Sarcopenic obesity has been associated with greater disability and morbidity and mortality. However, at present, there are few studies regarding the role of sarcopenia in the evolution of the comorbidities associated with obesity in individuals less than 65 years of age. The pathogenesis is multifactorial and uncompletely ilucidated, but it seems that inflammatory mediators and insulin resistance play an important role. Although there is no clear consensus on its definition and diagnostic methods, there is a growing interest in finding biomarkers useful for the detection and monitoring. Regarding the treatment, a multimodal approach is recomended, basically based on dietary recommendations, exercise and eventually bariatric surgery.
La obesidad sarcopénica se ha asociado con mayor discapacidad y morbi-mortalidad. Sin embargo, todavía existen pocos estudios sobre el papel de la sarcopenia en las comorbilidades asociadas a la obesidad en individuos con edad inferior a 65 años. La etiopatogenia es multifactorial pero parece que los mediadores inflamatorios y la resistencia a la insulina desempeñan un papel relevante. Aunque no existe un consenso claro sobre su definición y métodos diagnósticos, hay un creciente interés por disponer de biomarcadores que ayuden a su detección y seguimiento. Respecto al tratamiento se postula el abordaje multimodal, que básicamente se fundamenta en recomendaciones dietéticas, ejercicio y eventualmente cirugía bariátrica.
Sarcopenic obesity (SO) is a clinical and physiopathological entity that has been associated with greater disability and morbidity and mortality than the two entities (obesity and sarcopenia) considered separately. This study summarizes the recent concepts on SO: definition, criteria and diagnostic methods, as well as pathogenic factors. In addition, a review is made of the role of SO in obesity-related comorbidities (type 2 diabetes, cognitive impairment and bone involvement). Finally, the current status of SO management is analyzed from the dietary viewpoint and referred to the recommendations based on physical exercise and the role of bariatric surgery.
Definition and diagnosisThe term sarcopenia comes from the Greek wordsarx, which means meat, and penia, which means poverty. Sarcopenia is a relatively recent concept coined by Irwin Rosenberg in 19891, and is encompassed within the range of muscle diseases. It has been coded as a pathological condition in the ICD-10 classification since 20162. Despite its recent formal acceptance, the presence of sarcopenia is gaining exponential clinical relevance, since it represents a very cross-sectional physiopathological situation that may concern many areas in medicine.
Sarcopenia is defined as a complex syndrome characterized by loss of skeletal muscle mass (SMM) and particularly function. Although there is no clear consensus on its definition, the different working groups appear to agree that the most relevant factor in defining sarcopenia should be loss of function, as described in the guide developed by the European Working Group on Sarcopenia in Older People (EWGSOP)3. When loss of muscle function or mass is associated to preserved or even increased fat mass, the condition is referred to as sarcopenic obesity (SO). There is sufficient evidence to affirm that the association of obesity and sarcopenia confers a poorer prognosis in terms of morbidity and mortality than either of these conditions alone4.
Loss of SMM is a physiological situation that occurs within specific contexts such as aging. People are considered to reach a maximum level of SMM and strength between 30-50 years of age, after which a progressive loss of MME is observed, together with greater difficulties for muscle synthesis. This situation is classified as primary sarcopenia, since there is no other underlying cause other than aging itself. When muscle loss occurs in the context of a pathological or dysfunctional process such as cancer, malnutrition or obesity, among others, we speak of secondary sarcopenia5.
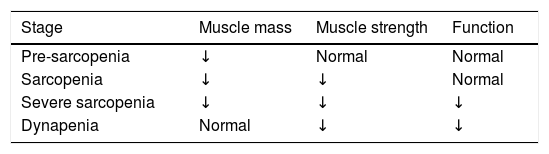
Another classification is referred to its severity (Table 1).
