Thyroglobulin antibodies (TgAb) trend may be considered a surrogate marker for thyroglobulin in differentiated thyroid carcinoma. The aim of this study is to analyse, in cases with positive TgAb, trend over time and its relationship with response to treatment.
Material and methodsRetrospective and descriptive study of 100 patients with differentiated thyroid carcinoma and positive TgAb (measured by electrochemoluminiscense) after thyroidectomy. Assessment of response to initial treatment was performed 6–24 months after surgery. Status at last follow-up was evaluated.
ResultsAfter the first year nearly half of the patients showed a reduction in TgAb levels ≥50%, in 91% of these patients, status at last follow up was excellent response (65%) or indeterminate response due to decreasing TgAb levels (26%). At first assessment, indeterminate responses were found in 49% of cases, without significant differences among initial risk of recurrence category or whether radioiodine ablation was performed. At last evaluation (median 53.5 months), 15% of ablated low-risk patients had an indeterminate response (due to declining TgAb), vs 62% in the non-ablated low-risk group (p 0.03). Median time to negativization for post-surgical TgAb levels<100UI/ml was 11 months [3–94] vs 31 months [8–119] for patients with TgAb≥100UI/ml (p 0.0003).
ConclusionA reduction of ≥50% in TgAb levels during the first year correlated with favourable outcomes. Non-ablated patients and patients with higher levels of post-surgical TgAb may need a longer time to achieve negative conversion.
La tendencia de los anticuerpos antitiroglobulina (TgAb) podría considerarse un marcador sustituto de la tiroglobulina en carcinoma diferenciado de tiroides (CDT). El objetivo del trabajo fue analizar, en casos de TgAb positivos, la tendencia en el tiempo y su relación con la respuesta al tratamiento.
Material y métodosEstudio retrospectivo descriptivo de 100 pacientes con CDT y TgAb positivos post tiroidectomía. Se evaluaron la repuesta inicial al tratamiento entre los seis y 24 meses postquirúrgicos y la respuesta final.
ResultadosLuego del primer año, aproximadamente la mitad de los pacientes mostraron reducción de los niveles de TgAb ≥ 50%, en 91% de estos, el estado final fue excelente (65%) y/o indeterminado por niveles de TgAb descendentes (26%). Tras la primera evaluación a los seis y 24 meses, las respuestas indeterminadas se detectaron en 49%, sin diferencias significativas según riesgo de recurrencia o antecedentes de ablación con radioyodo. En la evaluación final (mediana de seguimiento de 53,5 meses), 15% de los pacientes de bajo riesgo ablacionados presentaron respuesta indeterminada (TgAb en descenso) vs. 62% en no ablacionados (p = 0,03). La mediana de tiempo para la negativización de TgAb fue de 11 meses [3-94] para aquellos con valores postquirúrgicos < 100 UI/mL vs. 31 meses [8-119] para pacientes con TgAb ≥ 100 UI/mL (p = 0,0003).
ConclusiónUna reducción de ≥ 50% de los TgAb durante el primer año post tiroidectomía se correlaciona con evolución favorable. Los pacientes no ablacionados y aquellos con elevados niveles postquirúrgicos de TgAb podrían requerir mayor tiempo para alcanzar la negativización.
