and outcomes was found (χ2=29.400, p=0.001), with patients with neck reoperation showing a higher rate of indeterminate or excellent responses.
A significant association between ATA response to second RAIT and absence of structural progression was found (χ2=44.914, p<0.001), with less structural progression in patients with downgrading on ATA response (χ2=30.914, p<0.001). There was also significant interdependence to some clinical variables, such as AJCC stage (χ2=8.460, p=0.015), ATA risk classification (χ2=10.694, p=0.005) and initial N stage (χ2=8.485, p=0.004).
ConclusionsIn selected cases, a second RAIT could lead to more robust responses with a potential improvement in prognosis in patients with incomplete response to initial DTC treatment.
Una respuesta incompleta al tratamiento inicial del cáncer de tiroides se puede manejar con observación o terapia adicional.
Nuestro objetivo fue evaluar el efecto de un segundo tratamiento con yodo radiactivo (RAIT) y su relación con causas y variables clínicas.
Materiales y métodosSe incluyeron pacientes (n=120) que recibieron un segundo RAIT por respuesta estructural o bioquímica incompleta tras la terapia inicial del cáncer diferenciado de tiroides (CDT).
Fueron categorizados según la clasificación de American Thyroid Association (ATA) y fueron reclasificados después del segundo RAIT mediante hallazgos de imagen y niveles de tiroglobulina y anticuerpos antitiroglobulina.
Se evaluó la asociación entre la mejoría en la categoría de respuesta, la supervivencia libre de progresión (SLP) y variables relacionadas.
ResultadosUn total de 66 pacientes (55%) tuvieron una mejoría en la categoría de respuesta ATA tras el segundo RAIT. Se encontró asociación significativa entre causas del segundo tratamiento y los resultados (X2=29,400, p=0,001), con mayor número de respuestas excelentes o indeterminadas en aquellos con reintervención previa.
Se halló asociación significativa entre la respuesta ATA al segundo RAIT y la ausencia de progresión estructural (X2=44,914, p<0,001), con mejores resultados en usuarios con mejoría en la categoría de respuesta ATA (X2=30,914, p<0,001). Se observó una relación significativa con respecto a algunas variables, como el American Joint Committee on Cancer (AJCC) (X2=8,460, p=0,015), la clasificación ATA (X2=10,694, p=0,005) y el estadio ganglionar inicial (X2=8,485, p=0,004).
ConclusionesEn casos seleccionados, un segundo RAIT podría llevar a respuestas más sólidas y a una potencial mejora en el pronóstico de los pacientes con respuesta incompleta al tratamiento inicial del CDT.
Radioactive iodine treatment (RAIT) is a clear indication after thyroidectomy in differentiated thyroid cancer (DTC),1 but its usefulness has not been established when there is biochemical or structural incomplete response without evidence of distant metastases.2
With increasing American Thyroid Association (ATA) risk level, the relative risk (RR) of having structural persistent/recurrent disease augments. On the other hand, ATA low-risk patients appear to have the highest RR of isolated increased level of thyroglobulin (Tg).2 In addition, after the first RAIT, all clinical, biochemical, imaging (structural and functional) and histopathologic findings obtained during follow-up should be used to redefine the clinical status and the individual response to therapy of patients. Based on that, four response-to-therapy categories were described by Tuttle et al.3 and modified by Vaisman et al.4 The ATA thyroid cancer guidelines suggest that either biochemical incomplete response (BIR) or structural incomplete response (SIR) patients can be managed with ongoing observation or potentially additional therapies such as second RAIT.2 However, only a few studies5–7 have investigated the effect of a second RAIT in these patients and the multiple clinicopathological factors that could mark that decision.
Based on the limited experience, the aim of this study was to assess the effect of a second RAIT in patients with DTC and incomplete response to the first RAIT and its relationship with causes and clinical variables.
