Hereditary fructose intolerance is a metabolic disease due to an aldolase B deficiency. Our objective was to ascertain the social and health care needs of those with this deficiency.
Material and methodsA prospective, observational study was performed. A survey of social and health care needs was conducted to hereditary fructose intolerance patients living in Spain.
ResultsMost patients had been diagnosed, mainly by genetic analysis in children and based on fructose overload in adults. Population surveyed had no sequelae (72.34%) or disability (64%), and 83.33% of children and 52.38% of adults were taking drugs (p<.05) (2.06 drugs on average). Most patients had attended medical visits in the past two years, mainly in metabolic disease units (42.5%) and/or nutrition units (42.5%), but less than a half attended reference centers (mostly children [p<0.05]). Although 48% were satisfied with health care, they felt discriminated in recreational activities, school, health and/or daily activities. The most reliable sources of information were the specialized care physician (69.39%) and patients’ association (59.18%). Fifty-five percent reported no problem in any quality of life dimension, although some had problems in daily activities, pain, and anxiety.
ConclusionsAlthough hereditary fructose intolerance is less disabling than other rare diseases, it is important to know the needs of those who suffer from it. Although time to diagnosis has shortened, the poorer health care and satisfaction with it perceived in adults makes it necessary to emphasize the needs of this population, and the critical need of training and information of health care professionals.
La intolerancia hereditaria a la fructosa es una enfermedad metabólica debida a una deficiencia en la aldolasa B. Nuestro objetivo es conocer las necesidades sociosanitarias del colectivo.
MetodologíaEstudio observacional prospectivo en el que se difundió una encuesta de necesidades sociosanitarias a pacientes con intolerancia hereditaria a la fructosa residentes en España.
ResultadosLa mayoría disponían de diagnóstico, confirmado principalmente por análisis genético en menores y sobrecarga de fructosa en adultos, no padecían secuelas (72,34%) ni discapacidad (64%) y el 83,33% de niños tomaban medicamentos frente al 52,38% de adultos (p<0,05) (2,06 medicamentos de media). La mayoría acudieron a consultas en los dos últimos años, principalmente unidades de enfermedades metabólicas (42,5%) y/o nutricionista (42,5%), aunque menos de la mitad eran atendidos en centros de referencia (mayoritariamente niños [p<0,05]). El 48% estaban satisfechos con la atención sanitaria aunque se sintieron discriminados en actividades de ocio, escolares, sanitarias y/o cotidianas. Las fuentes más fiables de información fueron el médico de atención especializada (69,39%) y la asociación de pacientes (59,18%). El 54% no indicaron ningún problema en ninguna de las dimensiones de calidad de vida, aunque algunos tuvieron problemas en actividades cotidianas, dolor y ansiedad.
ConclusionesAunque su perfil no sea tan discapacitante como el de otras enfermedades raras, es importante conocer las necesidades del paciente con intolerancia hereditaria a la fructosa. Aunque se han reducido los tiempos en el diagnóstico, la menor atención y satisfacción sanitaria en adultos hace necesario incidir en las necesidades de esta población, siendo clave la formación e información de los profesionales sanitarios.
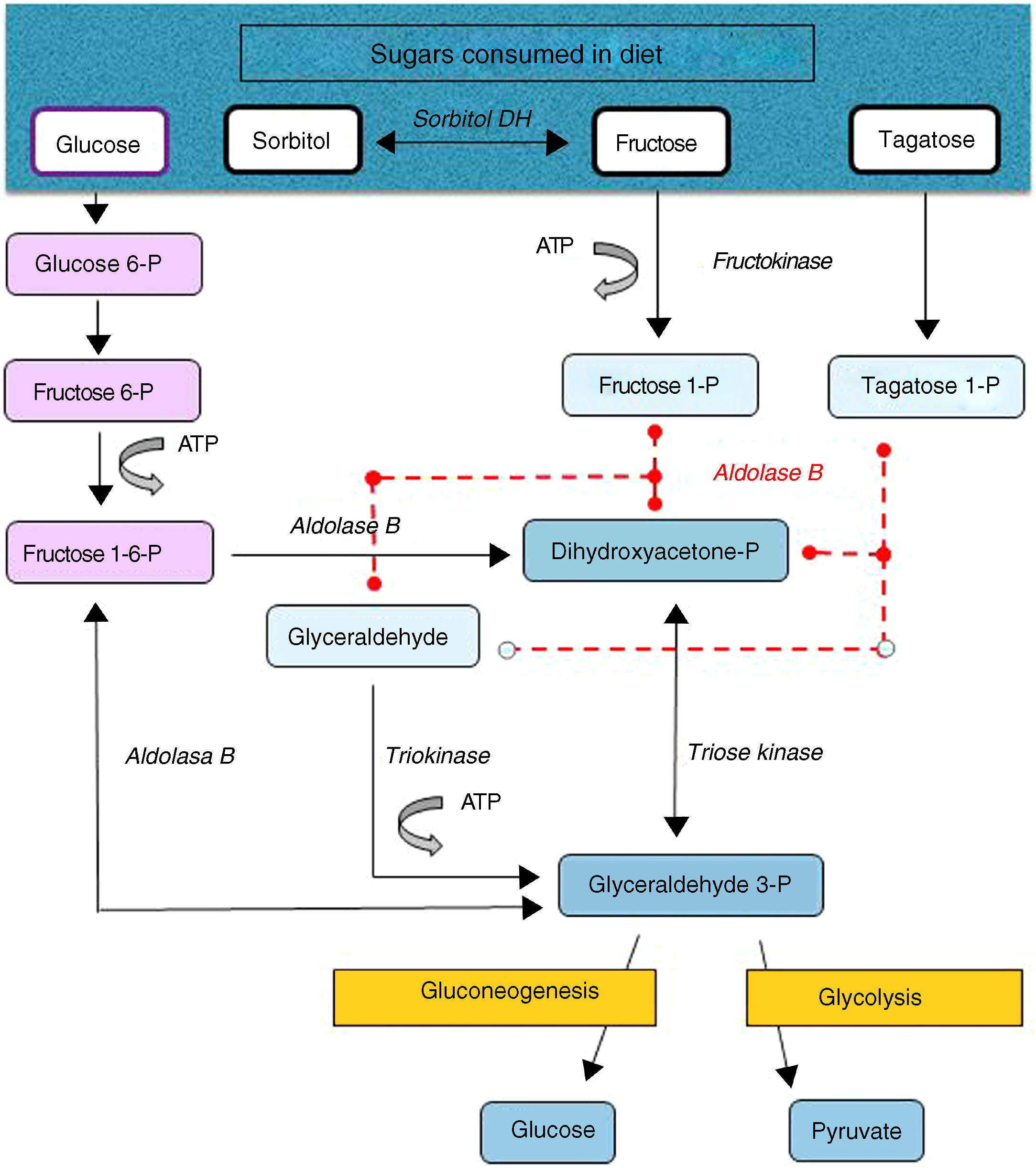
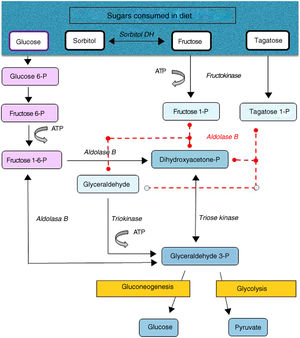
