Graves’ disease (GD) is an autoimmune thyroid disease, common in adults but rare in children. The best therapeutic approach remains controversial.
ObjectivesTo ascertain the current treatment of pediatric GD in Portugal and to assess the clinical and biochemical factors that determine definitive/long-term remission after treatment with antithyroid drugs (ATDs).
Patients and methodsA retrospective analysis of data about pediatric GD treatment collected from a nationwide survey conducted by the Portuguese Society of Pediatric Endocrinology and Diabetology from May to August 2013. Population was categorized based on sex, age, use of ATDs, dosage, treatment duration, adverse reactions, thyrotropin receptor-stimulating antibody (TRAB) titer, remission and remission/relapse rates, and definitive treatment, and divided into group A (with ongoing treatment) and group B (with treatment stopped). Group B was subdivided into ‘Remission’, ‘Remission+relapse’ and ‘No remission’ subgroups based on the course of disease. The same parameters were compared between both groups.
ResultsSurvey response rate was 77%; 152 subjects, 116 female, mean age at diagnosis 11.23±3.46 years. They all started treatment with ATDs, 70.4% with thiamazole, with a mean treatment duration of 32.38±28.29 months, and 5.9% had adverse effects. Remission rate was 32.6%. Lower age at diagnosis correlated with higher remission rates. Treatment duration was longer when propylthiouracil was used. Initial TRAB titer was significantly higher in the ‘No remission’ group. Surgery and radioiodine were used as second-line treatments.
ConclusionOur study results were similar to those reported in the literature. Age and TRAB titer were identified as potential clinical and laboratory determinants of remission. Based on risk/benefit analysis, it was concluded that treatment should be individualized based on age, accessibility to treatments, and physician's experience.
La enfermedad de Graves (EG) es una enfermedad tiroidea autoinmune frecuente en el adulto pero rara en edad pediátrica. La mejor opción terapéutica sigue siendo controvertida.
ObjetivosConocer su tratamiento en Portugal, y evaluar factores clínicos y bioquímicos determinantes de la remisión definitiva/prolongada con fármacos antitiroideos (AT).
Pacientes y métodosAnálisis retrospectivo de los datos obtenidos mediante un cuestionario nacional realizado por la Sociedad Portuguesa de Endocrinología Pediátrica y Diabetología entre mayo y agosto de 2013. Caracterizamos la población por sexo, edad, uso de AT, dosis, duración del tratamiento, reacciones adversas, thyrotropin receptor-stimulating antibody (TRABs), tasas de remisión y remisión/recaída, y tratamiento definitivo. Se definieron los siguientes grupos: grupo A (mantiene tratamiento) y grupo B (tratamiento detenido), el cual se subdividió en «remisión», «remisión+recaída» y «no remisión»; se compararon los parámetros entre los diferentes grupos.
ResultadosLa tasa de respuesta al cuestionario fue del 77%: 152 sujetos, 116 de ellos mujeres, con un promedio de edad al diagnóstico de 11,23±3,46años. Todos iniciaron tratamiento con AT (70,4% con tiamazol), con una duración media del tratamiento de 32,38±28,29meses; un 5,9% presentaron efectos adversos y la tasa de remisión fue del 32,6%. Las edades menores al diagnóstico se correlacionaron con mayor índice de remisión. La duración del tratamiento fue mayor con propiltiouracilo. El título inicial de TRABs fue significativamente mayor en el grupo sin remisión. Cirugía y yodo radioactivo se utilizaron en segunda línea.
ConclusiónSe obtuvieron resultados similares a los de la literatura. Como posibles determinantes de la remisión, se identificaron la edad y el título de TRABs. Considerando los riesgos/beneficios, se concluye que la terapéutica debe ser individualizada, teniendo en cuenta la edad, la accesibilidad a las terapias y la experiencia del médico.
Graves’ disease (GD) is an autoimmune disease that causes hyperthyroidism through the stimulation of TRABs (thyrotropin receptor-stimulating antibody). The estimated incidence in the adult population is 1–2 cases per 1000 people per year1 and in the pediatric population 0.1 cases/100,000 children before puberty and 3 cases/100,000 children during adolescence.2
There are three therapeutic options for GD: medical treatment with antithyroid drugs (ATD), radioactive iodine (131-I) and surgery. However, the most efficient therapy remains controversial as pediatric age is a restrictive factor in this decision.