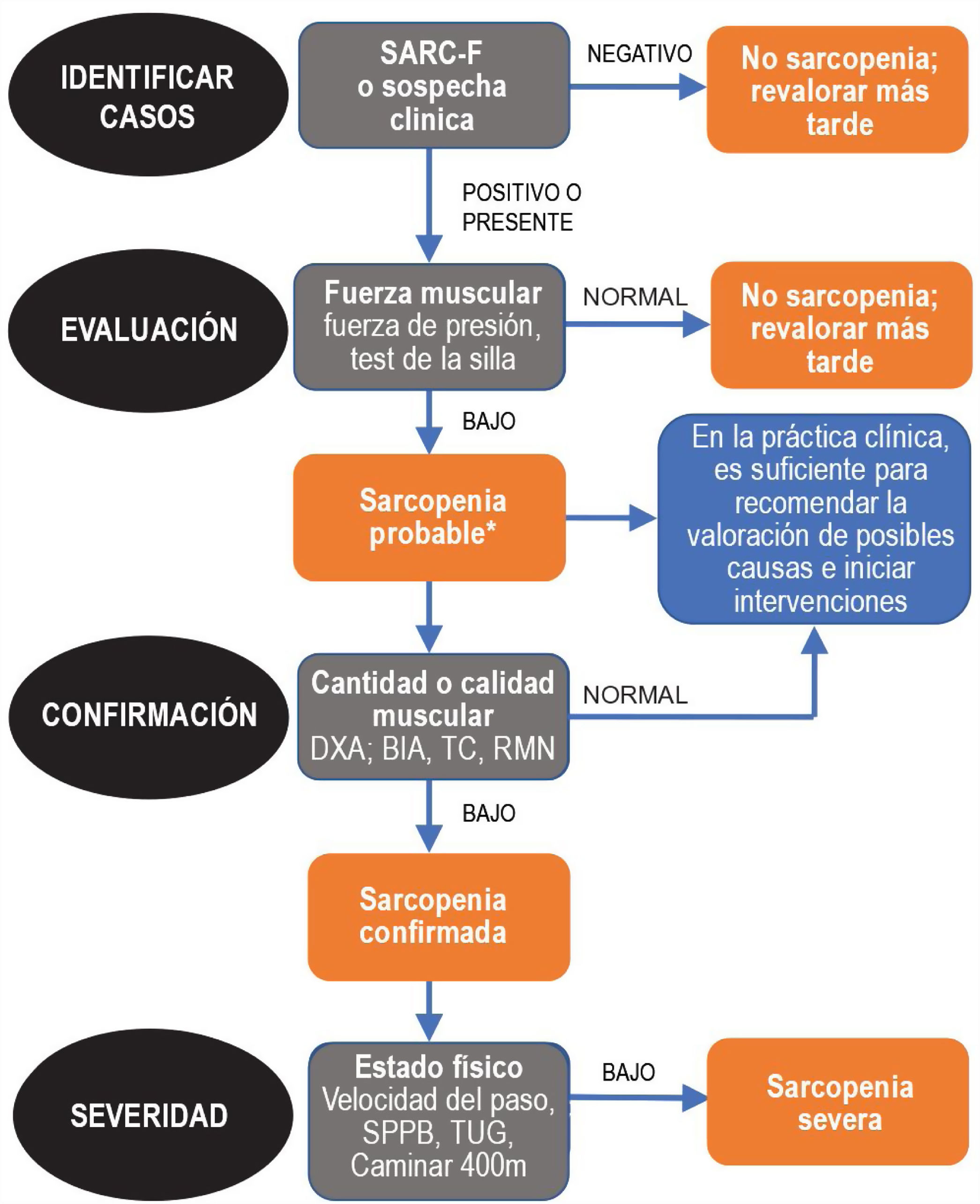
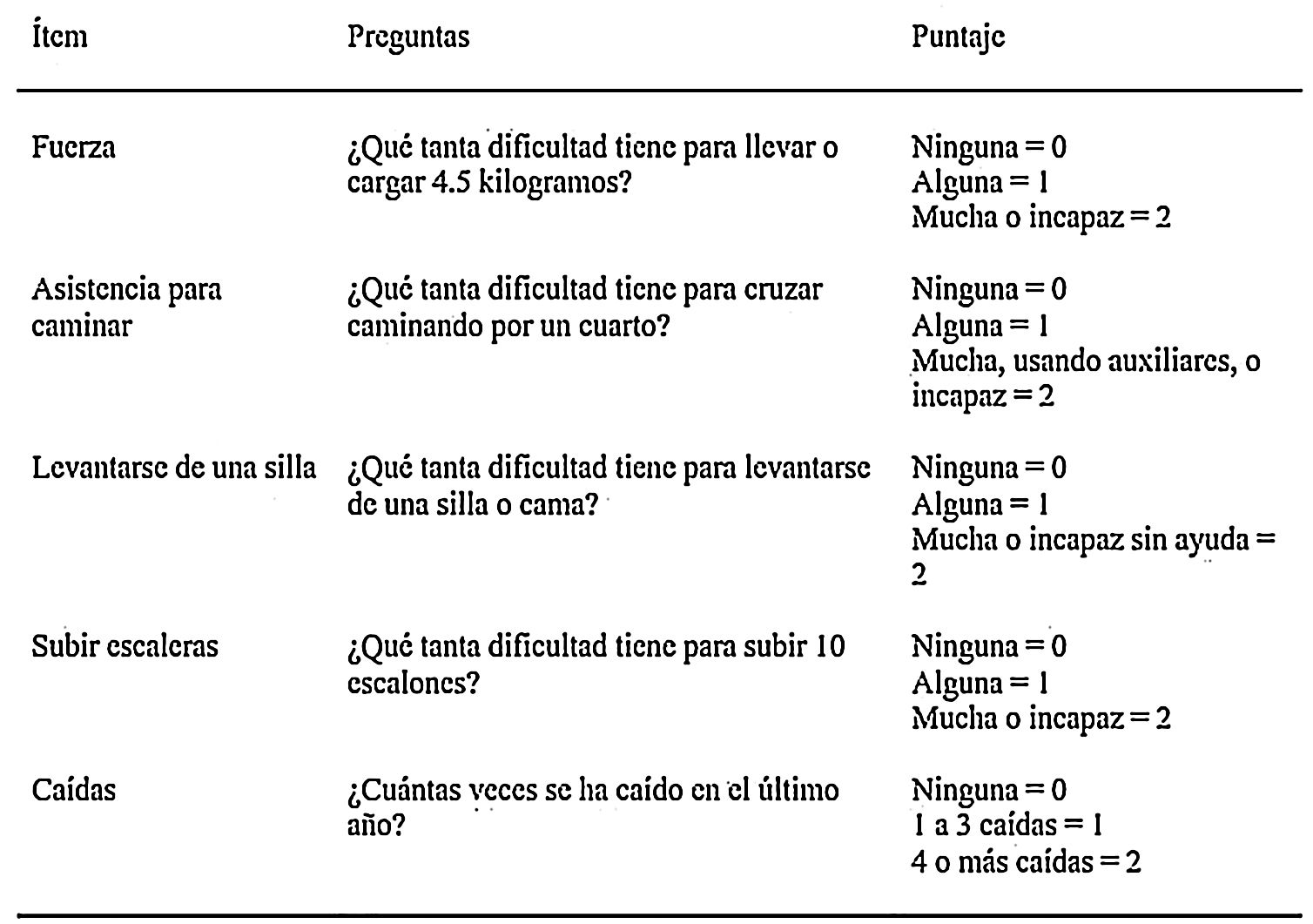
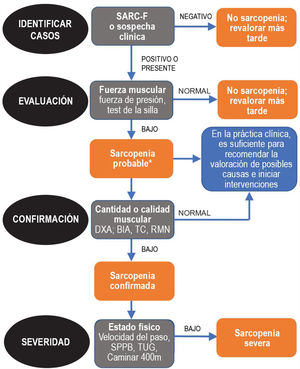
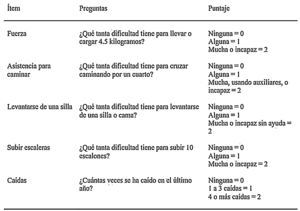
Since sarcopenia comprises loss of both muscle mass and function, the related diagnostic methods can be differentiated into quantitative and qualitative or functional. The EWGSOP23 consensus document recommends a method for identifying and diagnosing sarcopenia (Fig. 1). The first diagnostic step is to identify patients at risk of sarcopenia using questionnaires such as the SARC-F.6 This questionnaire (Fig. 2) is a 5-question test designed to assess the ability to perform routine tasks such as walking, rising from a chair and climbing