Hereditary fructose intolerance (HFI) (OMIM #229600) is a low prevalence autosomal recessive metabolic disorder (1–9 cases per 100,000 inhabitants) resulting from a deficiency in the activity of aldolase B, a key enzyme in the metabolism of fructose occurring mainly in the liver, and to a lesser extent in the renal cortex and bowel (Fig. 1). In these patients, nausea, vomiting, perspiration, lethargy, shock, dehydration, hepatorenal impairment and/or postprandial hypoglycemia rapidly occur when large amounts of fructose are consumed (4–6g/kg/day). This situation may lead to coma and even death, and can cause irreversible neurological, hepatic and renal sequelae. However, the ongoing consumption of small amounts of fructose (due to dietary transgressions or lack of a diagnosis) equivalent to over 40mg of fructose/kg/day can also give rise to feeding difficulties, vomiting, hepatomegaly, edema, ascites and lack of weight gain (in children). The metabolic and/or laboratory changes are mainly due to the accumulation of the substrate of aldolase B, fructose-1-phosphate, causing the inhibition of liver glycogenolysis, the inhibition of gluconeogenesis, the inhibition of phosphomannose isomerase, partial fructokinase inhibition, liver alterations, renal tubular damage or the depletion of ATP or inorganic phosphorus, with hypophosphatemia, hypermagnesemia and increased magnesium loss in urine, hyperuricemia, uricosuria, metabolic acidosis, etc.1
After a dietary restriction of all fructose sources, recovery and the disappearance of signs and symptoms occur rapidly, with an excellent long-term prognosis, though some patients maintain liver steatosis, with normalization of the liver enzymes, for years. The only currently available management is a lowering in the diet of all fructose sources to <1–2g of fructose/day in adults and <20–40mg/kg/day in children.1 Fructose is naturally found in fruits and vegetables, saccharose (disaccharide of fructose and glucose) and honey. Other sweeteners to be avoided are tagatose (a fructose isomer metabolized by aldolase B) and sorbitol (as well as sweeteners containing it, such as maltitol, lactitol or isomaltitol), which is converted into fructose by the enzyme sorbitol dehydrogenase.2
Understanding the sociosanitary needs of patients with low-prevalence or rare diseases (RDs) is a starting point for identifying and measuring the impact and burden of these disorders on the affected individuals and their families, and social and health institutions. Spanish national studies such as the ENSERio trial of the Spanish Federation of Rare Diseases (Federación Española de Enfermedades Raras [FEDER])3,4 reflect public awareness of the importance and sociosanitary repercussions of these diseases upon clinicians, investigators and politicians.5 To date, no studies have been published on the sociosanitary needs of patients with HFI. The present study was designed to analyze such needs in people with this disease in Spain.
Material and methodsA prospective observational study was carried out involving the distribution of an anonymous survey assessing the social and health needs among patients with HFI residing in Spain (June 2016–February 2017). The survey design was based on the questionnaire of the “Study on the sociosanitary needs of people with rare diseases in Spain: the ENSERio Study (FEDER)”, conducted on a sample of 715 individuals with RDs and their families (November 2008–January 2009).3 The questions were adapted to our population, respecting the structure and semantics in order to preserve their validity, with the addition of further questions regarding improvements to the current situation, the use of medicines, information sources and health-related quality of life (HRQoL) surveys (EQ-5D-Y and EQ-5D). The final survey consisted of 40 questions addressing sociodemographic aspects, diagnosis, the degree of disability, healthcare, disease-related expenses, patient associations and disease perception (Annex I, Supplementary material). The study was approved by the Ethics Committee of Hospital Universitario Central de Asturias (Spain).