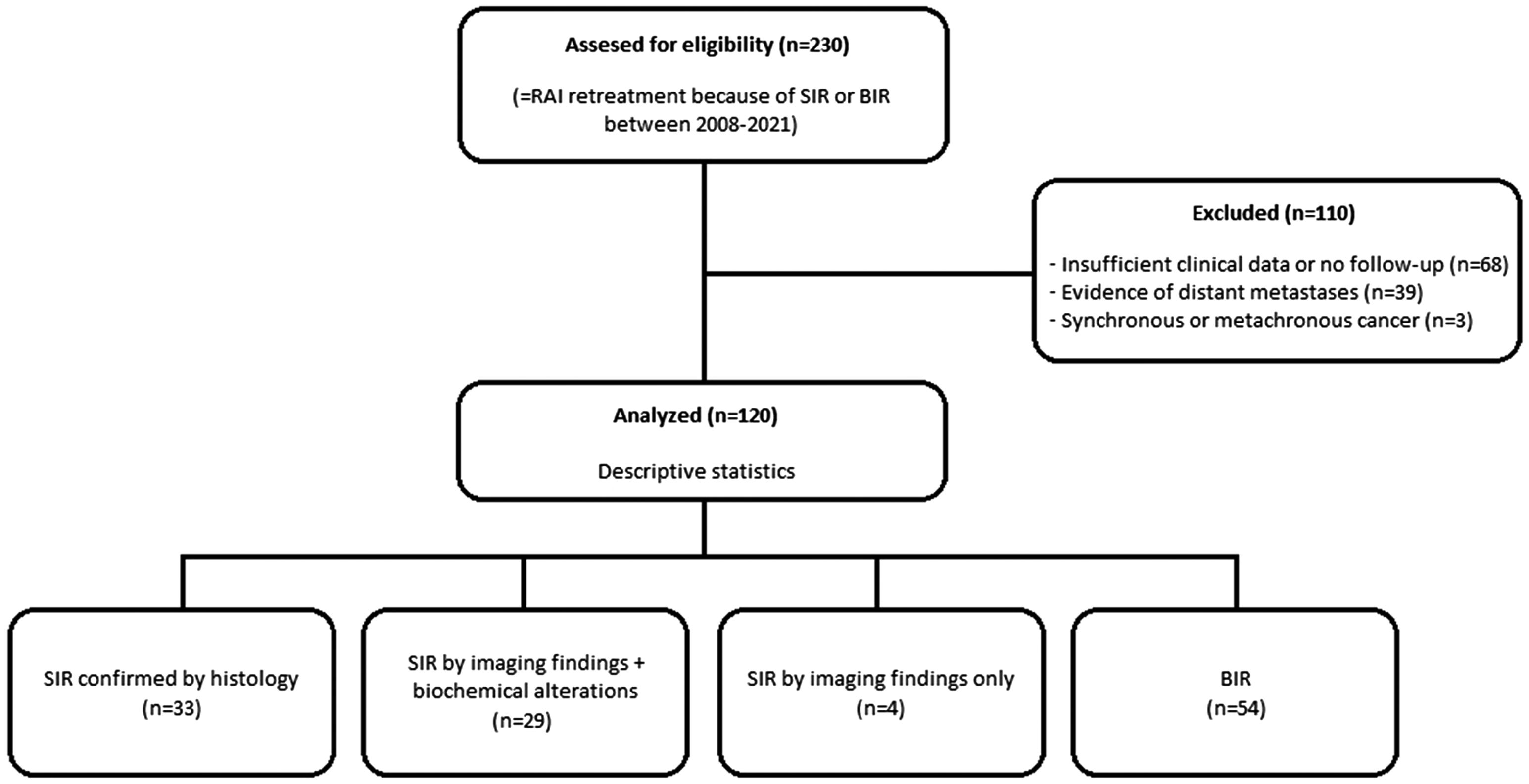
Material and methodsPatientsThe nuclear medicine local registry for metabolic therapy of our reference centre, which attends to a population of two million inhabitants, was searched for patients who underwent a second RAIT between April 2008 and June 2021 for BIR or locoregional structural incomplete response (LSIR) of a DTC, defined by the 2015 ATA guidelines as abnormal Tg or rising anti-Tg antibody levels in the absence of localisable disease or with persistent or newly identified locoregional disease on diagnostic imaging, respectively.2 In addition, a minimum follow-up time of 12 months after the second RAIT was required.