stairs, and the presence of falls. Patients can perform the test on their own without external help, and a score of four or higher is considered suggestive of sarcopenia.
Sarcopenia: EWGSOP2 algorithm. Modified from Cruz-Jentoft A.J. et al.3.
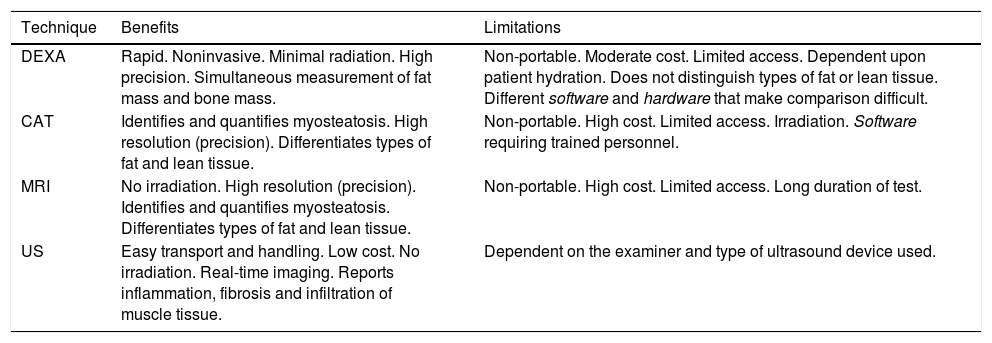
This is a low-cost technique, but requires a considerable amount of time as well as trained staff, since it relies on the correct obtainment of different measures, and is observer-dependent (Table 2). Such measurements are particularly useful in studies conducted in athletes, but are generally not considered to be a good measure of muscle mass in elderly patients with obesity, particularly when associated to sarcopenia5.