Follow-up strategies after initial treatment of differentiated thyroid carcinoma (DTC) are based on thyroglobulin (Tg) measurement and cervical ultrasound, as the combination of both methods has shown to be highly sensitive and specific to detect recurrent or persistent disease.1
It is well known that approximately 25–30% of patients with DTC have positive serum thyroglobulin antibodies (TgAb),2,3 which can be a marker of autoimmune thyroid diseases or immune reaction to DTC or multinodular goitre.4
The detection of TgAb interferes with Tg measurement,5 rendering it unreliable as an oncological marker. However, when positive, TgAb may be considered a surrogate marker for Tg and its trend over time is applied to assess the dynamic risk during the follow-up of DTC.1,6
As TgAb are not direct tumor markers but rather reflect the immune response of the patient to an antigen (i.e.: Tg), the interpretation of TgAb results is often challenging in the clinical practice. The implementation of fixed cut-off value in TgAb testing may lead to misclassifications (false positive or negative). Measurement of TgAb should be performed longitudinally in the same laboratory and using the same assay for each patient.6,7
Declining levels of TgAb over time are considered indicators of a favourable outcome; however, the average time needed for negative conversion in patients without evidence of disease is uncertain. Several authors reported that a median of 2–3 years is required after radioiodine ablation (RA),8,9 but this interval could be longer (up to 5 years) in patients who were not submitted to RA.10
The aim of this study is to evaluate, in patients with DTC and positive TgAb, a) the trend of TgAb levels over time and b) their relationship with response to treatment both in patients who underwent RA and those who did not.
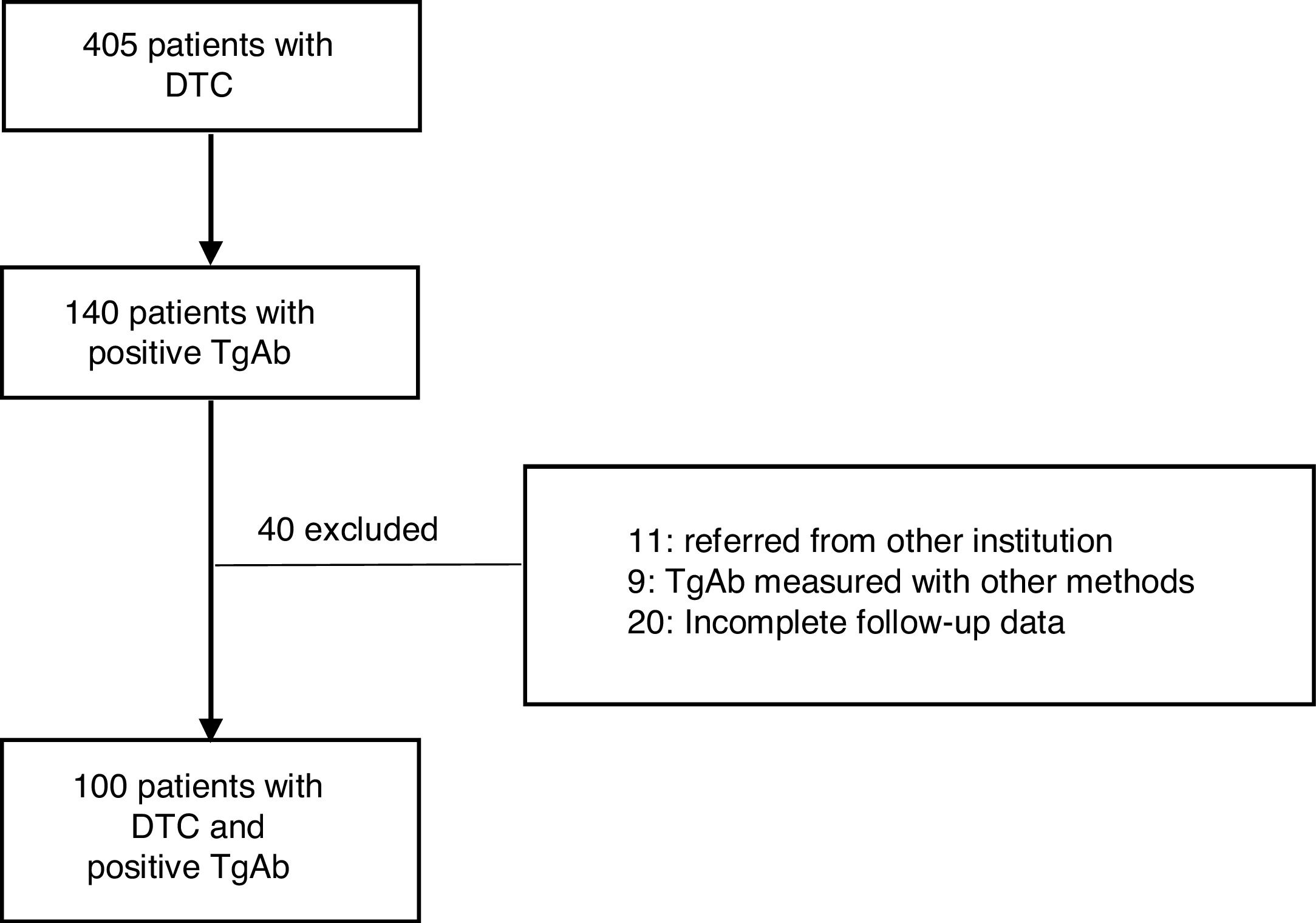
Material and methodsThis retrospective and descriptive study included patients diagnosed with DTC from September 2004 to February 2019. All cases were initially treated at Instituto de Oncología AH Roffo (IOAHR), Buenos Aires, Argentina. Inclusion criteria were as follows: (1) total thyroidectomy with (RA) or without RA (NRA) (according to current protocols), (2) positive TgAb in the first determination after surgery, (3) a minimum follow-up of 12 months, performed at IOAHR, and, (4) plasma levels of Tg and TgAb measured in the same institution and with the same method. Exclusion criteria were: (1) patients referred to the study centre later during follow-up, (2) de novo appearance of previously undetectable TgAb during follow-up, (3) TgAb measured by other methods, and (4) incomplete follow-up data.