The local ethics committee approved this retrospective study.
Exclusion criteria were as follows: (i) evidence of distant metastases on the whole-body scan (WBS) after first RAIT or on CT and/or 18F-FDG PET/CT scans performed before the second RAI dose, (ii) previous neoplasm or diagnosis of another synchronous or metachronous cancer during the follow-up and (iii) insufficient clinical data for analysis.
The eligible LSIR patients were further divided into those with or without neck reoperation before the second RAIT.
In addition, variables such as age on diagnosis, sex, tumour histology, AJCC-8th edition stage,5 initial N stage, initial risk stratification (low, intermediate or high) attending to ATA guidelines, time from first to second RAIT and cumulative RAI dose of both treatments, were collected.
Follow-up and endpointsA post-therapy WBS, performed 5–10 days after the second RAIT, was evaluated by two experienced nuclear physicians. In case of discordance, a consensus agreement was reached.
Follow-up data of the patients were retrospectively collected from the medical files. Anti-Tg antibodies and serum basal or stimulated Tg levels were collected six-monthly. Also, an expert radiologist performed neck ultrasonography. In some cases, patients underwent supplementary tests such as CT scan, diagnostic RAI WBS and/or 18F-FDG PET/CT scan.
After the second RAIT, patients were reclassified as: (i) SIR, determined as structural or functional evidence of disease with any Tg level; (ii) BIR, which should have negative imaging and suppressed Tg ≥1ng/mL, stimulated Tg ≥10ng/mL or rising anti-Tg antibody levels; (iii) indeterminate response (IR), defined as having biochemical, structural or functional findings that cannot be confidently classified as either excellent response or persistent disease and (iv) excellent response (ER), which was defined as negative imaging and either a TSH-stimulated Tg <1ng/mL or suppressed Tg <0.2ng/mL.
The endpoints considered were category downgrading, defined as the de-escalation of at least one ATA response category after the second RAIT (from SIR to BIR, IR or ER, or from BIR to IR or ER), and the progression-free survival (PFS), defined as the time since the diagnosis to a structural progression by any diagnostic imaging technique (morphological or metabolic) or a new surgical procedure or biopsy.
Statistical analysisStatistical analysis was performed using IBM SPSS Statistics for Windows v.28 (IBM Corp., Armonk, NY). In the descriptive analysis, categorical variables were described with absolute and relative frequencies, while mean and standard deviation were used with quantitative variables. Association between categorical variables were studied with Pearson's chi-square test (χ2 test). A survival analysis of the progression-free survival was performed by means of Kaplan–Meier test and using Log-rank test for the categories’ comparison. p-Value <0.05 was considered statistically significant.
ResultsA data set of 2236 patients that underwent a RAIT from April 2008 to June 2021 was revised. After the review of the clinical data, 230 patients received a second RAIT. Finally, the cohort included 120 patients (Fig. 1), 70 women and 50 men, with a mean age of 47.46±17.23 years (14–82).
A median time of 14 months elapsed between first and second RAIT. A median follow-up time of 121 months and mean cumulative RAI doses of 7.65±2.09GBq (2.22–12.58) were recorded. The demographic and clinical characteristics of the patients are shown in Table 1.