Main characteristics of the quantitative methods.8,16
| Technique | Benefits | Limitations |
|---|---|---|
| DEXA | Rapid. Noninvasive. Minimal radiation. High precision. Simultaneous measurement of fat mass and bone mass. | Non-portable. Moderate cost. Limited access. Dependent upon patient hydration. Does not distinguish types of fat or lean tissue. Different software and hardware that make comparison difficult. |
| CAT | Identifies and quantifies myosteatosis. High resolution (precision). Differentiates types of fat and lean tissue. | Non-portable. High cost. Limited access. Irradiation. Software requiring trained personnel. |
| MRI | No irradiation. High resolution (precision). Identifies and quantifies myosteatosis. Differentiates types of fat and lean tissue. | Non-portable. High cost. Limited access. Long duration of test. |
| US | Easy transport and handling. Low cost. No irradiation. Real-time imaging. Reports inflammation, fibrosis and infiltration of muscle tissue. | Dependent on the examiner and type of ultrasound device used. |
Sarcopenic obesity was first defined in 1996 by Heber et al. using electrical bioimpedance (EB); subsequently, an equation based on EB was developed by Andaluza et al.7 This technique calculates lean mass, fat mass and body water based on electrical conductivity. Muscle mass is not measured directly, but the use of a series of equations and mathematical adjustments affords an estimate quite similar to that obtained by body densitometry7. It is an inexpensive, simple and easily transportable test. However, the measurements must be made under similar conditions so that the samples are comparable. In addition, the results may be influenced by the hydration status of the patient. On the other hand, in patients with body mass index (BMI) > 34 kg/m2 the technique may overestimate lean mass and underestimate fat mass4.
Imaging techniquesMuscle mass may be assessed by dual-energy X-ray absorptiometry (DEXA), computed tomography (CT), magnetic resonance imaging (MRI) and ultrasound (US).
Dual-energy X-ray absorptiometry (DEXA)Dual-energy X-ray absorptiometry (DEXA) measures the absorption of two X-ray photons, typically at a setting of 40-47 keV and 70-80 keV, which allows the differentiation of bone and soft tissue fat mass and lean mass, based on their different X-ray attenuation characteristics. The technique uses a two-dimensional projection and provides information about the amount of fat and lean tissue in each part of the body8. However, one of its main disadvantages is its lack of accuracy in quantifying body composition in the context of higher BMI values, which limits its use in obese individuals4.
Computed axial tomography (CAT)In a study carried out by Shen et al.4 in 2004, muscle mass measurement at lumbar level L3 was found to be significantly correlated to total body muscle mass9. Since then, level L3 measurement obtained by CAT has been used to determine body composition in clinical research, particularly in those disease conditions in which CAT forms part of the routine patient study, such as in certain types of cancer10–12.
Decreased muscle strength is not only due to a decrease in muscle mass, but also to a loss of muscle quality. Both aging and obesity are typically characterized by a decrease in lean mass and an increase in adipose tissue, which can infiltrate muscle, thereby decreasing its functional capacity. This concept is referred to as myosteatosis. Computed axial tomography is able to identify and even quantify myosteatosis through the observation of a decrease in muscle tissue Hounsfield units (HUs) - this being significantly correlated to poorer clinical outcomes and greater mortality in cancer patients13.
Magnetic resonance imaging (MRI)Magnetic resonance imaging (MRI) affords very precise images, similar to those of CAT, capable of distinguishing in detail between fat and lean tissue, and of identifying fat infiltration. The costs of this technique are greater in comparison with other methods, and there are still too few data in the literature to allow a comparative cost-benefit analysis4,5.
Muscle tissue ultrasound (US)Ultrasound (US) is a useful initial method for evaluating muscle mass and quality in all clinical settings. Since a decrease in muscle mass does not occur homogeneously in all anatomical regions, US allows the detection of "site-specific sarcopenia" or "regional sarcopenia". Ultrasound measurement of the thickness of the four heads of the quadriceps femoris muscle is highly reproducible in sarcopenic and non-sarcopenic patients, and correlates to maximum voluntary isometric contraction force14. In addition, an inverse relationship has been demonstrated between muscle echogenicity and muscle strength. In this regard, increased muscle echogenicity has been related to the presence of intramuscular fat in biopsies from patients15.
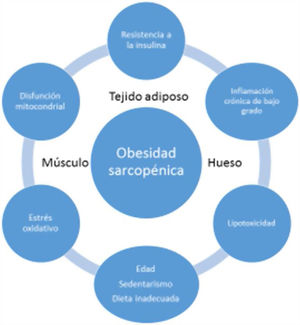
EtiopathogenesisThe etiopathogenesis of SO is multifactorial, and there is a relationship between age, a sedentary lifestyle, unhealthy eating habits and factors such as insulin resistance (IR), systemic inflammation and oxidative stress. All this results in a quantitative and qualitative decrease in muscle mass and strength, as well as in a concomitant increase in fat mass17.