ATD is universally recommended as the first-line treatment. Nevertheless, the relapse rate remains high as only 30% of children achieve prolonged remission after 2 years of treatment.3,4 Some authors advocate for longer treatment as first course intending to increase definitive remission rates or more prolonged remission.5
It is difficult to anticipate the disease course and there is no certainty about the clinical or biochemical factors that predict remission with ATD. Some studies point to older age, lower thyroid hormone concentrations at diagnosis and a faster response to ATD as predictive factors to an early remission.6 Others highlight the initial titer of TRABs.7 There is no evidence-based consensus on the clinical utility of these factors as remission determinants.
The present study was designed to assess how pediatric GD is treated in Portugal, investigating the biochemical and clinical factors associated with prolonged or definitive remission after ATD treatment.
Material and methodsThe Portuguese Society of Pediatric Endocrinology and Diabetology (SPEDP) conducted a national data survey on pediatric GD. It was a retrospective study, between May and August 2013, including all the national public hospitals with Pediatric and/or Endocrinology departments.
For this purpose, a questionnaire (Supplementary file 1) was designed and distributed to all the hospitals with Endocrinology and Pediatric departments and a SPEDP member was responsible for the data collection in each center. The answers, given anonymously, were collected by mail or e-mail. After that, they were assembled and analyzed by a committee designated by the SPEDP.
All the included patients were younger than 18 years old with at least 6 months of follow-up, independent of time of diagnosis. Neonatal GD cases were excluded.
In this study we intended to identify the hospital centers and medical units where these patients are treated and the aims were to characterize the population about gender, age, age at diagnosis, first treatment, drug's choice and its initial dose, combined therapy with levothyroxine, use of ß-blockers, initial TRABs titer, complete blood count and hepatic enzymes levels during therapy, adverse drug reactions, remission and relapse rates, definitive treatment and its adverse effects and complications.