Patients’ characteristics.
| Characteristics | n (%) |
|---|---|
| Sex | |
| Male | 50 (41.7) |
| Female | 70 (58.3) |
| Age (years) | |
| <55 | 80 (66.7) |
| ≥55 | 40 (33.3) |
| Histologic type | |
| Papillary | 112 (93.3) |
| Follicular | 8 (6.7) |
| Initial N stage | |
| Positive | 78 (65) |
| Negative | 42 (35) |
| AJCC stage | |
| I | 96 (80) |
| II | 18 (15) |
| III | 6 (5) |
| ATA risk classification | |
| Low | 33 (27.5) |
| Intermediate | 71 (59.2) |
| High | 16 (13.3) |
| WBS after 1st RAIT | |
| Thyroid bed uptake | 92 (76.7) |
| Lymph node metastases | 21 (17.5) |
| Negative | 7 (5.8) |
| Reoperation before 2nd RAIT | |
| Yes | 33 (27.5) |
| No | 87 (72.5) |
| Causes for 2nd RAIT | |
| SIR (surgically confirmed) | 33 (27.5) |
| SIR (imaging and biochemical) | 29 (24.2) |
| SIR (only imaging) | 4 (3.3) |
| BIR | 54 (45) |
| Causes for 2nd RAIT (simplified) | |
| SIR | 66 (55) |
| BIR | 54 (45) |
| WBS after 2nd RAIT | |
| Thyroid bed uptake | 44 (36.7) |
| Lymph node metastases | 14 (11.7) |
| Distant metastases | 2 (1.6) |
| Negative | 60 (50) |
| ATA responses after 2nd RAIT | |
| SIR | 31 (25.8) |
| BIR | 33 (27.5) |
| Indeterminate response | 39 (32.5) |
| Excellent response | 17 (14.2) |
| Downgrading on response | |
| Yes | 66 (55) |
| No | 54 (45) |
1st: first; 2nd: second; AJCC: American Joint Committee on Cancer; ATA: American Thyroid Association; BIR: biochemical incomplete response; RAIT: radioactive iodine treatment; SIR: structural incomplete response; WBS: whole-body scan.
In first RAIT, mean administered activity was 3.84±1.21GBq, and the individual activity was assigned according to initial risk after surgery and the state-of-the-art practice at decision time. In second RAIT it was 3.81±1.19GBq (not shown in Table 1).
At initial N staging, 78 patients were diagnosed as N-positive: 73 confirmed after lymphadenectomy and 5 based on lymph node uptake on WBS after the first RAIT.
Among patients with LSIR, 33 (50%) underwent neck reoperation before the second RAIT.
Initial N stage was the most robust factor to predict a RAI retreatment (χ2=20.198, p<0.001). No other statistically significant associations were found within the other clinical characteristics.
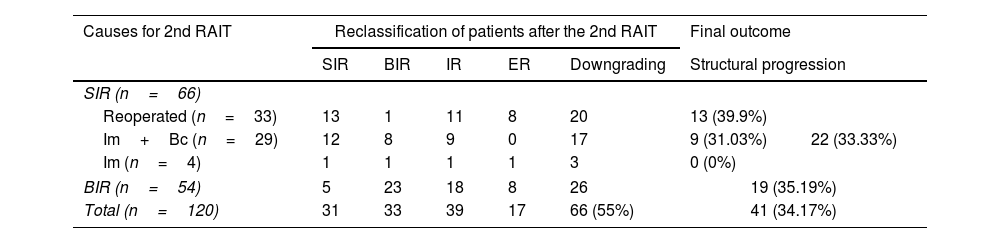
Response categories after the second RAIT are referred in Table 2. Sixty-six out of 120 patients had a downgrading on their previous ATA response category after the second RAIT, which is shown in a representative clinical case in Fig. 2, although without significant association with the cause for the second RAIT (χ2=1.862, p=0.172, not shown in the table).