Age-associated sarcopenia and obesity are usually due to a progressive decrease in physical activity and protein intake. This would result in a reduction of energy expenditure and an increase in IR - generating a series of changes in adipose tissue. More specifically, the adipocytes would increase in size and number, and immune cells would infiltrate the adipose tissue, resulting in an inflammatory response. Adipocytes and immune cells would produce adipokines (e.g., leptin, chemerin, resistin, and decreased adiponectin) and cytokines (e.g., TNF-α, interleukins [ILs], interferon-ϒ), thereby generating a low-grade inflammatory status. Such inflammation would not be limited to the adipose tissue but would be widespread, since most adipokines and cytokines enter the systemic circulation. This unfavorable adipokine/cytokine profile would further increase IR, which in turn would amplify inflammation and oxidative stress, but would also contribute to ectopic fat accumulation18.
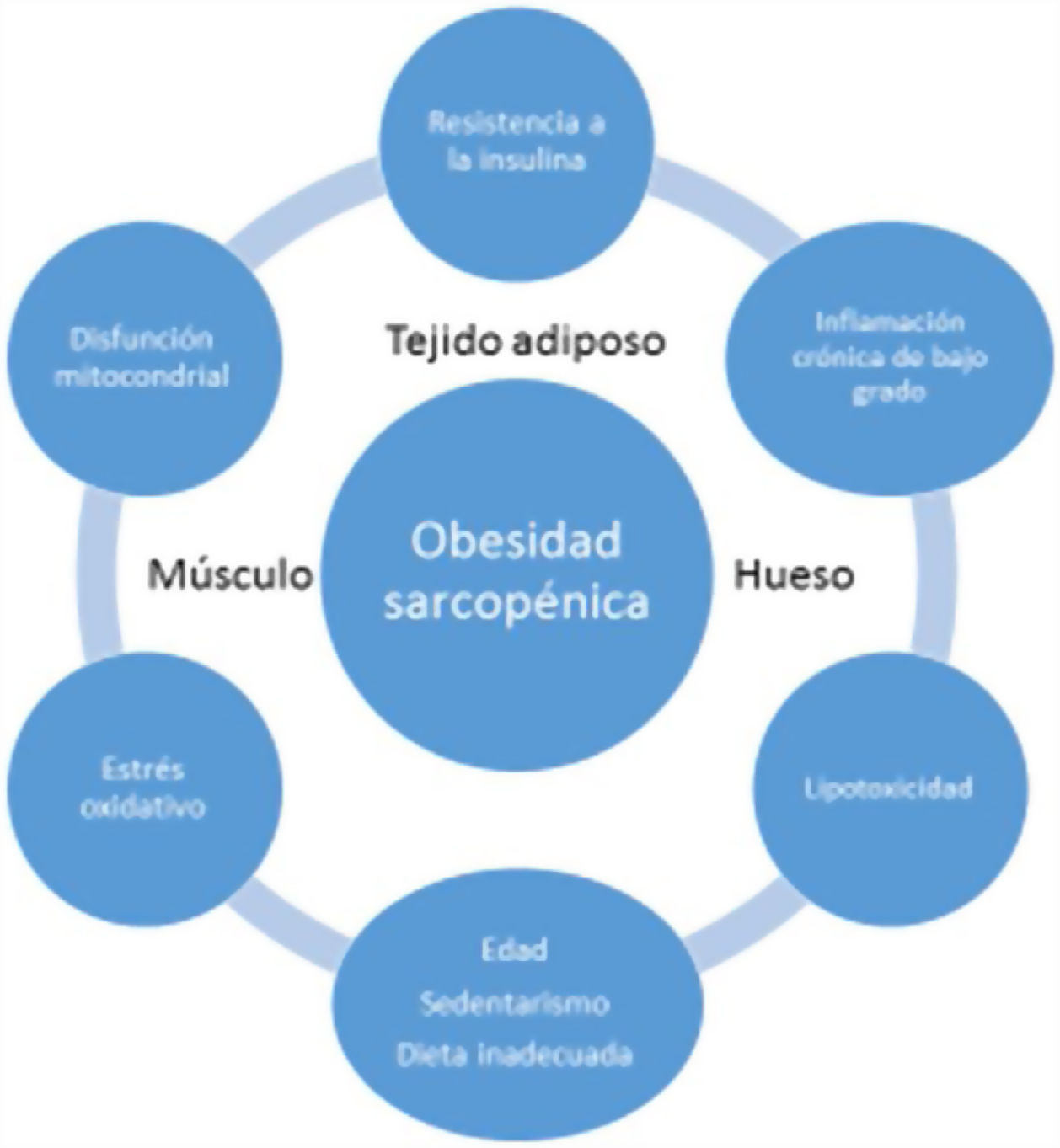
Low-grade systemic inflammation and intramuscular fat accumulation would result in mitochondrial dysfunction and in an imbalance of myocyte-secreted myokines (myostatin, irisin, TNF-α and ILs). More specifically, mitochondrial βoxidation would be impaired, leading to increased lipid peroxidation. This in turn would increase the accumulation of intermediate lipids and free radicals, which would further worsen IR, inflammation, oxidative stress and lipotoxicity within the myocyte - causing its dysfunction and apoptosis. In addition, myokines may exacerbate IR in adipose tissue and in other tissues. A vicious cycle would therefore be established in which a negative interaction would be maintained not only between muscle and adipose tissue19, but also with bone (Fig. 3). It has been reported that increased IL-6 and C-reactive protein (CRP) levels are associated to a two- to three-fold increased risk of significant loss (> 40%) of muscle strength, and these therefore may be regarded as risk factors for the development of sarcopenia. It has also been suggested that TNF-α may be a potential risk biomarker for sarcopenia20.