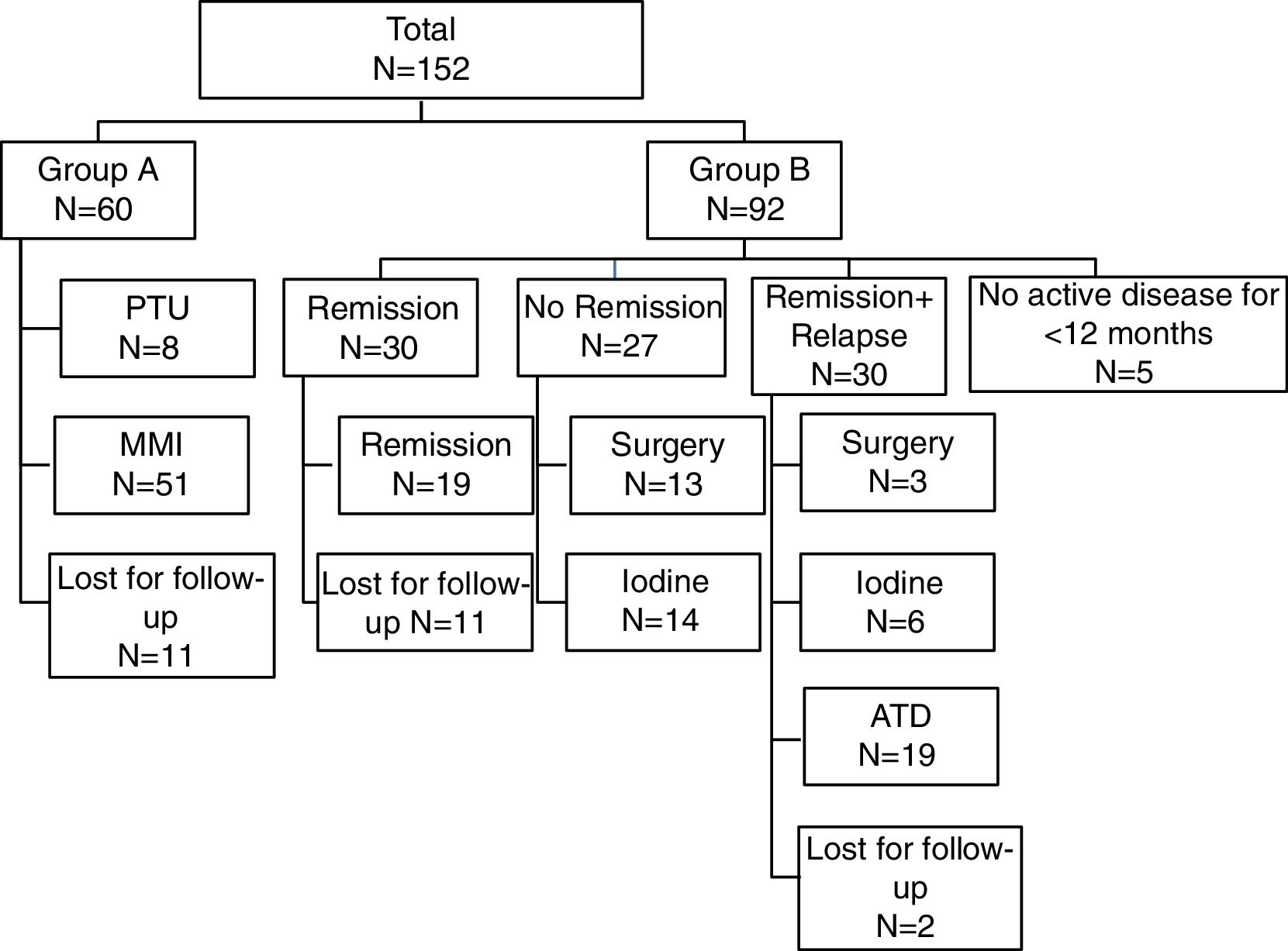
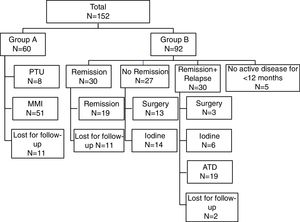
According to the clinical outcome after first course of medical therapy the population was divided in two groups: group A – for those maintaining treatment; group B – for those that stopped treatment. Group B was then divided into three subgroups according to the clinical status: ‘Remission’, ‘Remission and relapse’ and ‘No remission’ – (Fig. 1). As most often used in published series, we defined ‘Remission’ as disease-free time, without therapy, for 12 or more months.8 Patients with no active disease but less than 12 months of follow-up were excluded. In the ‘Remission’ group, only patients that achieved remission and maintained regular follow-up were considered for statistical analysis. Relapse was defined as recurrence of disease after 12 months of ATD withdrawal. If recurrence occurred before that period, they were classified in the “No remission” subgroup. The 3 subgroups were compared about the same parameters mentioned above, in order to determine possible predictive factors for disease remission.
Data analysis was performed using the Statistics Package for Social Sciences, the program IBM SSPS version 20. We used descriptive statistics to characterize the population. The results for continuous variables were expressed as mean and median, and for categorical variables as percentages.
The characteristics of the different groups were tested for statistically significant differences by Student's t-test or Mann–Whitney U test for continuous variables and by the χ2 test for categorical variables. Kruskal–Wallis test was used to compare the three groups. Statistical significance was accepted at the level of p<0.05.
The data collection was conducted according to Regional Ethical Committee proceedings and the Declaration of Helsinki.
ResultsEighty-seven hospital centers were contacted including 57 Pediatrics and 30 Endocrinology departments. A 77% response rate was attained and 42 centers reported no follow-up for pediatric GD patients (27 Pediatrics and 15 Endocrinology). A total cohort of 152 cases was identified in 25 centers (14 Pediatrics and 11 Endocrinology).