Demographic, clinical, histopathological data (AJCC/UICC 8th edition)11 and risk stratification (American Thyroid Association (ATA) 2015)1 were collected in all cases. Follow-up was performed, with Tg and TgAb measurement and neck ultrasound in all cases initially every 6 months, and later annually. Tg and TgAb measurement in the needle washout after fine needle aspiration of suspicious lesions found in neck ultrasound was performed. Other complementary studies (CT scan, PET/CT, whole body radioiodine scan) were indicated according to the criteria of the attending physician.
Tg and TgAb were measured by electrochemiluminescense® (Elecsys Roche, cobas e411). For this method, functional sensitivity for Tg was 0.2ng/ml and for TgAb 22IU/ml.7 Values of TgAb above 22IU/ml were considered positive. Changes in TgAb levels over the first year of follow-up were considered as (a) increasing (rise of ≥50%), (b) decreasing (reduction of ≥50%) and (c) stable (elevation or decrease<50%).
Assessment of response to initial treatment was conducted 6 to 24 months after surgery and at the last evaluation of follow-up using ATA dynamic risk stratification scale1 in patients with RA and Momesso's criteria for assessment in NRA cases.12 Patients were classified as having excellent, structural incomplete response (with no distinction between persistent or recurrent disease), biochemical incomplete and indeterminate response.
Statistical analysisDescriptive analyses were performed using median and range for quantitative variables, and comparisons were performed using Student test (or non-parametric Wilcoxon test if non-normally distributed). Qualitative variables were expressed in percentage and comparisons were performed using chi-square test. Values were considered statistically significant at p<0.05. Statistical analysis was performed using SPSS software (version 21: SPSS Chicago, IL).
ResultsA total of 405 patients with DTC were treated at the IOAHR from September 2004 to February 2019; 140 (34.5%) of them had positive TgAb on the first measurement after surgery. Forty patients were excluded (Fig. 1). Finally, 100 cases were included in the study. Most of the patients were women (94%), median age at diagnosis was 46 years [20–80], and papillary thyroid carcinoma (PTC) was the most frequently found histotype (97%). Among these, the classical subtype was detected in 76% of cases (Table 1).
