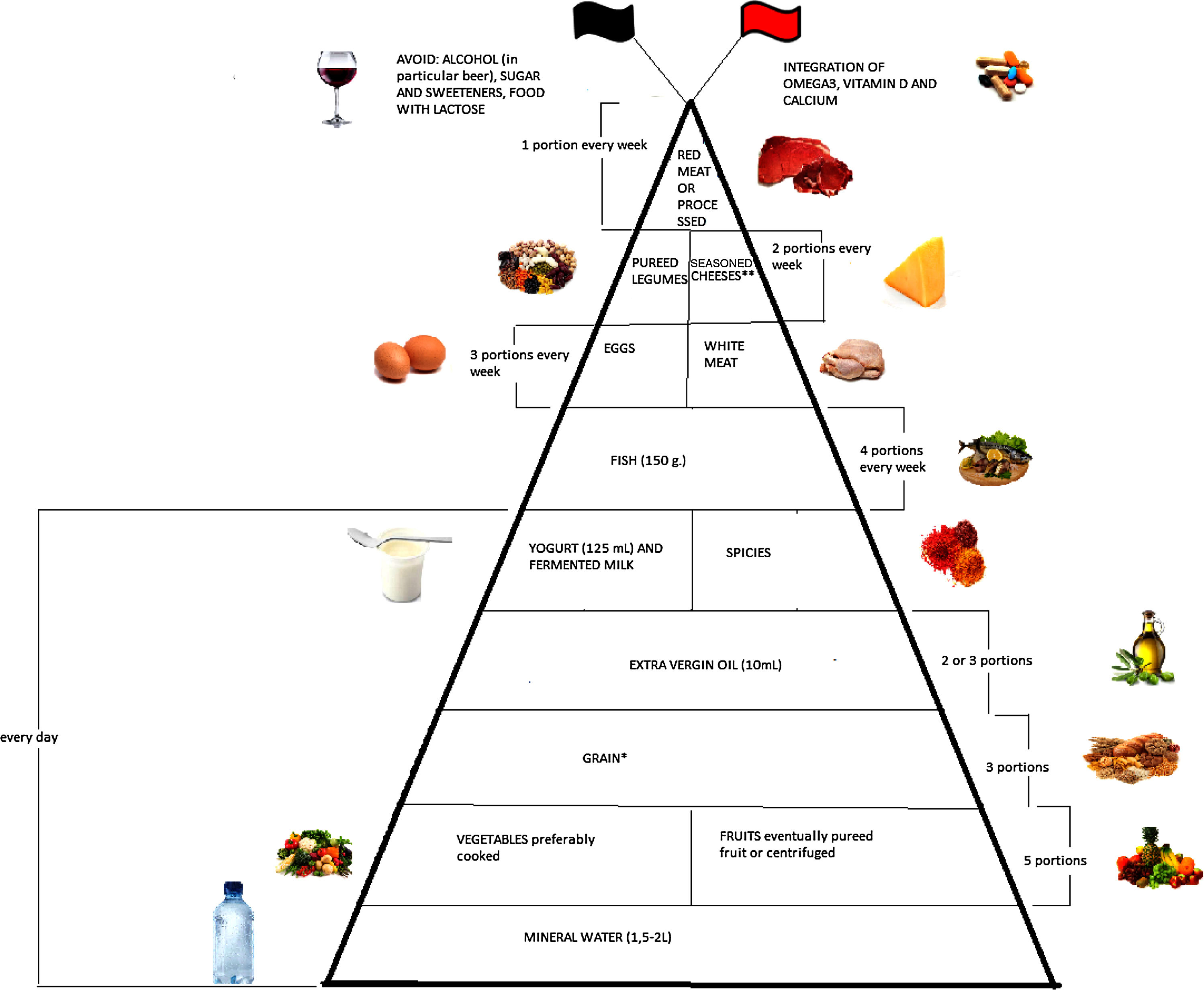
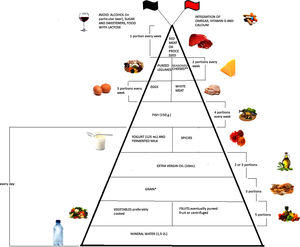
Emerging literature suggests that diet plays an important modulatory role in inflammatory bowel disease (IBD) through the management of inflammation and oxidative stress. The aim of this narrative review is to evaluate the evidence collected up till now regarding optimum diet therapy for IBD and to design a food pyramid for these patients. The pyramid shows that carbohydrates should be consumed every day (3 portions), together with tolerated fruits and vegetables (5 portions), yogurt (125ml), and extra virgin olive oil; weekly, fish (4 portions), white meat (3 portions), eggs (3 portions), pureed legumes (2 portions), seasoned cheeses (2 portions), and red or processed meats (once a week). At the top of the pyramid, there are two pennants: the red one means that subjects with IBD need some personalized supplementation and the black one means that there are some foods that are banned. The food pyramid makes it easier for patients to decide what they should eat.