Non-probabilistic sampling was performed in an attempt to reach as many HFI patients in Spain as possible. The questionnaire was distributed through the Association of Patients with Hereditary Intolerance to Fructose (Asociación de Afectados por Intolerancia Hereditaria a la Fructosa [AAIHF]) via e-mail (asociacionihf@gmail.com), website (http://www.aaihf.com/) and social networks (Facebook® and Twitter®), as well as through other associations of patients and physicians specializing in metabolic diseases.
Patients living in Spain with HFI diagnosed by genetic testing, liver biopsy, intestinal biopsy or fructose overload were included in the study. Patients in the process of establishing the prognosis but with a clear suspicion of HFI based on the clinical symptoms were also included, while patients with a diagnosis other than HFI (e.g., other congenital metabolic errors or congenital or acquired fructose malabsorption) and individuals with doubts about their diagnosis were excluded from the data analysis of the study.
Qualitative variables were reported as absolute numbers and percentages, while quantitative variables were presented as the mean and standard deviation (SD) and/or the median (Md) and interquartile range (IQR) in the event of an asymmetrical distribution. The chi-squared test or Fisher's exact test was used to compare qualitative variables, while the Student t-test or nonparametric tests (in the event of a non-normal distribution) were used in application to quantitative variables. Statistical significance was considered for p<0.05. Correlations between two variables were explored using Pearso's R statistic, Spearman's rho (ρ) or Kendall's tau (τ) statistic. The SPSS version 20.0 statistical package was used throughout.
ResultsA total of 72 surveys were completed. Practically incomplete or duplicate cases were excluded, together with those corresponding to patients residing outside Spain or with a diagnosis other than HFI. Fifty questionnaires were accepted, mostly corresponding to females (n=34/49; 69.39%), subjects over 16 years of age who answered the questionnaires by their own means (n=26/50; 52%), with a mean age of 22.92 (15.04) years [Md=23; IQR=27], and of Spanish citizenship (n=49/50; 98%). In the case of patients under 16 years of age (n=24/50; 48%), a relative was asked to answer the questionnaire. The respondents came from 11 Autonomous Communities, mainly Galicia (n=10/50; 20%), Andalusia (n=7/50; 14%), the Community of Madrid (n=7/50; 14%) and the Basque Country (n=6/50; 12%). Forty-two percent (n=21/50) reported that some other member of their family also had HFI, mainly siblings (n=18/21; 85.71%).
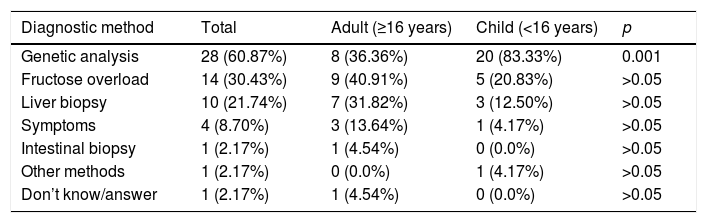
Most reported having had a diagnosis (confirmed [n=40/50; 80%] or not yet confirmed [n=6/50; 12%]). All the underage patients had a diagnosis, mainly based on genetic tests (second in order of frequency among adults [p<0.001], after fructose overload). Symptoms started in infancy after intake of the first fruit. In most cases the first symptoms manifested before three years of age (67.39%), though a positive correlation was observed between age and a later diagnosis (τ=0.365 and p=0.001), since most children had their symptoms earlier (before one year in 58.33% of the cases) than the adults (Table 1). The respondents considered that the consequences of diagnostic delay or the lack of a diagnosis were mainly “receiving no support or treatment” (n=15/34; 44.12%) and worsening of the disease (n=13/34; 38.23%), though 29.41% (n=10/34) reported no consequences due to such delay or were not aware of any consequences. Most suffered no sequelae (n=34/47; 72.34%) and had no disability status or had not requested official disability status (n=32/50; 64%).
Diagnostic method and time to diagnosis from symptoms onset in HFI.