In recent years, there has been growing interest in clarifying the role played by proinflammatory cytokines, myokines and adipokines in the physiopathology of sarcopenia and SO. The myokines that have generated most interest in recent years are myostatin and irisin, which are mainly produced in skeletal muscle. A number of studies have suggested that myostatin (which downregulates muscle mass) would be increased and irisin (which is positively associated to muscle mass) would be decreased in sarcopenia21,22.
Fatty acid binding protein (A-FABP) is an adipokine involved in fatty acid absorption and subsequent transport to the mitochondrial oxidation system. Some studies have shown A-FABP to be positively associated to body fat and negatively correlated to muscle mass, and in humans it is moreover associated to the severity of IR18.
It has been suggested that the measurement of plasma transthyretin (TTR) may represent a biomarker of sarcopenia in elderly subjects. Liver TTR synthesis decreases in protein depletion states and is suppressed in inflammatory states induced by cytokines such as IL-6. Recent data suggest that TTR decreases in parallel to the loss of lean mass seen in inflammatory processes23.
The levels of soluble receptor for advanced glycation end products (sRAGE) play a protective role in the development of chronic age-related disorders, neutralizing the action of advanced glycation end products (AGEs). It has recently been suggested that decreased circulating levels of sRAGE could constitute an independent risk factor for sarcopenia24.
Some endocrine diseases such as hypogonadism (Aguirre et al.), hypo- and hyperthyroidism, adult growth hormone deficiency and Cushing's syndrome could play a role in the development of SO. It is clear that additional studies are needed to analyze the contribution of each of these disorders to the pathogenesis of SO25. With regard to dietary factors, inadequate protein intake and vitamin D deficiency have been shown to worsen SO26,27.
Role of sarcopenic obesity in comorbidities associated to obesityIt has been hypothesized that SO significantly increases the harmful effects of obesity upon health. In fact, several studies described below have recently related this condition to increased morbidity and mortality and poorer quality of life, as well as to the risk of type 2 diabetes, cognitive impairment and osteoporosis.
Type 2 diabetesA recent meta-analysis28 has shown the presence of SO to be associated to an increased risk (odds ratio [OR] 1.38 [95% confidence interval [95%CI] 1.27-1.50]) of type 2 diabetes. The underlying mechanism is still unclear, but there appears to be a bidirectional relationship, with chronic inflammation and insulin resistance as the main factors. An interesting study by Srikanthan et al.29 found obese patients with sarcopenia to have higher HOMA-IR (homeostatic model assessment of insulin resistance) and glycosylated hemoglobin (HbA1c) levels than obese patients without sarcopenia. This effect was age-related, and only occurred in the group of patients under 60 years of age. A possible explanation would be that in age-related muscle atrophy, the type II muscle fibers, which are less responsive to the metabolic effect of insulin30, are lost to a greater extent than type I fibers. However, the limited data are still controversial, and further studies are needed to clarify the influence of SO as a pathogenic factor of type 2 diabetes. A recent study has shown glucose fluctuations in patients with type 2 diabetes to be associated to sarcopenia31. In addition, in that same study, the prevalence of sarcopenia was seen to be higher in patients with type 2 diabetes and cognitive impairment than in those with normal cognitive function. This finding is interesting, for although the relationship between type 2 diabetes and cognitive impairment is known32,33, little is known about the role of sarcopenia. It should be noted that few studies to date have assessed the relationship between sarcopenia and glycemic variability, and all of the existing studies have been conducted in non-obese patients over 65 years of age. In fact, glucose variability - particularly acute hyperglycemia - increases beta-amyloid (Aβ) production and alters insulin signaling in both brain34 and muscle, leading to impaired peripheral glucose metabolism35 that contributes to sarcopenia.
Cognitive impairmentIt has recently been seen that the presence of obesity is related to mild global cognitive impairment, even at an early age, with executive function and information processing speed being particularly affected36,37. In addition, several studies reported lower brain volumes in obese patients as compared to patients with normal weight38,39. It should be noted that histopathological findings specific of Alzheimer's disease (e.g., cortical gliosis, hippocampal atrophy, Aβ deposits) have been reported in experimental models of obesity40,41.