La literatura emergente sugiere que la dieta resulta ser un importante papel modulador en la enfermedad inflamatoria intestinal (EII), a través del manejo de la inflamación y el estrés oxidativo. El objetivo de esta revisión narrativa es evaluar la evidencia hasta la fecha con respecto a la EII óptima de la terapia dietética, y construimos una pirámide de alimentos sobre este tema. La pirámide muestra que los hidratos de carbono deben consumirse todos los días (3 porciones), junto con las frutas y verduras toleradas (5 porciones), el yogur (125ml) y el aceite de oliva virgen extra; semanalmente, pescado (4 porciones), carne blanca (3 porciones), huevos (3 porciones), puré de legumbres (2 porciones), quesos condimentados (2 porciones) y carnes rojas o procesadas (una vez por semana). En la parte superior de la pirámide hay 2 banderines: uno rojo significa que los sujetos con IBD necesitan una suplementación personalizada y un negro significa que hay algunos alimentos que están prohibidos. La pirámide alimenticia permite a los pacientes descubrir fácilmente qué comer.
Inflammatory bowel disease (IBD) includes two chronic inflammation of the gastrointestinal tract, ulcerative colitis (UC) and Crohn's disease (CD). Both diseases are characterized by alternating periods of remission and flare up with symptoms of abdominal pain, diarrhea, extra-intestinal manifestations, and malnutrition.1 IBD is a complex and multifactorial disease resulting from the interplay of the genetic background of individual, environmental triggers and alterations in the intestinal microbiome, that together stimulate an aberrant immune response which drives chronic intestinal inflammation. The localization of inflammatory process is limited to the mucosa of the colon and continuous in UC. In contrast to UC, CD shows transmural inflammation and skip lesions without a specific localization in the gastrointestinal tract.2 Genome-wide association studies have identified over 163 loci linked to increased IBD susceptibility, mapping an array of genes that regulate processes involved in IBD, including microbe recognition, lymphocyte activation and intestinal epithelial defense. However, they only account for about 13% of CD and 7% of UC disease variance.3 Furthermore, studies focused on the level of concordance of CD or UC between identical twins estimated the maximum contribution of genetic factors to IBD to be approximately 10% for UC and 30%–40% for CD. Therefore, environmental factors are likely the largest contributor to the risk of IBD.4 Nearly 2 million people worldwide are affected by IBD. It is most prevalent in northern Europe and North America and less common in the Asia-Pacific region, with the exception of Australia. IBD is no longer a rare condition but affects up to 0.5% of the population, as indicated by several studies on children and adults in Western Europe and North America.5,6 Epidemiological and clinical evidences suggest that IBD is linked to several environmental factors, such as a wrong diet, smoking, use of drugs (non-steroidal anti-inflammatory drugs and oral contraceptives), geographical location, and social status.4 Further confirmation is given by the increased evidence of IBD in developed countries and urban populations unlike rural populations.7,8 The adoption of Western diet and lifestyle is correlated with the increased prevalence that is characteristic of the population of developing countries. Some studies reiterate the importance of environmental factors showing that individuals emigrating from low incidence regions to countries with higher IBD prevalence are at an increased risk of developing IBD, especially among the first-generation children.9 The management of IBD includes pharmacological, nutritional, and surgical therapy, with the main goals of treatment being the induction and maintenance of remission, the correction and abolition of nutritional deficiencies, and the prevention of complications.1 Although the development of highly active drugs like anti-tumor necrosis factor alpha (anti-TNF-α) antibodies has changed the short-term prognosis of severe IBD, there is still a need for low risk alternative approaches or adjuvant therapies. Nutritional intervention represents a support for traditional therapies and cannot be seen as a source of therapeutic strategies on its own right. In recent years, researchers tried to find a suitable diet for IBD patients, in order to either provide a correct and balanced intake of all essential nutrients or to find a specific combination of food able to help restore normal physiology of the