| Diagnostic method | Total | Adult (≥16 years) | Child (<16 years) | p |
|---|---|---|---|---|
| Genetic analysis | 28 (60.87%) | 8 (36.36%) | 20 (83.33%) | 0.001 |
| Fructose overload | 14 (30.43%) | 9 (40.91%) | 5 (20.83%) | >0.05 |
| Liver biopsy | 10 (21.74%) | 7 (31.82%) | 3 (12.50%) | >0.05 |
| Symptoms | 4 (8.70%) | 3 (13.64%) | 1 (4.17%) | >0.05 |
| Intestinal biopsy | 1 (2.17%) | 1 (4.54%) | 0 (0.0%) | >0.05 |
| Other methods | 1 (2.17%) | 0 (0.0%) | 1 (4.17%) | >0.05 |
| Don’t know/answer | 1 (2.17%) | 1 (4.54%) | 0 (0.0%) | >0.05 |
| Time to diagnosis | Total | Adult (≥16 years) | Child (<16 years) | p |
|---|---|---|---|---|
| <6 months | 9 (19.56%) | 1 (4.54%) | 8 (33.33%) | <0.05 |
| 6 months–1 year | 11 (23.91%) | 5 (22.73%) | 6 (25.0%) | >0.05 |
| 1–3 years | 11 (23.91%) | 2 (9.09%) | 9 (37.5%) | <0.05 |
| 4–9 years | 7 (15.22%) | 6 (27.27%) | 1 (4.17%) | <0.05 |
| >10 years | 8 (17.39%) | 8 (36.36%) | 0 (0.0%) | 0.001 |
| Total surveyed subjects | 46 (100%) | 22 (100%) | 24 (100%) | – |
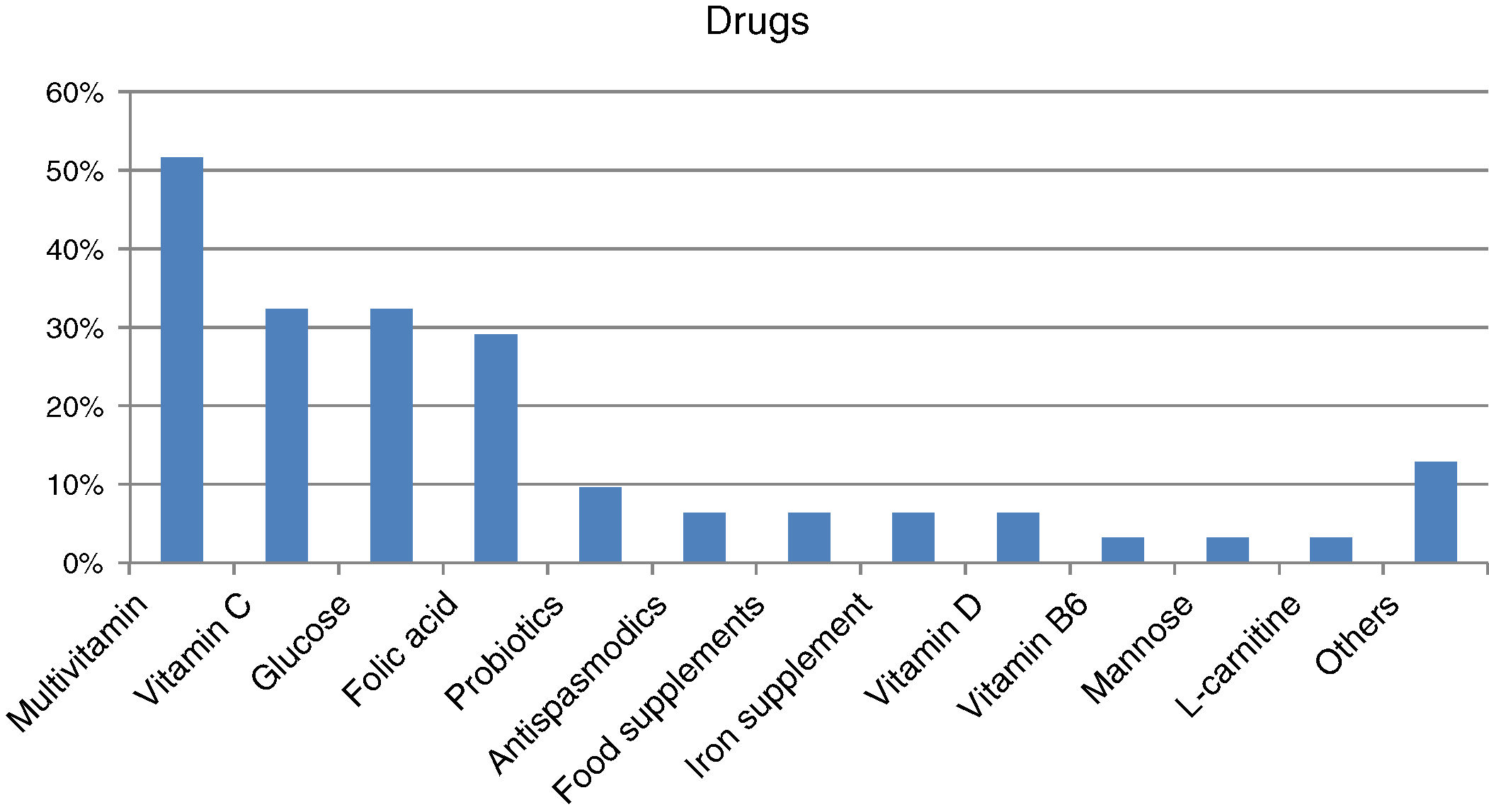
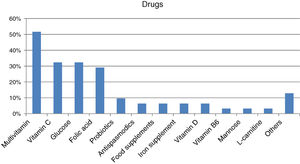
Forty-four percent of those surveyed reported receiving the treatment they need for the disease (n=22/50), though almost one-quarter required no treatment (n=12/50; 24%). Care was provided mainly by specialized physicians in the public healthcare setting (n=27/32; 84.37%), followed to a much lesser degree by private specialists (n=8/32; 25%) or public primary care (PC) physicians (n=7/32; 21.87%). Management mainly consisted of continuous long-term or permanent treatments (n=22/28; 78.57%). Most reported taking some kind of medication (n=31/45; 68.89%; mean per patient 2.06 [0.85]; Md=2, IQR=2), with greater medication in children (n=20/24; 83.33%) than in adults (n=11/21; 52.38%) (p<0.05). The most commonly used medications were multivitamins, vitamin C, glucose, and/or folic acid (Fig. 2). Most subjects had no difficulties in relation to access (n=25/31; 80.64%), though concerns were expressed regarding the non-financing of treatment and difficulties and/or delays in inspection visas. In turn, 31.11% (n=14/45) reported that they did not take any medication, and none of the respondents used medical devices for HFI.