A retrospective data analysis of the clinical files of the 152 children and adolescents was performed. There were 116 female and 36 male patients, with a mean age of 11.25±3.44 years (3–18 years), and mean age at diagnosis of 11.23±3.46 years (3–18 years). There were no cases diagnosed before 3 years of age. We included all registered GD patients and the oldest diagnosis was in 1990 and the latest in 2012.
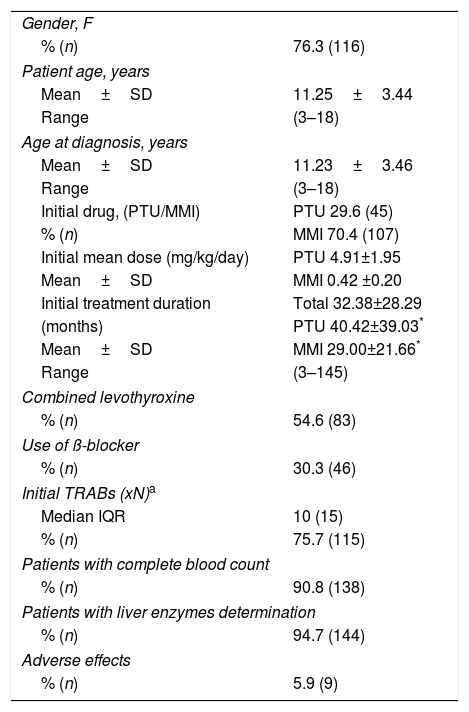
The patients’ characteristics at the time of diagnosis and the initial treatment are described in Table 1. ATD was the first therapeutic option in all patients: thiamazole (MMI) in 70.4% (n=107), and propylthiouracil (PTU) in 29.6% (n=45). After 2010, when INFARMED (National Authority of Medicines and Health Products)9 published a recommendation regarding the high risk of hepatotoxicity with PTU, only 2 patients started therapy with this drug.
Patients characteristics at time of diagnosis and initial therapy.
| Gender, F | |
| % (n) | 76.3 (116) |
| Patient age, years | |
| Mean±SD | 11.25±3.44 |
| Range | (3–18) |
| Age at diagnosis, years | |
| Mean±SD | 11.23±3.46 |
| Range | (3–18) |
| Initial drug, (PTU/MMI) | PTU 29.6 (45) |
| % (n) | MMI 70.4 (107) |
| Initial mean dose (mg/kg/day) | PTU 4.91±1.95 |
| Mean±SD | MMI 0.42 ±0.20 |
| Initial treatment duration | Total 32.38±28.29 |
| (months) | PTU 40.42±39.03* |
| Mean±SD | MMI 29.00±21.66* |
| Range | (3–145) |
| Combined levothyroxine | |
| % (n) | 54.6 (83) |
| Use of ß-blocker | |
| % (n) | 30.3 (46) |
| Initial TRABs (xN)a | |
| Median IQR | 10 (15) |
| % (n) | 75.7 (115) |
| Patients with complete blood count | |
| % (n) | 90.8 (138) |
| Patients with liver enzymes determination | |
| % (n) | 94.7 (144) |
| Adverse effects | |
| % (n) | 5.9 (9) |
PTU: propylthiouracil; MMI: thiamazole; IQR: interquartile range.
Patients using PTU had a significantly longer treatment course than those treated with MMI, in average, 40.42±39.03 months vs. 29.00±21.66 months, respectively (p=0.013). Propranolol was the ß-blocker drug mostly used. Bisoprolol and atenolol were rarely used. We found 56.4% cases treated with combination therapy with levothyroxine (block and replace therapy).
The adverse effects of ATD were rare and mild in severity, except for a case of hepatitis with PTU that reverted after drug withdrawal. There was a transient elevation of liver enzymes in 6 cases. Other 6 patients presented with mild severity leukopenia and did not require drug interruption.
Data concerning initial treatment and treatment results are described in Fig. 1. Of total population, 39.5% (n=60) maintained treatment with ATD (15 patients for less than 12 months, 16 patients for 13–24 months and 29 patients for more than 24 months).