intestinal mucosa. The various gastroenterology organizations that have formulated the recommendations for IBD do not refer to dietary restriction during remission, a common practice by many patients with IBD.10 For instance, the National Clinical Guideline Center11 recommends a diverse and well balanced diet for CD patients. Proper diet is also essential in IBD patients in order to avoid malnutrition. According to the study by Nguyen et al.,12 the rate of adult patients suffering from protein-energy malnutrition was significantly higher among IBD patients (OR=5.57, 95% CI: 5.29–5.86) than it was among non-IBD cases, for both CD (P<0.0001) and UC patients (P<0.0001). Another study showed that among 76 IBD patients (23 CD and 53 UC), 52 (68.4%) met the criteria for malnourishment, and 24 (31.6% of the entire cohort) were severely malnourished.13 On the other hand, a recent study in the U.S. showed that obesity prevalence in IBD patients reflects the obesity index in the general population, while clinical outcomes in obese patients are better than in non-obese patients with IBD, and that obesity [defined using body mass index (BMI)] is a marker of a less severe disease course in IBD.14 A relatively high percentage of obese patients during diagnosis could be related to an increase in obesity in the general population, accompanied by earlier IBD recognition. IBD children patients showed malnutrition and disturbed body composition due to a lower energy input during active disease.15 Another study found mesalamine was predictive of lean mass for height Z-score less than −1.00.16 Bile acid malabsorption is common in IBD patients, independent of the intestinal localization of the disease, leading to fat malabsorption and subsequent steatorrhea, impaired intestinal motility, and/or significant changes in the intestinal microflora environment. The presence of fat in stool could also be a result of a deficit in pancreatic enzyme secretion. Gastric acid and pancreatic enzyme impaired secretion were observed in 80% of CD patients.17,18 Loss of nutrients can also occur as a result of protein enteropathy from a ruptured, permeable gut. Additionally, studies utilizing whole gut lavage have demonstrated that disease activity closely paralleled gastrointestinal protein loss.19 Given this background, the aim of this review is to evaluate the evidence to date regarding the ideal dietary therapy for the management of IBD to reduce the risk of relapse and obstruction and counteract malnutrition, and to construct a food pyramid for patients with IBD.
Materials and methodsThis narrative review was performed following these steps: 1. Configuration of a working group: three operators skilled in clinical nutrition (one acting as a methodological operator and two participating as clinical operators). 2. Formulation of the revision question on the basis of considerations made in the abstract: “the state of the art on management of dietary approach in IBD.” 3. Identification of relevant studies: a research strategy was planned on PubMed [Public MedIine run by the National Center of Biotechnology Information (NCBI) of the National Library of Medicine of Bethesda (USA)] as follows: (a) definition of the keywords (IBD, foods, inflammation, oxidative stress, nutrients, malnutrition), allowing the definition of the interest field of the documents to be searched, grouped in inverted commas (“…”) and used separately or in combination; (b) use of: the Boolean (a data type with only two possible values: true or false) AND operator, that allows the establishments of logical relations among concepts; (c) Research modalities: advanced search; (d) Limits: time limits: papers published in the last 20 years; humans; languages: English; (e) Manual search performed by senior researchers experienced in clinical nutrition through the revision of reviews and individual articles on management of inflammation and oxidative stress by dietary approach in IBD published in journals qualified in the Index Medicus. 4. Analysis and presentation of the outcomes: the data extrapolated from the “revised studies” were collocated in tables; in particular, for each study we specified the author and year of publication and the study characteristics. 5. The analysis was carried out in the form of a narrative review of the reports. At the beginning of each section, the keywords considered and the kind of studies chosen have been reported. We evaluated, as suitable for the narrative review, the studies of any design, which considered the relevance of diet, foods, or nutrients for IBD management.