Most of the patients had visited a clinic due to HFI in the previous two years (n=40/50; 80%), mainly metabolic disease units (n=17/40; 42.5%), dieticians or nutritionists (n=17/40; 42.5%), endocrinology (n=15/40; 37.5%), hepatology (n=13/40; 32.5%), gastroenterology (n=12/40; 30%), allergology (n=11/40; 27.5%), genetics (n=10/40; 25%) or general pediatric consultations (n=10/40; 25%). The patients that did not visit such clinics or who failed to answer were all adults. Less than half (n=18/40; 45%) were seen in public reference centers, with most visits being by children (n=14/24; 58.33%), since only one fourth of the adults visiting clinics were seen in these units (p<0.05). Most of the affected patients had not been hospitalized in the past two years because of HFI (n=40/50; 80%), and had not needed to travel outside their province (n=34/50; 68%). Eighty-four percent reported having undergone medical tests in the previous two years, particularly biological or biochemical tests (n=39/42; 92.86%), ultrasound (n=34/42; 80.95%) and, to a lesser extent, other imaging tests (n=12/42; 28.57%).
Overall, less than half of the respondents (n=24/50; 48%) were satisfied with the healthcare received (62.5% in children under 16 years of age, n=15/24 versus 34.61% in adults, n=9/26 [p>0.05]), and most reported a lack of knowledge of the disease on the part of the health professionals (n=36/50; 72%). The causes of dissatisfaction were mainly related to a lack of knowledge about drug composition, errors or delays in diagnosis, a lack of information about suitable or contraindicated foods, or confusion with other disease conditions.
According to a subjective assessment of the respondents, the percentage of household income allocated to disease-related care was mostly less than 20% of annual income (38% less than 10%, n=19/50 and 22% between 10 and 20%, n=11/50). The expenses referred to the purchase of dietary or specific foods for HFI (n=38/42; 90.48%), medication (n=30/42; 71.43%) and healthcare (n=18/42; 42.86%).
More than half of the respondents (n=28/50; 56%) belonged to the patient association of the disease (AAIHF), specifically 75% of the children (n=18/24) versus only 38.46% of the adults (n=10/26) (p<0.05). Most were satisfied with the services and activities offered (n=25/28; 89.29%).
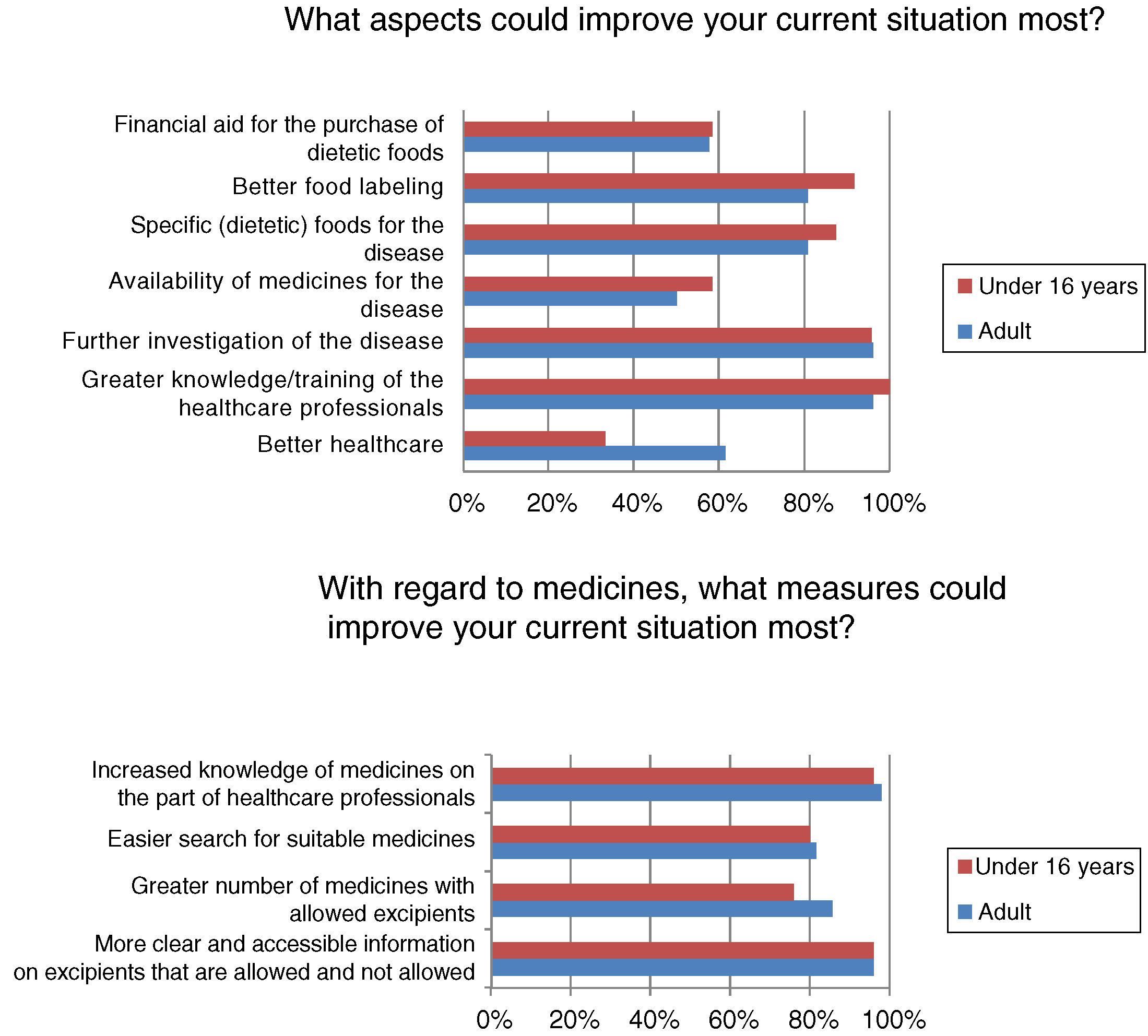
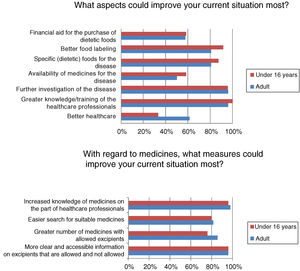