General characteristics of patients with DTC and positive TgAb.
| General characteristics | N=100 |
|---|---|
| Median age (years) | 46 [20–80] |
| <55 years | 74 |
| ≥55 years | 26 |
| Female/male | 94/6 |
| Histology | |
| Papillary carcinoma | 97 |
| Classic variant | 73 |
| Follicular variant | 17 |
| Other variants | 7 |
| Hürthle cell carcinoma | 2 |
| Follicular carcinoma | 1 |
| T | |
| 1a | 17 |
| 1b | 38 |
| 2 | 22 |
| 3a | 11 |
| 3b | 3 |
| 4a | 8 |
| 4b | 1 |
| N | |
| 0 | 52 |
| 1a | 30 |
| 1b | 18 |
| M | |
| 0 | 97 |
| 1 | 3 |
| Thyroiditis in surgical piece | 48 |
| Stage | |
| I | 87 |
| II | 7 |
| III | 3 |
| IVa | 1 |
| IVb | 2 |
| ATA recurrence risk | |
| Low | 47 |
| Intermediate | 35 |
| High | 18 |
| Radioiodine therapy yes/no | 86/14a |
DTC: differentiated thyroid cancer; TgAb: thyroglobulin antibodies.
More than 70% of patients presented tumors of up to 4 centimetres and 48% had thyroiditis in the surgical specimen. Almost 90% of patients were stage I and nearly 50% had low risk of recurrence according to ATA classification. After total thyroidectomy, 14 patients were not submitted to RA; 13 of them were low-risk patients. The remaining case was a 63-year old woman with diagnosis of classic PTC and extensive lung metastases with severely impaired respiratory function, leading to contraindication of RA. Sorafenib treatment was prescribed and her TgAb levels remained stable throughout follow-up.
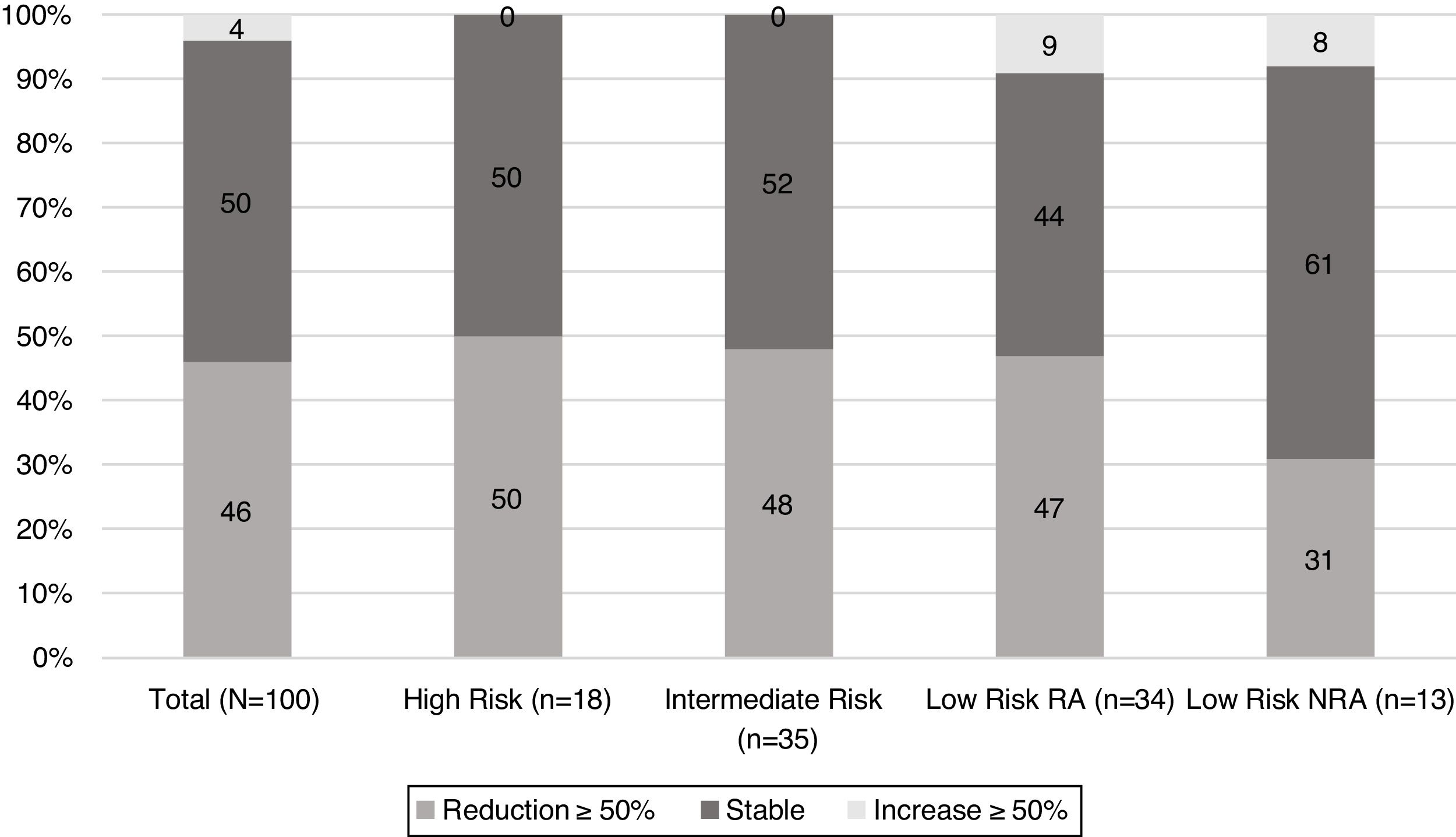
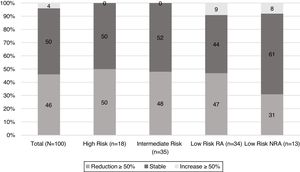