ResultsThis review included eligible studies, and the dedicated flow-chart is shown in Fig. 1.
Moreover, it is therefore thought to represent graphically, in a simple and intuitive way, what should be proper nutrition for IBD patients, specifying the quality and amount of food, in order to counter the states of chronic inflammation and increased oxidative stress. As shown in Fig. 2, the pyramid is divided into:
- -
foods that should be consumed daily;
- -
foods that must be consumed once, twice, or four times a week;
- -
foods to be eaten occasionally.
The pyramid shows the correct nutrients distribution for IBD patients.
*Only if patients experience repeated and severe symptoms after carbohydrate intake, specific diets can be taken, such as the low-FODMAP (Fermentable Oligo-, Di-, and Mono-saccharides and Polyols) diet, the Specific Carbohydrate Diet (SCD).
**Without lactose.
At the top of the pyramid there are two pennants: one red indicating subjects with IBD requiring some personalized supplementation including vitamin D, omega 3 fatty acids, calcium and probiotics and the other black indicating the complete avoidance of particular food items such as those containing lactose, sweeteners and alcohol.
DiscussionWaterThis research has been carried out based on the keywords: “water intake” OR “drinking water” OR “hydration” AND “colitis” OR “crohn's disease” OR “ulcerative colitis” OR “IBD” AND “water MAP”. As shown in Table 1, six articles were sourced: two review papers, a cohort study, a case–control study, a dietary guideline and a clinical trial.
The onset of CD is favored by environmental factors in genetically susceptible individuals. Some data suggest that habitual drinking of well water, as opposed to tap water, constitutes a risk factor for CD.20 A study from southeastern Norway reported a strong asscoiation between iron content in drinking water and incidence rates of IBD.21 The authors of that study suggested the increase of oxidative stress or the increase in bacterial growth that raise the likelihood of adverse immune responses in genetically predisposed individuals. As stressed by the authors, the main strength of their study is the relatively large population-based cohort living in an area with different water suppliers, and with a daily consumption of drinking water of approximately 2L per person.21 However, as shown by the Mediterranean food pyramid, water should be consumed daily at every meal in large quantities (at least 1.5–2L per day) by all populations and all studies agree with the recommendation to drink adequate amounts of water, little consumption but more times during the day, to improve symptoms in patients with CD and UC.22,23 Nevertheless, the case–control study of Abubakar et al.,24 did not support a role for water or dairy products potentially contaminated with Mycobacterium avium paratuberculosis (MAP) in the etiology of Crohn's disease. Despite initial observation of an association with drinking unboiled water and a negative association with the use of a water filter in the univariable model, the effect was not significant. The authors also examined the risks associated with various water treatment measures, including comparisons of different types of treatment, surface water and groundwater, and animal density and none of them were statistically significant.24 A recent review examining risk factors for Crohn's disease highlights significant associations with the consumption of processed meats and cheeses, while milk consumption and drinking water, direct contact with ruminants and high risk occupations (farmer, veterinarian) are factors not associated with the disease and/or MAP exposure status.25 As such, MAP contamination and risk of IBD is controversial and therefore still under careful scrutiny. In conclusion, concerning the association between water supply and risk of IBD, some data suggest that the drinking of well water, as opposed to tap water, constitutes a risk factor for CD. However, all studies agree with the recommendation to drink adequate amounts (at least 1.5–2L per day) of water in order to improve symptoms in patients with CD and UC. Furthermore, given the background regarding the use of well versus tap water in order to prevent risk of IBD, the use of tap water could be recommended.
CarbohydratesThis research has been carried out based on the keywords: “Carbohydrate” OR “FODMAPs” OR “The Specific Carbohydrate Diet” OR “IBD-Anti-Inflammatory diet” AND “colitis” OR “crohn's disease” OR “ulcerative colitis” OR “MICI”, AND “Gluten free diet” AND “fiber”. As shown in Table 2, 16 articles were sourced: a multicenter prospective study, two pilot studies, a clinical trial, five reviews and a retrospective chart review, a case study and a case control study, a case report and a case series report, a cohort study and a prospective randomized double blinded placebo controlled trial.