Most respondents had felt discriminated against because of their disease at some time, often or continuously (n=39/50; 78%). Regardless of the frequency of discrimination, it was mainly experienced in leisure or cultural settings (n=31/40; 77.5%), school activities (n=22/40; 55%), healthcare (n=19/40; 47.5%) and/or in daily life activities (n=19/40; 47.5%). Among other aspects, many adults (n=16/26; 61.54%) but fewer children (n=8/24; 33.33%) (p<0.05) felt that better healthcare would improve the current situation (Fig. 3).
The most reliable sources of information on disease-related issues were the specialist (n=34/49; 69.39%) and the patient association (n=29/49; 59.18%) and, to a lesser extent, the Internet (n=19/49; 38.78%) or nutritionist (n=18/49; 36.73%). The pharmacist and primary care physician were reliable sources for only 8.16% of the respondents (n=4/49), and the social networks or nursing professionals ranked even lower (n=2/49; 4.08% and n=1/49; 2.04% respectively). Adults rated the specialist as less reliable compared with the group of children (56% [n=14/25] versus 83.33% [n=20/24], respectively [p<0.05]).
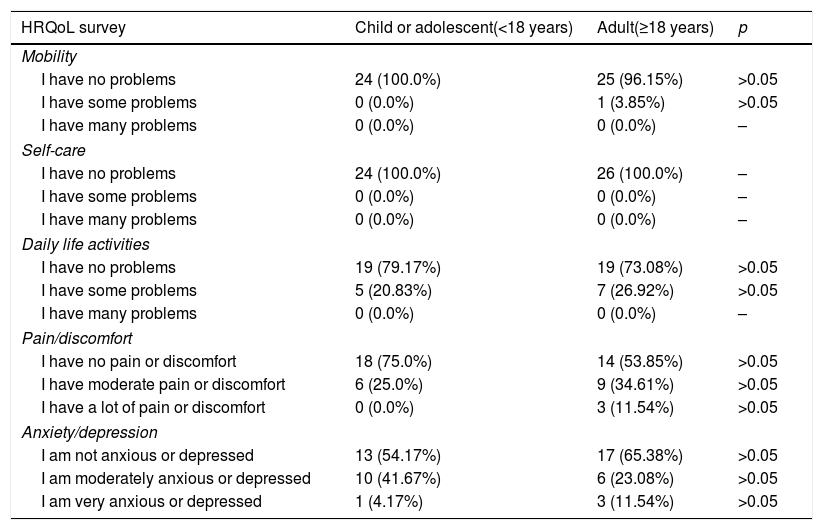
With regard to HRQoL, 54% of the surveyed patients (n=27/50) reported no problems in any of the dimensions. Most had no problems with mobility or self-care. The patients did report some problems with daily activities and the perception of pain or discomfort, and 11.54% of the adults even reported severe pain or discomfort. With regard to anxiety or depression, 41.67% of the children and 23.08% of the adults experienced moderate problems of this kind, and in both groups a small percentage reported a greater intensity. The overall health score according to the visual analog scale (VAS) was 74.84 (18.86) (Md=80 and IQR=20), and proved higher in the children, with a score of 81.33 (13.06) (Md=80 and IQR=15), than in adults, with a score of 68.85 (21.51) (Md=70 and IQR=40) (p<0.05) (Table 2). The correlation between age and the VAS score was not statistically significant (ρ=−0.265; p=0.063). No statistically significant differences were found between gender and quality of life, though significant differences were observed between patients whose diagnosis was confirmed before three years of onset of the first symptoms (79.10 [16.47]; [Md=80 and IQR=20]) and those in which diagnostic confirmation took place later (67.67 [19.07]; [Md=70 and IQR=35]). Since the EQ-5D-Y scale has been validated in children between 8 and 18 years of age, suppression from the analysis of the 10 children under 8 years of age resulted in a VAS score in this group of 81.07 (13.61) (Md=80 and IQR=20), the correlation between VAS and age being statistically nonsignificant (ρ=−0.234; p=0.146). Likewise, no statistically significant differences were found between a diagnosis confirmed before or after three years.