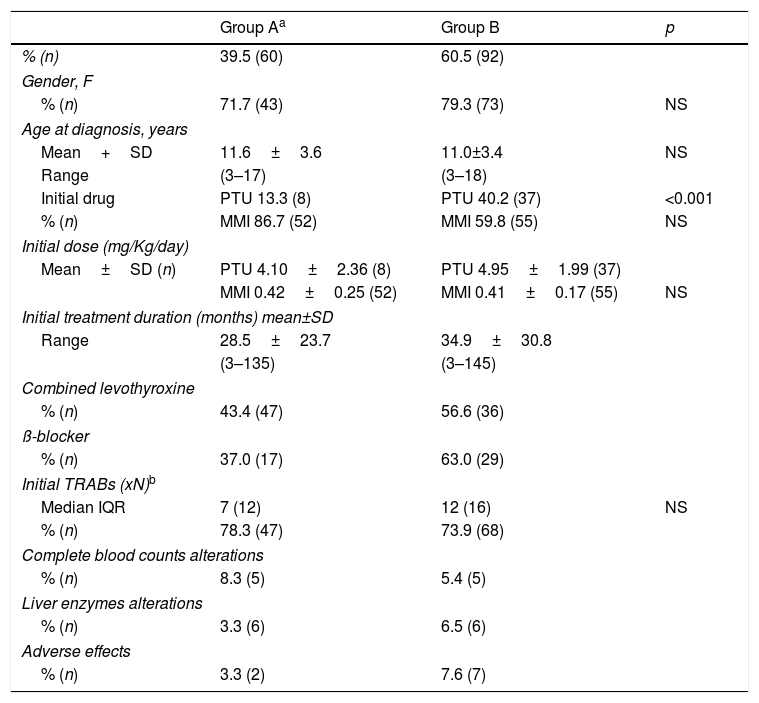
In Table 2, the comparative data between first therapeutic approach in Group A (maintain treatment) and Group B (stopped treatment) is presented.
Comparison between Groups A and B.
| Group Aa | Group B | p | |
|---|---|---|---|
| % (n) | 39.5 (60) | 60.5 (92) | |
| Gender, F | |||
| % (n) | 71.7 (43) | 79.3 (73) | NS |
| Age at diagnosis, years | |||
| Mean+SD | 11.6±3.6 | 11.0±3.4 | NS |
| Range | (3–17) | (3–18) | |
| Initial drug | PTU 13.3 (8) | PTU 40.2 (37) | <0.001 |
| % (n) | MMI 86.7 (52) | MMI 59.8 (55) | NS |
| Initial dose (mg/Kg/day) | |||
| Mean±SD (n) | PTU 4.10±2.36 (8) | PTU 4.95±1.99 (37) | |
| MMI 0.42±0.25 (52) | MMI 0.41±0.17 (55) | NS | |
| Initial treatment duration (months) mean±SD | |||
| Range | 28.5±23.7 | 34.9±30.8 | |
| (3–135) | (3–145) | ||
| Combined levothyroxine | |||
| % (n) | 43.4 (47) | 56.6 (36) | |
| ß-blocker | |||
| % (n) | 37.0 (17) | 63.0 (29) | |
| Initial TRABs (xN)b | |||
| Median IQR | 7 (12) | 12 (16) | NS |
| % (n) | 78.3 (47) | 73.9 (68) | |
| Complete blood counts alterations | |||
| % (n) | 8.3 (5) | 5.4 (5) | |
| Liver enzymes alterations | |||
| % (n) | 3.3 (6) | 6.5 (6) | |
| Adverse effects | |||
| % (n) | 3.3 (2) | 7.6 (7) | |
PTU: propylthiouracil; MMI: thiamazole.