When TgAb dynamics after the first year from initial treatment were analysed (Fig. 2), it was found that nearly half of the patients in each risk category showed a reduction in TgAb levels ≥50%, with the exception of low-risk patients who were not submitted to RA. In this group, the proportion of patients with a declining TgAb trend was lower (31%); however, this difference did not reach statistical significance.
At first assessment, 30% of patients showed an excellent response to initial treatment. Of note, excellent responses were not found in the high-risk category. Indeterminate responses were found in 49% of patients, ranging from 44% in the low-risk RI patients to 69% in low-risk NRA (p 0.1) (Table 2). Structural incomplete response was found in 13% of the population. When each group was evaluated, structural incomplete response was found in 25% (n=1) of patients with increasing levels of TgAb, 20% (n=10) of those with stable levels of TgAb and 4% (n=2) of patients with decreasing TgAb.
Initial response to treatment in patients with DTC and positive TgAb.
| N (%) | Total(n=100) | High risk(n=18) | Intermediate risk(n=35) | Low risk RA(n=34) | Low risk NRA(n=13) |
|---|---|---|---|---|---|
| Structural incomplete | 13 | 7 (39) | 3 (9) | 3 (9) | 0 |
| Biochemical incomplete | 5 | 2 (11) | 1 (3) | 1 (3) | 1 (8) |
| Indeterminate | 49 | 9 (50) | 16 (45) | 15 (44) | 9 (69) |
| Excellent | 30 | 0 | 13 (37) | 14 (41) | 3 (23) |
| NA | 3 | 0 | 2 (6) | 1 (3) | 0 |
Data are expressed as number (%). DTC: differentiated thyroid cancer; TgAb: thyroglobulin antibodies.
Overall, during follow-up, 13% of patients were submitted to additional treatments (surgery, radioiodine and/or multikinase inhibitors). This included 4% (n=2) of patients in the group with decreasing TgAb levels, 18% (n=9) in the group with stable levels of TgAb and 50% (n=2) in the group with increasing levels of TgAb.