Clinical studies have described an association between sarcopenia and impaired global cognition42,43, executive function, information processing speed, global memory and brain atrophy. In addition, the presence of sarcopenia was identified as a predictor of impaired cognitive function44. Tolea et al.42 conducted a study of 353 patients aged 69 years on average and divided them into three groups: obesity without sarcopenia, SO and sarcopenia without obesity. The SO group showed a greater degree of cognitive impairment, with sarcopenia being a more potent predictor of cognitive function loss than obesity.
Since SO is associated to IR, this mechanism may contribute to the cognitive alterations observed in these patients. In this regard, it is interesting to note that sarcopenia exacerbates IR and dysglycemia related to obesity41. It should be mentioned that altered insulin signaling in the brain (which leads to IR) plays a key role in the pathogenesis of dementia, and precedes cognitive dysfunction and pathological processes even by decades45.
However, it must be noted that most studies linking SO to cognitive impairment have been carried out in patients over 65 years of age. Therefore, additional studies are needed to evaluate the relationship between SO and cognitive function in younger individuals.
Osteosarcopenic obesityBone and muscle are tissues that are coupled physically and functionally. The relationship between overweight and bone metabolism remains controversial, but a trend is seen that suggests the protection of bone mineral density in the context of non-sarcopenic obesity - probably because there is an increase in lean mass that may partly explain the beneficial mechanical effect of obesity upon bone46. However, SO is clearly related to an increased incidence of osteoporosis, fractures and fragility, probably due to the loss of mechanical impact of muscle on bone, the loss of the positive effects of insulin on bone tissue, and to the fact that this is a physiopathological situation related to an increased proinflammatory state47–49. "Osteosarcopenic obesity" (OSO) has been regarded as a disease entity in its own right for a number of years50,51. A distinction has even been made between two different conditions: osteosarcopenic visceral obesity (OSVAT) and osteosarcopenic subcutaneous obesity (OSSAT)52. It has been seen that patients with OSVAT present a significantly greater risk of fractures and inflammation and a poorer metabolic profile, and it has been concluded that the identification and classification of patients within these types of risk profiles could contribute to define different treatment plans53.
Although the close relationship between muscle and bone has been known for quite some time, the inclusion of fatty tissue (in both generalized obesity and fatty infiltration of tissues) has recently become increasingly relevant52. Nevertheless, further studies considering OSO as a single entity are needed in order to accurately quantify the deleterious effect it exerts per se47.
Impact of SO upon morbidity and mortality and quality of lifeAs has been commented above, SO affects the physiology of energy balance, muscle function and physical capacity17. It seems logical to assume that subjects with muscle strength proportionally low to their body mass are at a greater risk of developing physical disability in the future - with consequences for functionality and quality of life. Recent studies54,55 evidence that SO increases mortality due to all causes. However, in the subgroups analysis of the meta-analysis, all-cause mortality depended on the criteria used for the diagnosis of SO - again underscoring the need to homogenize these criteria.
Rantanen et al.56 recorded an increased mortality risk (OR 1.39) associated to obese subjects (BMI > 30 kg/m2) with lesser grasping strength, in contrast to subjects with normal weight and greater grasping strength. To date, few studies have evaluated the combined effect of obesity and low muscle strength (as a marker of sarcopenia) upon different daily physical activities. Stenholm et al.54 found individuals with both conditions to have greater gait limitations than those with only a high degree of body fat or low muscle strength. In sum, obesity may lead to reduced physical activity and fat accumulation, as well as to impaired muscle mass and function. Sarcopenic obesity precedes the disability to perform instrumental activities of daily living57, limiting quality of life. In addition, a recent study has found the presence of SO to be associated to poorer quality of life according to the health-related quality of life (HRQoL) questionnaire, reaching clinical and statistical significance as compared to obesity alone. In that study, 64 of the 130 participants who met the criteria for SO (49.2%) yielded significantly higher scores than the group without SO (p = 0.001)58.
Approach to therapy and management planNutritional interventionSeveral factors are involved in muscle formation, development and maintenance. The most widely recognized management strategy is therefore based on a multimodal regimen comprising adequate calorie and protein intake, physical activity, and the administration of certain specific amino acids, fish oil, vitamins and trace elements. However, specific treatment has yet to be defined, due to the current lack of consensus and adequate evidence59.
It is generally advised to provide a protein supply of 0.8-1.0 g/kg/day in healthy adults, 1-1.2 g/kg/day in elderly people, and even > 1.2 g/kg/day in elderly patients with physical activity and associated acute or chronic disease conditions60. Although some studies have reported a better muscle anabolic rate with the supply of larger amounts at dinner time61, uniform distribution over meals is generally recommended (25-30 g per meal)60,62. With regard to the type of protein, the consumption of high biological value proteins is advised, particularly leucine63.