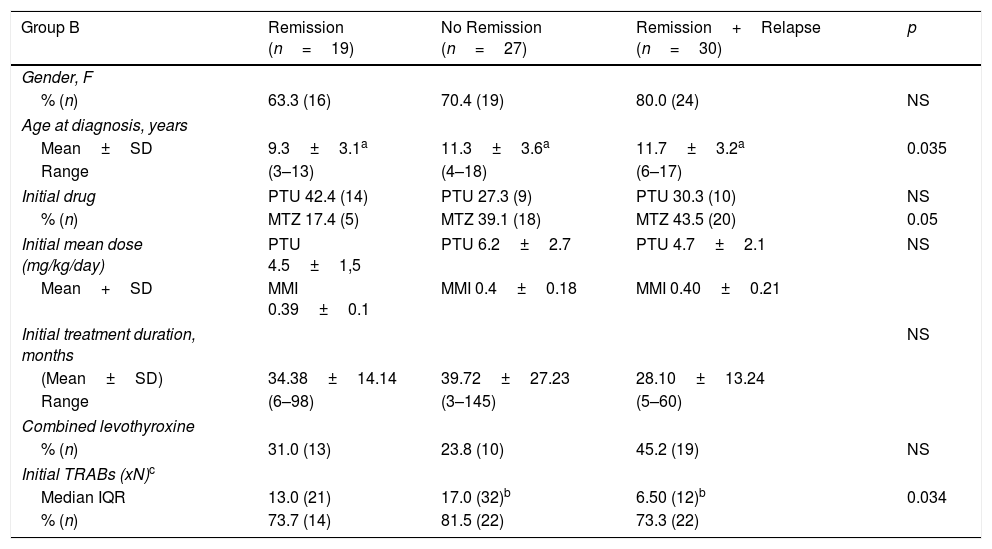
Table 3 shows the comparative results between the subgroups of patients that stopped initial therapy with ATD. The three subgroups were compared regarding gender, age at diagnosis, ATD use and dose, duration of initial treatment, combined therapy with levothyroxine, initial serum TRABs titer. Remission rate was 32.6%. We found significant differences between the three groups concerning the age at diagnosis of GD. In the ‘Remission’ group, age was significantly lower than in ‘Remission+Relapse’ and ‘No Remission’ groups (p=0.035). We obtained no statistical difference but a borderline result (p=0.05) for MMI in favor of ‘No remission’ and ‘Remission+Relapse’. In the ‘No remission’ group, we found that the initial TRABs titer was statistically higher than ‘Remission+Relapse’ group (p=0.034), but not to the ‘Remission’ group (p=0.32). The initial TRABs titer was more than ten times normal in 63.3% of patients in the ‘No remission’ group.
Comparison between patients that stopped initial ATD therapy.
| Group B | Remission (n=19) | No Remission (n=27) | Remission+Relapse (n=30) | p |
|---|---|---|---|---|
| Gender, F | ||||
| % (n) | 63.3 (16) | 70.4 (19) | 80.0 (24) | NS |
| Age at diagnosis, years | ||||
| Mean±SD | 9.3±3.1a | 11.3±3.6a | 11.7±3.2a | 0.035 |
| Range | (3–13) | (4–18) | (6–17) | |
| Initial drug | PTU 42.4 (14) | PTU 27.3 (9) | PTU 30.3 (10) | NS |
| % (n) | MTZ 17.4 (5) | MTZ 39.1 (18) | MTZ 43.5 (20) | 0.05 |
| Initial mean dose (mg/kg/day) | PTU 4.5±1,5 | PTU 6.2±2.7 | PTU 4.7±2.1 | NS |
| Mean+SD | MMI 0.39±0.1 | MMI 0.4±0.18 | MMI 0.40±0.21 | |
| Initial treatment duration, months | NS | |||
| (Mean±SD) | 34.38±14.14 | 39.72±27.23 | 28.10±13.24 | |
| Range | (6–98) | (3–145) | (5–60) | |
| Combined levothyroxine | ||||
| % (n) | 31.0 (13) | 23.8 (10) | 45.2 (19) | NS |
| Initial TRABs (xN)c | ||||
| Median IQR | 13.0 (21) | 17.0 (32)b | 6.50 (12)b | 0.034 |
| % (n) | 73.7 (14) | 81.5 (22) | 73.3 (22) | |
A definitive treatment was used in 23.7% of the patients. Surgery was performed in 16 patients, the majority (n=13) in the group that did not achieve remission, and 131-I was used in 20 patients, 14 within the same group. Regarding those treated with 131-I, 6 presented with hypothyroidism and 4 needed a second dose therapy. The outcome in the other 10 was unknown. There were no cases of acute adverse effects. The type of surgery was total or subtotal thyroidectomy. We found two cases of persistent hypoparathyroidism and one case of dysphonia. There were no data justifying criteria for treatment relapses and definitive treatment choice.