After a median follow-up of 55.3 months [9.9–184.1], structural disease was found in 5 patients, all of them with high initial risk of recurrence and structural incomplete response at assessment of initial treatment. Overall, 60% and 30% of patients respectively showed an excellent and indeterminate response. When comparing final responses according to initial risk of recurrence, 15% of low-risk RA patients had an indeterminate response, vs 62% in the low-risk NRA group (p=0.03), (Table 3).
Final status of patients with DTC and positive TgAb.
| N (%) | Total(n=100) | High risk(n=18) | Intermediate risk(n=35) | Low risk RA(n=34) | Low risk NRA(n=13) |
|---|---|---|---|---|---|
| Structural incomplete | 5 | 5 (28) | 0 | 0 | 0 |
| Biochemical incomplete | 2 | 1 (6) | 0 | 1 (3) | 0 |
| Indeterminate | 30 | 6 (33) | 11 (31) | 5 (15) | 8 (62) |
| Excellent | 60 | 6 (33) | 22 (63) | 27 (79) | 5 (38) |
| NA | 3 | 0 | 2 (6) | 1 (3) | 0 |
Data are expressed as number (%). DTC: differentiated thyroid cancer; TgAb: thyroglobulin antibodies.
Median follow-up was longer in patients who achieved a final excellent response to treatment (76.3 months [28.1–176.8] vs 43.8 months [14.4–121] in patients with a persistent indeterminate response at last evaluation) (p=0.0004).
When patients with initial decreasing levels of TgAb were assessed, no evidence of disease was found in 65% (n=30) while 26% (n=14) had an indeterminate response (which was due to a declining trend of TgAb) at last follow-up visit. None of these patients had received additional treatment during follow-up.
As for those patients with stable TgAb during the first year after initial surgery, 58% (n=29) evolved to an excellent response and 30% (n=15) to an indeterminate response. Among them, most displayed decreasing TgAb levels (≥70%). Median follow-up interval was longer in patients without evidence of disease at last follow-up than in those with indeterminate response (76.3 months [20.2–183.8] vs 54 months [14.4–184.1], p=0.04). In 6% of the cases (n=3) structural disease was found; all of them were high-risk patients, and in two cases, metastatic disease was present at diagnosis. At last follow-up, 75% (n=3) of patients in the group with rising levels of TgAb had an indeterminate response and 1 had no evidence of disease.