Distribution of patients attending to causes for a 2nd RAI treatment, reclassification of patients and final outcome.
| Causes for 2nd RAIT | Reclassification of patients after the 2nd RAIT | Final outcome | |||||
|---|---|---|---|---|---|---|---|
| SIR | BIR | IR | ER | Downgrading | Structural progression | ||
| SIR (n=66) | |||||||
| Reoperated (n=33) | 13 | 1 | 11 | 8 | 20 | 13 (39.9%) | 22 (33.33%) |
| Im+Bc (n=29) | 12 | 8 | 9 | 0 | 17 | 9 (31.03%) | |
| Im (n=4) | 1 | 1 | 1 | 1 | 3 | 0 (0%) | |
| BIR (n=54) | 5 | 23 | 18 | 8 | 26 | 19 (35.19%) | |
| Total (n=120) | 31 | 33 | 39 | 17 | 66 (55%) | 41 (34.17%) | |
2nd RAIT: second radioactive iodine treatment; BIR: biochemical incomplete response; ER: excellent response; Im+Bc: imaging plus biochemical; IR: indeterminate response; SIR: structural incomplete response.
A 47-year-old man with PTC underwent total thyroidectomy plus central and left functional dissection, classified as pT1bN1b (AJCC I-stage, intermediate ATA risk). A radioiodine dose of 4.44GBq was administered, showing inferior cervical foci of uptake on post-RAIT whole-body scan (WBS) (A) and SPECT/CT (B) which corresponded with thyroid remnants plus a lymph node at IV right cervical level. During follow-up, non-stimulated Tg level reached 19.9ng/mL. Neck ultrasound (C) showed a suspicious lymph node at IV left cervical level which was not accessible for fine needle aspiration biopsy (FNAB). Patient was classified as SIR and a 4.44GBq second radioiodine dose was administered. Post-treatment WBS (D) and SPECT/CT (E) only showed physiological mediastinal uptake. After the follow-up non-stimulated Tg decreased to 4.0ng/mL and no pathological findings were observed on neck ultrasound and 18F-FDG PET/CT (F), being reclassified as BIR. The patient had no structural progression and has not needed additional treatment.
However, a significant association of the causes for a second RAIT and response to therapy categories was found (χ2=29.400, p=0.001) (Table 3). Moreover, we found significant interdependence between the ATA response to the second RAIT and their correspondent WBS (χ2=23.366, p=0.005).
Association between clinical characteristics and response to 2nd RAI treatment.
| Characteristics | χ2 | p-Value |
|---|---|---|
| Age | 1.883 | 0.597 |
| Sex | 5.084 | 0.166 |
| Histologic type | 2.652 | 0.487 |
| AJCC stage | 9.592 | 0.143 |
| ATA risk classification | 6.043 | 0.428 |
| Initial N stage | 7.789 | 0.051 |
| WBS after 1st RAIT | 10.285 | 0.107 |
| Causes for 2nd RAIT | 29.400 | 0.001 |
| Causes for 2nd RAIT (simplified) | 18.623 | <0.001 |
| Reoperation before 2nd RAIT | 16.422 | 0.001 |
| WBS after 2nd RAIT | 23.366 | 0.005 |
1st: first; 2nd RAIT: second radioactive iodine treatment; AJCC: American Joint Committee on Cancer; ATA: American Thyroid Association; BIR: biochemical incomplete response; SIR: structural incomplete response; WBS: whole-body scan.
After second RAIT, patients with neck reoperation had a higher rate of indeterminate or excellent responses with respect to non-reoperated SIR and BIR patients (57.6%, 33.3% and 48.1%, respectively; χ2=16.422, p=0.001). A downgrading of the response was observed in the same proportion for SIR reoperated and non-reoperated patients (in both cases 20/33, 60.6%), although with more BIR among non-reoperated ones. In BIR patients, second RAIT led to downgrading in 26/54 (48.1%) (Table 2). However, no significant association of reoperation with a downgrading of the response was observed (χ2=0.578, p=0.447).
Twenty-three out of 33 reoperated patients (69.7%) had detectable Tg before receiving the second RAIT. Eighteen of them (78.26%) still presented detectable Tg after treatment. No SIR patient reoperated with undetectable Tg after second RAIT (8 ER) progressed. On the other hand, from 10 reoperated patients with non-detectable Tg before the second RAIT, 2 progressed on the follow-up. The outcomes of the different groups are also referred in Table 2.