DiscussionThe treatment of pediatric GD differs between institutions, depending on the availability of therapies and the physician's experience.8,10–12 Up to this moment, in 2016 American Thyroid Association (ATA) published the GD therapy guidelines, including the therapy during pediatric ages, but evidence about the best treatment remains a controversial topic.4 For the first time in our country, we performed a nationwide data collection about pediatric GD treatment and study the influence of biochemical and clinical factors on disease remission, following ATD treatment.
All the public treatment centers offering Pediatric and Endocrinology care were invited to participate and we obtained a response rate of 77%. The contributing centers were distributed along the national territory.
The patients’ characteristics (gender and age at diagnosis) were similar to those found in the literature.
As it is recommended by the ATA and present throughout almost all the series published in literature,4,7,8,13 ATD was the initial treatment. Despite MMI and PTU are used for the treatment of hyperthyroidism for more than half a century, in 2010, INFARMED advised against the use of PTU due to the high risk of hepatotoxicity. MMI was the most frequently used ATD in our population, according to this national8 and other recommendations.14 The drug doses used were similar to the recommended ones.3 Contrasting with literature reports, adverse effects recorded in our study were uncommon (5.9%), of mild severity and did not require ATD withdrawal, except for a case of toxic hepatitis by PTU. In a meta-analysis of 550 PTU-treated patients, Rivkees et al. reported adverse effects in 15–35% of children and found that 4% of children on MMI developed adverse effects after 18 months of therapy.15 Very few studies report low PTU adverse effects as did Lippe et al., who found 1 case with arthritis out of 63 patients.16
One of the goals of this study was to identify one or more predictive factors for ATD treatment definitive response. In that way, we evaluated the influence of clinical and biochemical factors, such as age at diagnosis, initial TRABs titer and ATD treatment (drug, treatment duration and combination with levothyroxine). There are studies indicating that the response to ATD may vary according to the child's age and, although children of younger age are treated for longer periods with ATD, the response is better in older, pubertal than in prepubertal children.6,17,18 In this study, patients achieving remission were significantly younger (p=0.035) than those who did not achieve remission or relapsed. Also, Shulman et al. reported that prepubertal children continued to remit after prolonged medical therapy (>6 years) whereas pubertal patients did not.19 A possible explanation for this finding is that more patients in subgroup ‘Remission’ used PTU than MMI and, in our cohort, we found that MMI favored no remission or relapse, even though this difference had a borderline significance (p=0.05). We may also speculate that younger patients had a better compliance and a more rigorous monitoring, as they depend on their caregivers surveillance. Nakamura et al. compared treatment with MMI or PTU and concluded that treatment should not be initiated with PTU due to its lesser efficacy in severe disease and greater hepatotoxicity.20 Our study revealed PTU as more effective in achieving remission.
The ATA and the American Association of Clinical Endocrinologists guidelines recommend that if MMI is chosen as primary therapy for GD, it should be maintained for 12–18 months and progressively decreased, or withdrawn if TSH levels are normal. This is a strong recommendation with high quality evidence.4 Léger, in a study with 154 children in France, suggested that longer treatment with carbimazole (8–10 years) results in higher remission rates (up to 50%), without major adverse effects and with good compliance.5,21 Others have observed that extending the treatment with ATD allows for remission without subsequent relapse.22 We observed that patients on PTU vs. MMI were treated during a significant longer period. It can be justified by the fact that this drug was mostly used in patients diagnosed a long time ago, for prolonged treatment periods, as used to be at that time. The treatment duration in our study was very long, without evident higher remission rate, which was shown to be 32.6%, similar to that described in literature.6,7,23
A study published in 2014 found a positive predictive value for TRABs titer at the time of diagnosis regarding achievement of remission, being considered a specific and sensible marker for diagnosis in children and reflecting the disease's activity and severity.18 In our group, higher initial TRABs titer favored the non-remission of hyperthyroidism but they were also present in the ‘Remission’ group. We could not find a valuable explanation to this finding but we may expect that different laboratory assays had interfered. Higher levels are supposed to be detected in all assays and are correlated with no remission as it was found; lower levels could translate the possible differences between them.