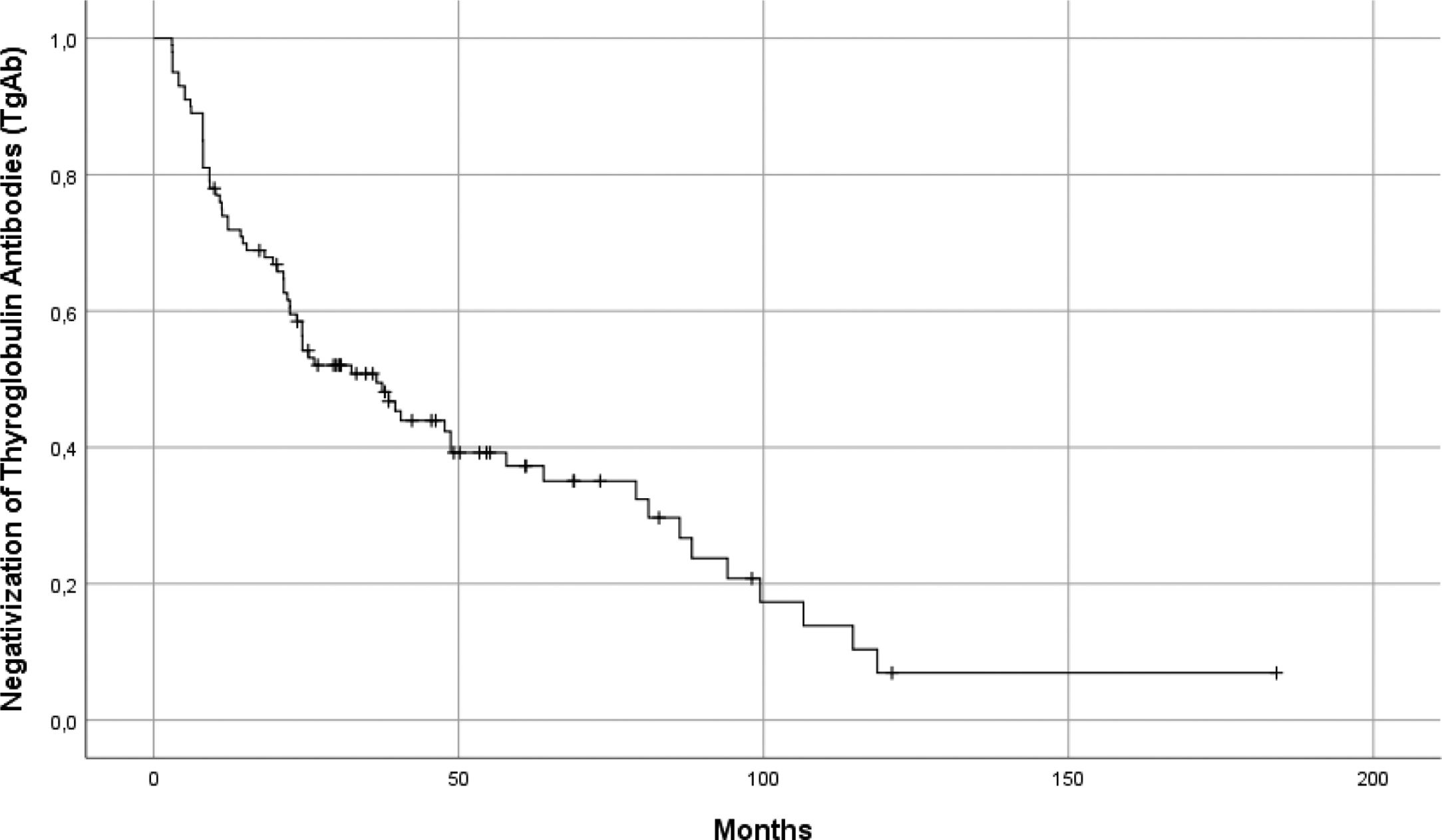
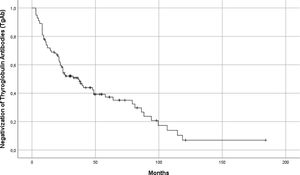
Globally, median time to negative conversion was 20.23 months [3–119] (Fig. 3). Patients who became TgAb negative during follow-up (n=67) were distributed in two groups, according to the levels of circulating TgAb after surgery: Group 1: TgAb<100UI/ml (n=43) and Group 2: TgAb≥100UI/ml (n=24). Median time to negativization for patients in group 1 was 11 months [3–94] vs 31 months [8–119] (p=0.0003).
DiscussionThis study analysed the trend over time and the clinical impact of TgAb in a cohort of DTC patients with detectable TgAb after total thyroidectomy. Clinical and pathological characteristics were similar to those previously reported for the Argentinian DTC population.13
After 12 months of surgery, 50% of patients showed a decline in TgAb levels (≥ 50%); 91% of them evolved to an excellent or indeterminate response with a decreasing trend of TgAb at the end of follow-up, without receiving additional therapies. This data, as well as the previously reported data, suggests that a significant reduction of TgAb during the first year after initial treatment is a favourable prognostic factor in patients with detectable TgAb.14–16 In fact, Zavala et al. propose that a decline of ≥50% in TgAb levels together with a negative ultrasound could be considered an excellent response.17 Similarly, Rosario et al. also found structural recurrences in <5% of patients with declining trends of TgAb.18 Conversely, persistent levels or a rising trend of TgAb point towards the presence of Tg-producing tissue, which could be related to persistent/recurrent DTC.14,19 It is worth noting that a similar proportion of patients with stable and decreasing TgAb levels during the first year after initial treatment achieved indeterminate or excellent response at final visit of follow-up (nearly 60 and 30% respectively). However, additional therapies were needed in the stable TgAb group to reach this status, while this was not necessary in the decreasing TgAb group. This is probably due to the higher incidence of structural disease found in patients with stable TgAb levels at assessment of response to initial treatment.