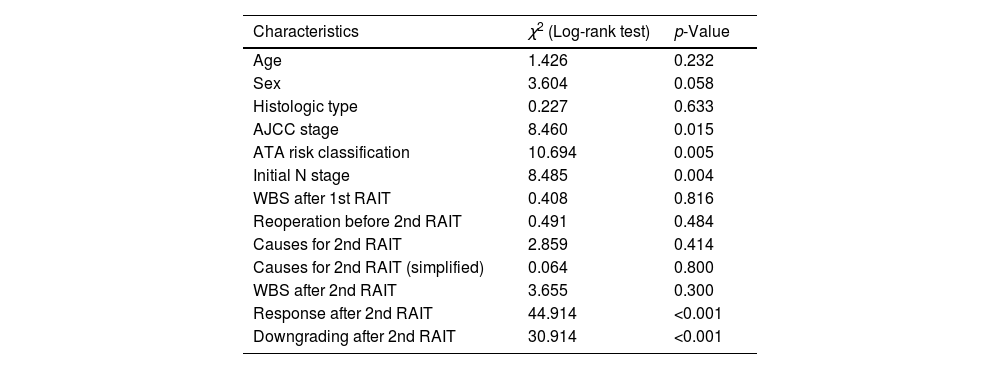
Regarding survival analysis, on the follow-up forty-one patients (34.2%) had a structural progression; after 60 months the PFS was 67.1%. Patients classified as AJCC stage I (χ2=8.460, p=0.015) and as low ATA risk (χ2=10.694, p=0.005) had a lower progression rate. On the other hand, there was a higher progression rate associated to positive initial N stage (χ2=8.485, p=0.004) (Table 4).
Association between clinical characteristics and structural progression on the follow-up.
| Characteristics | χ2 (Log-rank test) | p-Value |
|---|---|---|
| Age | 1.426 | 0.232 |
| Sex | 3.604 | 0.058 |
| Histologic type | 0.227 | 0.633 |
| AJCC stage | 8.460 | 0.015 |
| ATA risk classification | 10.694 | 0.005 |
| Initial N stage | 8.485 | 0.004 |
| WBS after 1st RAIT | 0.408 | 0.816 |
| Reoperation before 2nd RAIT | 0.491 | 0.484 |
| Causes for 2nd RAIT | 2.859 | 0.414 |
| Causes for 2nd RAIT (simplified) | 0.064 | 0.800 |
| WBS after 2nd RAIT | 3.655 | 0.300 |
| Response after 2nd RAIT | 44.914 | <0.001 |
| Downgrading after 2nd RAIT | 30.914 | <0.001 |
1st and 2nd RAIT: first and second radioactive iodine treatment; AJCC: American Joint Committee on Cancer; ATA: American Thyroid Association; BIR: biochemical incomplete response; SIR: structural incomplete response; WBS: whole-body scan.
A significant association was found between ATA response to second RAIT and the PFS (χ2=44.914, p<0.001), with a worse outcome in those with structural incomplete response and a better one in those with indeterminate or excellent response. There was also a higher PFS in patients with downgrading of ATA response after second RAIT (χ2=30.914, p<0.001) (Table 4).
Death from disease occurred in six patients (5%), four of them with SIR and two with BIR.
Finally, a multivariate analysis assessing the correlations between the clinical characteristics and the response to second RAIT and the structural progression was performed. A statistically significant association was found with age, but not with sex. Regarding the response to the second treatment, a hazard ratio (HR) of 7.538 was observed for an incomplete structural response compared to an excellent response. In addition, a HR of 5.861 was found for intermediate ATA risk compared to low-risk category.
No association with response to second RAIT or structural progression on the follow-up was found for other analysed variables, such as time from first to second RAIT and cumulative RAI dose of both treatments (not shown in Tables 3 and 4).