Results of the EQ-5D-Y (children and adolescents) and EQ-5D (adults) health-related quality of life (HRQoL) survey.
| HRQoL survey | Child or adolescent(<18 years) | Adult(≥18 years) | p |
|---|---|---|---|
| Mobility | |||
| I have no problems | 24 (100.0%) | 25 (96.15%) | >0.05 |
| I have some problems | 0 (0.0%) | 1 (3.85%) | >0.05 |
| I have many problems | 0 (0.0%) | 0 (0.0%) | – |
| Self-care | |||
| I have no problems | 24 (100.0%) | 26 (100.0%) | – |
| I have some problems | 0 (0.0%) | 0 (0.0%) | – |
| I have many problems | 0 (0.0%) | 0 (0.0%) | – |
| Daily life activities | |||
| I have no problems | 19 (79.17%) | 19 (73.08%) | >0.05 |
| I have some problems | 5 (20.83%) | 7 (26.92%) | >0.05 |
| I have many problems | 0 (0.0%) | 0 (0.0%) | – |
| Pain/discomfort | |||
| I have no pain or discomfort | 18 (75.0%) | 14 (53.85%) | >0.05 |
| I have moderate pain or discomfort | 6 (25.0%) | 9 (34.61%) | >0.05 |
| I have a lot of pain or discomfort | 0 (0.0%) | 3 (11.54%) | >0.05 |
| Anxiety/depression | |||
| I am not anxious or depressed | 13 (54.17%) | 17 (65.38%) | >0.05 |
| I am moderately anxious or depressed | 10 (41.67%) | 6 (23.08%) | >0.05 |
| I am very anxious or depressed | 1 (4.17%) | 3 (11.54%) | >0.05 |
| VAS scale | Mean (SD) | Mean (SD) | |
|---|---|---|---|
| 81.33 (13.06) | 68.85 (21.51) | <0.05 | |
| Total surveyed subjects | 24 | 26 | – |
The characteristics of the population with HFI in our study are similar to those of other rare diseases (RDs) in Spain (ENSERio 2016–2017)4 as regards the sociodemographic profile of the patients. In addition, the diagnosis is also predominantly established in less than three years from the onset of the first symptoms, though the diagnostic rate in the first 6 months was higher in the ENSERio study than in our population, possibly because of the rapid physical and/or intellectual impairment that occurs in some rare RDs and the low specificity of the symptoms in HFI. Patients with RDs suffer more hospital admissions and are more often in possession of a disability certificate than patients with HFI (81.23% versus 4%), the latter figure being even lower than in the general population (7.26%).6 The identification of HFI through fructose overload testing is the most common diagnostic method in adults, but is being decreasingly used because of its risk and low specificity. The increase in fructose malabsorption diagnostic tests using the exhaled hydrogen after oral fructose administration technique can cause serious problems in non-diagnosed HFI patients. The measurement of the enzyme activity of aldolase B (liver or intestinal biopsy) was a commonly used invasive test in past decades, but genetic testing is now the most common diagnostic method. Lack of support and treatment and/or a worsening of the disease due to a delayed diagnosis are problems affecting most patients with HFI, as it is with other RDs.4
Metabolic diseases, including HFI, can lead to acute liver failure in infants under 24 months of age.7 Although most patients reported no sequelae in our study, steatosis occurs in over 50% of all patients with HFI, and hepatomegaly in almost 20%.8 Cases of liver adenomatosis have even been reported, though the cause is unclear.9 Hereditary fructose intolerance has also been correlated to type 2 diabetes mellitus10 or celiac disease,11 though this was not recorded in our survey, since many patients did not consider these conditions to be related. In addition, there have been isolated reports of neurological problems secondary to acute intoxication or poor adherence to diet.1,12 In the present study sample, only three individuals reported some disability, and only one patient was using antiepileptic drugs.
Although almost half of the respondents were satisfied with their healthcare, satisfaction was higher among the children, who are those that use these resources most. In general, patients visiting metabolic units report a high degree of satisfaction with the healthcare system, considering the care received to be good or very good.13 The patients and their relatives consider that improved healthcare and greater knowledge and/or the training of healthcare professionals are aspects that would improve their situation. The advent of the “expert patient”, due to association affiliation and the empowerment of patients, has led to a change in patient attitude toward the professionals and the healthcare system, requiring the professionals to adapt accordingly.14 Studies on the training needs of healthcare professionals in RDs are mainly conducted in the primary care setting, where it is considered more important to focus on information needs than on training needs,15 since the professionals obtain information from the Internet, scientific bodies, journals and/or associations.16 Similarly, the activities of the AAIHF involve the distribution of written educational material providing information on medicinal products and excipients aimed at the healthcare professional caring for patients with HFI (Fig. 4). In addition, patients with HFI consider the specialist to be the most reliable source of information (more so in individuals under 16 years of age, who are the patients that most visit specialist clinics). The infrequency of the visits of patients with HFI to primary care centers or pharmacies, and the little specific training given, may be the reasons for there being less confidence in the pharmacist, primary care physician or nursing staff.