In the present series, structural disease on last evaluation was found exclusively in patients who also had incomplete structural response to initial treatment. Additionally, all of them had high risk of recurrence according to ATA classification. This data suggests that both initial and dynamic risk stratification, as well as the tendency of TgAb levels, should be taken into account when assessing DTC patients. After a median follow-up of 55.3 months, no patient in the ATA low-risk category had a structural incomplete response. This finding underscores the favourable prognosis in this group of patients, independently of whether RA was performed or not. However, there was a significant difference in final responses: low-risk patients NRA persisted with an indeterminate response in 62%, whereas patients with RA showed an indeterminate response in 15%; in both groups indeterminate response was due to the presence of TgAb with declining trend. Ernaga-Lorea et al. reported that in patients with RA the time needed to achieve negative TgAb was 2–3 years.9 Sun et al. found the median time needed for positive TgAb to become negative after RA was 15.8 months,20 while according to the findings of Matrone et al., in NRA patients, up to 5 years may be necessary to achieve negativization.10 TgAb levels in non-ablated patients may remain detectable due to the presence of reactive intrathyroidal lymphocytes and thyroidal tissue. Theoretically, patients with NRA have persistent follicular thyroid cells which may maintain immunoreactivity longer than in patients with RA.8 However, this may not be always the case. As was shown by Schlumberger et al.,21 undetectable stimulated Tg levels after surgery were found in 37-59% of DTC patients. As this suggests complete antigen removal, it is possible that TgAb levels may spontaneously decline in these patients without further intervention. However, dissimilar results were reported. Recently, Bueno et al. reported that patients with RA needed longer for negative conversion of TgAb than those with NRA.22 Tsushima et al.23 noted increasing levels of TgAb were more frequently found in patients submitted to RA than those NRA; however, this may be due to the higher incidence of high-risk features in the RA group (Tumor size ≥4cm and lateral lymph node metastases), which also consistently showed higher rates of local and distant recurrences. Further studies are needed to elucidate these findings; nevertheless, assessment of TgAb trend over time is clearly mandatory to evaluate this population.
Patients who achieved an excellent response (negative TgAb) at last evaluation had longer follow-up than those with indeterminate response (declining TgAb) (p=0.004). This could be related to several factors, one of them being the initial value of TgAb. Patients with higher initial TgAb levels needed longer to achieve negative conversion (p=0.0003). This observation is in agreement with Matrone et al., who reported that higher initial values of TgAb take longer to become negative, and this applies both for patients with RA and NRA.24 The time for negative conversion, according to the same author, is influenced by the degree of lymphocytic infiltration in surgical piece and the initial level of TgAb after the thyroidectomy.10 In the present series, 48% of patients had thyroiditis in the surgical specimen, which was similar to findings in other studies.10,22
The present study has several limitations, such as the retrospective design, the lack of data about the degree of lymphocytic infiltration and the small population of NRA patients. This last aspect may be related to the prolonged time frame our patients were included for and the relatively recent restriction of RA indications.
To summarise, the analysis of this series showed that a reduction of ≥50% in TgAb levels during the first year correlated with favourable outcomes. No structural incomplete responses were found in low-risk patients, whether they were submitted to RA or not; however, NRA patients may need longer to achieve negative conversion. The time needed to achieve negative conversion of TgAb was longer in patients with higher initial levels of TgAb. According to these findings, low-risk patients with decreasing trend of TgAb could be observed with ultrasound and plasma levels of Tg and TgAb, with no need for other interventions due to the favourable prognosis of this disease.
Conflicts of interestThe authors state that they have no conflicts of interest.