DiscussionIn DTC, approximately 8–40% of patients require an additional application (re-ablation) due to ablation failure. However, not many previous data exist regarding the percentage of second RAIT in BIR or SIR cases. Attending to our experience, 230/2236 (10.3%) of RAITs performed in our centre between April 2008 and June 2021 were retreatments because of BIR or SIR.
A diagnostic radioiodine WBS is not a systematically indicated follow-up procedure, especially in low-risk patients. Thus, patients undergo standard biochemical and ultrasonography follow-up until biochemical and/or structural signs of active disease appear. Although the higher the relative ATA risk, the higher the structural persistent/recurrent disease, the prevalence of BIR is similar among the three ATA risk groups.2 A SIR to initial therapy is seen in 2–6% of ATA low-risk, 19–28% of ATA intermediate-risk and 67–75% of ATA high-risk patients. On the other hand, a BIR is not an uncommon outcome and is seen in 11–19% of ATA low-risk, 21–22% of ATA intermediate-risk and 16–18% of ATA high-risk patients.3,8
In our study there was a significant association between cause for second RAIT and initial N stage (χ2=20.198, p<0.001), with a higher number of SIR in patients with N-positive status.
No consensus exists regarding the impact of the second RAIT in the disease status of patients with incomplete responses after the first RAIT.2 Hirsch et al.,6 evaluated 164 patients, 61 patients were categorised as BIR (group A), 50 as SIR with neck reoperation before second RAIT (group B) and 53 patients as SIR without neck reoperation (group C). At 1–2 years from the second RAIT, 73.3% of patients in group A still had an elevated Tg level, compared to 47.7% and 84.8% in groups B and C. On the other hand, in groups B and C, 47.7% and 93.6% of patients, respectively, still had positive locoregional imaging findings, compared to 15.5% in group A. Based on these results, it may be assumed that improvements in neck structural findings, when they occurred, were attributable largely to the surgery and not to the second RAI dose.
Moreover, Hirsch et al.6 found no association between positive RAI uptake in WBS after the second RAIT and long-term outcomes. On the contrary, we found a significant association between the WBS findings and the response to second RAIT (χ2=23.366, p=0.005), with a higher number of excellent and indeterminate responders among those patients with negative RAI uptake or only thyroid bed uptake.
Prpic et al., in a group of patients undergoing a second RAIT with the only purpose of re-ablation, evaluated initial N tumour status as a potential predictor of treatment outcome. Patients with N1a status had a significantly higher risk of treatment failure compared to N0 patients (27.8% vs. 9%, p=0.032).9 The clinical context of our patients was completely different; we did not exclude the SIR patients with high risk of recurrence and furthermore we included patients with BIR.
Another controversial issue is the adjuvant RAIT after neck reoperation for a locoregional recurrence. Yim et al.7 retrospectively evaluated 45 patients with DTC and persistent serum Tg elevation after reoperation for locoregional recurrence. The patients who received an empirical second RAIT showed no outcome benefit compared with untreated patients. In our sample, patients were derived to a second RAIT as an adjuvant procedure. Furthermore, in a retrospective multicentre study from Italy and Switzerland investigating the effect of an additional RAIT administration in DTC patients with negative imaging studies after neck reoperation for lymphadenectomy, the authors found no association between this treatment and improved overall or progression-free survival in the whole cohort of 113 patients (64 treated with RAIT). Nevertheless, subgroup analysis revealed better PFS in patients with an elevated Tg level after receiving the RAIT retreatment.10
In a retrospective study by Bouvet et al.,11 including 85 patients reoperated due to locally persistent or recurrent disease, 49 patients were re-treated with a second RAIT while the other 36 patients were only followed up. Disease remission rates in the re-treated RAI group and the follow-up group did not differ (61% vs. 69%), and recurrence rates were also statistically indifferent (39% vs. 34%). ATA guidelines suggest that the clinical outcome of the LSIR category is usually disease persistence or recurrence in about 50–85% of the patients despite further therapies.2 Moreover, these patients usually have worse clinical outcomes than the BIR ones.4 In our study, patients with neck reoperation had a higher rate of indeterminate or excellent responses with respect to SIR without prior reoperation or BIR (57.6%, 33.3% and 48.1%, respectively; χ2=16.422, p=0.001) (Table 2). In any case, we probably must assume that improvements in neck structural findings should be largely attributable to the surgery and not to the second RAI dose.