The association of ATD with levothyroxine favors a longer treatment period with ATD, without symptomatic hypothyroidism. Some authors advise against this association, claiming that it increases the possibility of drugs adverse effects24 or it offers little in the stabilization of thyroid function when compared to a progressive reduction of ATD regime.25 Recently, ATA guidelines suggest that clinical practice should change as this therapeutic approach does not favor compliance.4 In our study, the use of ‘block and replace’ was used very often and did not offer added value in achieving initial remission.
Surgery and 131-I therapy were always used as second-line treatment, preferentially in patients that did not achieve remission with medical therapy. Peroni et al. observed 27 patients undergoing total thyroidectomy, and concluded that surgical treatment, in GD, if performed by an experienced surgeon, is a safe, definitive and cost effective option.26 Breuer et al. also concluded that surgery in pediatric GD is technically more challenging, with transient hypocalcemia more common, even though the complication rate overlaps the rate seen in the adult population, providing it is performed by an experienced team with a high volume of surgeries.27 On the other hand, some Spanish authors, in a review of 20 cases, concluded that surgery, with experienced physicians, should be reserved for cases of hyperthyroidism relapse in children or young people with goiter of great volume or those with goiter with nodules.28 In our country, several centers can perform thyroid surgery in the pediatric age providing great surgeon's experience. The short number of cases did not allow us to infer the rate of complications.
Virtually all authors agree unanimously that 131-I is a therapeutic option for children older than 5 years old.10,29 There is evidence that the US medical community prefers 131-I therapy as a first-line treatment. This approach is being increasingly defended by various groups considering its low risk of malignancy, specifically thyroid cancer, and low complication rate, when compared to surgery.30 Use of an adequately high dose of iodine 131 should be highlighted so that the gland's ablation occurs and, subsequently, the risk of carcinogenesis is reduced.29 Only one patient had 131-I therapy shortly (3 months) after the beginning of oral treatment (the patient with PTU hepatitis).
We consider that the 77% response rate from the national data reflects a representative sample of our country and strengths this study. We recognize that, as a retrospective study, there were limitations that were challenging for the interpretation of results, such as different therapeutics approaches of the centers, different criteria in use and titration of drugs, different methods of blood analysis, absent time limits concerning diagnosis date and last observation and lack of data concerning compliance, orbitopathy and initial level of thyroid hormones.
As a conclusion, we emphasize that this is a relevant study about GD treatment in pediatric age in Portugal and demonstrates the effort and collaboration of the majority of Pediatric and Endocrinology departments and the changes in therapeutic practices all over the years. It highlights the fact that in general our results overlap those existing in literature and the low incidence of drug adverse effects in patients with long term medical therapy. In search for the identification of predictive clinical or biochemical factors for disease remission we found lower age and TRABs titer as significant factors. Being aware of the risks and benefits of the different therapeutic approaches, treatment should be chosen according to the patient's age, the availability of treatment options and the experience of the physician.
FundingThis work was funded by the Portuguese Society of Pediatric Endocrinology and Diabetology.
Conflict of interestsThe authors have no conflicts of interest to disclose.
SPEDP thanks the special contribution of Dr Filipa Espada e Dr Joana Guimarães in the conception and disclosure of this questionnaire and to all the participants responsible for the data collection, namely: Alice Mirante, Ana Monteiro, Carlos Vasconcelos, Carla Meireles, Carla Maria Brandão, Elisa Galo, Isabel Dinis, Isabel Fernandes, João Anselmo, Lurdes Lopes, Lurdes Sampaio, Marcelo da Fonseca, Margarida Almeida, Margarida Ferreira, Marisol Anselmo, Rosa Arménia Campos, Teresa Bernardo, Teresa Borges, Sónia Pratas, Susana Gama de Sousa, Tiago Nunes da Silva, Valeriano Leite.