There is no treatment to replace enzyme deficiency in HFI, but vitamin supplements are needed (vitamin C or folic acid) because of poor dietary intake due to the consumption restrictions regarding fruit and vegetables. Although most subjects have had no difficulties with access, they complain about the lack of financial assistance (since food supplements are involved) or delays in the inspection visa (e.g., vitamin C capsules), as in other RDs4 or metabolic diseases where there are difficulties and a limited availability of specific food products and drugs (e.g., because of the need for magistral formulas).13 The percentage use of medicines or supplements is higher in children than in adults, since they are supplements that do not improve the course or prognosis of the disease, or are not prescribed due to a lack of health attention in many of the adults. Nevertheless, the AAIHF receives many consultations related to medicines and/or sweeteners. These issues constitute the third leading cause of consultation, after diet and general consultations about the Association.17 In addition, despite the mandatory legal requirements referring to alerts in the package insert and the Summary of Product Characteristics when the drug contains excipients not suitable for patients with HFI (fructose, sucrose, invert sugar, sorbitol, maltitol, lactitol and isomaltitol),18 serious errors have been detected in the Summaries of Product Characteristics, such as stating glucose syrup (an excipient allowed in HFI) when in fact the product is fructose or maltitol syrup (hydrogenated glucose syrup), both of which are contraindicated in this disease.19
The leisure and cultural settings are often a cause of discrimination for patients with HFI, whereas in other RDs the daily setting is mainly affected because of greater patient disability.4 The reasons for discrimination are the lack of adapted meals (restaurants, schools, summer camps, etc.) or the fact that the disease is not taken seriously due to a lack of information, an increase in other more benign intolerances, or food fashions. The non-inclusion of these sugars or polyalcohols in food legislation on substances capable of producing food intolerances (such legislation being focused only on allergens)20 makes it difficult to provide adequate information on labeling or in restaurants and school canteens. Furthermore, it is not mandatory for the labeling to state the composition in terms of additives (aromas, flavorings, coloring agents, etc.) that can be included in large quantities and may be accompanied by large amounts of sugar. All of this reinforces the concept of the “expert patient” who, with accurate and reliable information, must proactively self-manage his or her own diet. The needs of patients with HFI are similar to those of celiac patients, such as better labeling, direct financial support, tax benefits and a greater offer of suitable products in restaurants.21
In the general population, 80% of all adults do not have any health problems.22 Patients with HFI have a poorer health status and suffer more anxiety and/or depression, and difficulties in daily life activities, though they suffer less mobility and personal care problems than the general population. The same is observed in the pediatric population (VAS score 87.40 [10.55] with KIDSCREEN-1023 versus 81.15 [14.16] between 8 and 14 years of age with EQ-5L-Y). Tools to measure HRQoL in children should be used with caution. In this regard, there are validated proxy versions from 6 years of age,24 but these were not available free of charge during the study.
The use of online surveys and social network dissemination is a simple way to contact as many affected patients as possible, this being essential in diseases with a low prevalence and wide geographic dispersion.25 Despite this, one of the limitations of our study is the limited sample size recruited despite diffusion among physicians, associations and social networks. The true number of cases of HFI in Spain is not known. A prevalence of 1–9 cases per 100,000 inhabitants26 (Spanish population census 46,449,874 inhabitants in July 2016 according to the National Statistical Institute) yields a theoretical number of 464–4180 cases. This is far higher than the number of cases recorded by the metabolic units or the patient association. Therefore, although the sample size was far from ideal (172–255 surveyed individuals for a margin of error of 5%, maximum heterogeneity assumptions and a confidence level of 90%), the sample may be representative of patients with HFI in Spain. Other factors may be underdiagnosis (instinctive fructose exclusion diet due to dislike of sweetness), a lack of regular visits to specialized physicians, deaths due to acute intoxications or sequelae (there are no published data on deaths in Spain, though withdrawal of fructose solutions did not occur until 200227), non-participation in patient associations, failure to use the Internet, etc.
In conclusion, the sociosanitary needs of patients with HFI are, in some aspects, unrepresented in other studies on RDs, and their profile may be more similar to that of other metabolic diseases and/or dietary intolerances. Although the disease is not particularly disabling, patients with HFI have problems which the general population does not have, mainly in relation to the leisure setting. The difficulty in establishing an adequate and correct exclusion diet can cause complications that may generate morbidities and associated healthcare costs. Although the times to diagnosis have decreased in recent years, less care and healthcare satisfaction in adults makes it necessary to emphasize their needs. In this respect, the provision of training and information to all healthcare professionals, and to society as a whole, is a key element.
Authorship/collaboratorsAll the authors participated in the analysis and interpretation of the results, as well as in the writing of the manuscript and the approval of the final version. E. Izquierdo-García, as study supervisor, conceived the design, conducted data collection methodology and analysis, and drafted the manuscript. I. Escobar-Rodríguez, J.M. Manuel Moreno-Villares and I. Iglesias-Peinado participated in the design of the study and in data analysis and interpretation.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Thanks are due to the patients, patient associations and metabolic units for their collaboration in this study.
Please cite this article as: Izquierdo-García E, Escobar-Rodríguez I, Moreno-Villares JM, Iglesias-Peinado I. Necesidades sociosanitarias en pacientes con intolerancia hereditaria a la fructosa en España. Endocrinol Diabetes Nutr. 2020;67:253–262.