Furthermore, in our study, 66 out of 120 patients had a downgrading on their previous ATA response category after a second RAIT, suggesting an outcome benefit. Additionally, an inverse relationship between ATA response to second RAIT and the existence of structural progression was found. Moreover, there was significant association between reoperation before the second RAIT and the response to it (χ2=16.422, p=0.001), simplified causes of second RAIT (χ2=18.623, p<0.001) and WBS findings after second RAIT (χ2=23.366, p=0.005).
Based on the controversial effect of reoperation in patients with SIR, recognising high-risk patients is of utmost importance to recommend a more aggressive approach and a closer follow-up to determine who will benefit from the treatment. There was a lower progression rate in patients classified as AJCC stage I (χ2=8.460, p=0.015) and as low ATA risk of recurrence (χ2=10.694, p=0.005) (Table 4). Furthermore, a higher structural progression rate was associated with initial N stage (χ2=8.485, p=0.004).
Retrospective studies validated the 2009 ATA risk of recurrence staging system.2 They reported estimates of no evidence of disease (NED), equivalent to ER, that were correlated to risk category (the lower the risk, the higher rate of NED). In the present study, we analysed the outcome of patients with DTC who were re-treated for BIR or LSIR. This research showed that a second RAIT did impact the downgrading on their previous ATA response category in DTC patients. In 9/66 (13.6%) of SIR patients and 8/54 (14.8%) of BIR patients a NED status (excellent response) was reached. This contrasts with what was reported by Vaisman et al.,4 who found that only 9% of the patients with SIR were reclassified to NED, despite additional therapies.8 Furthermore, in our series the rate of structural progression was similar between initial SIR and BIR patients (33.3% and 35.2%, respectively).
The present study supports the potential of a second RAIT in promoting more robust responses that may decrease the risk of recurrence in selected DTC cases. Nonetheless, prospective studies are needed to identify patients in whom a second RAIT should be indicated to avoid unnecessary radioactive exposure for the rest, analysing the clinical characteristics that may impact on the decision, especially in those patients with a previous neck reoperation.
The strengths of the current study are the high number of patients included with a relative long follow-up period, which provided an overview of the effect of a second RAIT in the management of different DTC patient subgroups. The availability of analytical and neck ultrasonographic data made it possible to compare the biochemical status and structural findings before and after administration of the second RAIT. In addition, the predictive dynamic classification after first RAIT proposed by Tuttle et al.3 and Vaisman et al.4 seems to work in a second RAIT attending to our results.
The limitations of this study include the non-randomisation and the absence of a control group of patients with DTC who were not treated with a second RAIT for incomplete response to initial therapy. Furthermore, selection bias could exist due to the retrospective nature of the study. Unfortunately, there is no randomised controlled trial evaluating the benefits of a second RAIT due to the difficulties in developing such a study that includes a heterogeneous group of patients, not all of them with demonstrable macroscopic disease (from some with BIR to others with SIR and significant disease burden), with different possible management approaches available, as must be the case in the era of individualised medicine.
ConclusionsDespite the retrospective nature of the study, we were able to identify some of the causes and effects related to second RAIT in DTC.
Downgrading of previous ATA response category was a relevant effect of the second RAIT in our population, potentially decreasing structural progression in the follow-up, although neck surgery represents a main issue and a probable confounding factor.
In selected cases, a second RAIT could lead to more robust responses with a potential improvement in prognosis in terms of recurrent disease, although further studies are needed to assess which patients might benefit from this approach.
Conflict of interestThe authors declare that they have no conflict of interest.