On the other hand, evidence is being gained on the benefits afforded by specific nutrients such as beta-hydroxy-methyl-butyrate (a leucine metabolite), vitamin D, creatine and omega-3 fatty acids49. However, further studies are needed to more firmly establish the advantages of these specific nutrients, and to define their ideal combination and doses60. Drugs seeking to promote muscle development, such as myostatin inhibitors, are also under clinical development63.
Prescription of physical exerciseThe data available to date suggest that resistance exercises appear to be more useful in counteracting sarcopenia, while aerobic training is more useful for obesity. It therefore seems reasonable to recommend a combination of both exercise modes in patients with sarcopenic obesity17,64,65. In a recent randomized controlled 6-month study in obese individuals between 60-80 years of age, better physical performance and quality of life were recorded in the group performing combined physical exercise than in those who only performed resistance exercises or only aerobic activity64. In addition, muscle strength increased more and lean mass decreased less in the combined exercise groups than in the aerobic exercise group. However, little information is available on the effect of exercise in the SO population, and in particular, studies in younger populations are lacking.
There are only limited data on the effectiveness of other types of exercise such as electrostimulation. The addition of a protein supplement to the prescription of physical exercise in subjects with SO also appears to be effective66. However, further studies are needed to define the optimum amount of protein supplement.
Bariatric surgery and sarcopenic obesityBariatric surgery (BS) is an effective treatment option for morbid obesity, and leads to very important improvements in patient associated comorbidities and quality of life67. Significant improvements have been reported in IR, as well as a post-BS type 2 diabetes remission rate of 60-70% after one year of follow-up68. Few studies are currently available on the role of BS in SO. A recent study in 69 morbidly obese patients showed BS to be effective in reaching the weight loss target in patients with SO, with remission rates of the main comorbidities and a safety profile similar to those recorded in the non-sarcopenic group69. However, muscle mass data were estimated using mathematical equations that could yield inaccurate results.
Another study, using DEXA70, identified two patient phenotypes after BS, based on the percentage of muscle mass lost referred to total body weight: "acceptable muscle loss" (< 15%) and "significant muscle loss" (> 15%). The patients with less muscle loss showed improvements in blood glucose three months after surgery, as compared to those with greater muscle mass loss. In addition, a negative correlation was found between the evolution of glycemia and muscle mass 12 months after BS. This observation once again supports the inter-relationship between muscle mass and metabolic profile.
On the other hand, some studies have described a harmful effect of preoperative SO upon the outcomes of BS, with an increased risk of vertical sleeve gastrectomy leak71, and a negative impact upon muscle structure72. The indication of bariatric surgery in elderly people is still subject to debate. In most centers, the indication of BS is limited to people under 65 years of age. Furthermore, people over 65 years of age have an increased risk of presenting sarcopenia. There are virtually no data in the literature on the impact of BS in this population. In a recent study, Voican et al. evaluated the evolution of muscle mass after vertical sleeve gastrectomy in patients with obesity under 45 years of age73 using lumbar CAT. These authors found that 8% of the patients had pre-BS sarcopenia while approximately 33% presented sarcopenia at 12 months postsurgery. In their study, the male gender, a lower BMI and pre-BS muscle mass index were identified as the significant predictors - underscoring the importance of conducting a muscle mass study before considering BS.
ConclusionsSarcopenic obesity (SO) has been associated with greater disability and morbidity and mortality than the two entities (obesity and sarcopenia) considered separately. It has also been associated to an increased risk of type 2 diabetes and cognitive impairment - with chronic inflammation and insulin resistance being important pathogenic factors. There is no clear consensus on its definition and diagnostic criteria. At present, DEXA, CAT and MRI are available as more precise techniques for measuring body composition; however, these tests are not easily available in daily clinical practice. We therefore believe that the combination of electrical bioimpedance (EB) and ultrasound (US) could be a good initial strategy for identifying patients with sarcopenia.
The most warranted treatment regimen would be a multimodal scheme with an adequate calorie-protein supply and the combination of resistance and aerobic exercises. Further studies are needed to determine the role of biomarkers in the early detection and follow-up of patients with sarcopenia, together with research on the impact of SO in younger populations (< 65 years of age). In addition, more in-depth investigation on the relationship between SO and bariatric surgery (BS) would be needed. It should be noted that most studies have not adequately assessed the presence of pre-BS sarcopenia. For this reason, we consider it essential to first standardize the diagnostic criteria and to determine the prevalence of sarcopenia in the obese population.
Research in this field will help avoid or alleviate the complications associated with SO, and which undoubtedly represent an emerging challenge in clinical practice.
Please cite this article as: Ciudin A, Simó-Servatd A, Palmasa F, Barahona MJ. Obesidad sarcopénica: un nuevo reto en la clínica práctica. Endocrinol Diabetes Nutr. 2020;67:672–681.