In a large multicenter prospective study, Chan et al.,26 found no association between total dietary complex and simple carbohydrates or starch intakes and the development of CD or UC. Similar results were also reported with carbohydrates intake greater than double the recommended daily intake (130mg total carbohydrates per day).27 However, patients with IBD often experience symptoms after carbohydrate intake, and therefore, specific diets have been developed, such as the low fermentable oligosaccharide, disaccharide, monosaccharide, and polyol (FODMAP) diet, the specific carbohydrate diet (SCD) and the IBD anti-inflammatory diet (IBD-AID).28 FODMAPs are a family of fermentable short-chain carbohydrates found in a wide variety of foods and include fructose (a monosaccharide), lactose (a disaccharide), fructans and galactans (oligosaccharides), and polyols. Their ingestion increases delivery of readily fermentable substrate and water to the distal small intestine and proximal colon, which are likely to induce luminal distension and induction of functional gut symptoms.29 There is a high prevalence of carbohydrate malabsorption in patients with diagnosed colectomy and ileoanal pouch anastomosis or ileorectal anastomosis (IRA). In this particular case, a fructose and lactose malabsorption indicates that a low intake of free fructose and lactose should be integral to the dietary approach. Croagh et al.,30 in a pilot study, demonstrated that reduction in dietary FODMAPs intake improved stool output and consistency in UC patients without pouchitis, depending on dietary adherence and baseline diet. Further, the reduction of FODMAP intake offers an efficacious strategy for patients with IBD who have concurrent functional gut symptoms. These dietary changes might play a significant role in the control of abdominal symptoms in IBD patients and the diet has a remarkable uptake by patients, in the long term, with durable apparent benefits.31 In addition, further evidence is provided by other studies showing that a low FODMAP diet enhances relief of global symptoms in the majority of patients with irritable bowel syndrome (IBS) and offers improvement in functional gut symptoms in patients with IBD, which in turn provides a therapeutic option in the treatment of IBD.29,32 A relatively recent case study conducted by Kakodkar et al.,33 also suggests that diet can be an effective treatment for some patients with IBD stems as it has the potential to change the intestinal luminal environment, specifically the intestinal microbiome. The SCD is a dietary approach that is proposed to induce and maintain drug-free remission in patients with IBD. This is not a low-carbohydrate diet, but rather a nutritional plan that is predominantly composed of monosaccharides, solid proteins, fats, a high ratio of amylose to amylopectin in vegetables, fruits, and nuts. Preliminary data have hinted that a change in the intestinal microbiome of IBD patients who follow the SCD may be an important intervention to induce and maintain remission with little or no known adverse reactions.33 Another case report demonstrated easy tolerability and lack of significant side effects of the SCD for an UC patient.34 According to the authors, the patient improved after following the highly restricted diet within a period of 3–6 months, with decreased frequency as well as firmer consistency of the stools, blood in the stools was absent and abdominal pain resolved. Subsequent colonoscopies showed resolution of the pancolitis and a remarkable absence of any inflammation.34 The SCD and other low complex carbohydrate diets may be possible therapeutic options for pediatric patients with CD. These diets may work by altering the dysbiosis to a more favorable bacterial milieu for individuals with IBD.35 Another similar diet protocol is the IBD-AID, a nutritional regimen for IBD that restricts the intake of certain carbohydrates. It is not designed around avoidance of gluten, and strives to address other micro- and macronutrients not addressed in the SCD. Oats and other fermentable grains are included. They are well tolerated and could help in regulation of bowel frequency and consistency, as probiotics use them as substrate. The carbohydrates allowed on the original diet are monosaccharides with a molecular structure that promotes intestinal absorption without additional enzymatic degradation, thus decreasing the risk of mucosal inflammation.36 IBD and celiac disease are two immune-mediated diseases characterized by chronic intestinal inflammation, but they are not strongly correlated with each other or with other immune-related disorders. The common clinical manifestations are probably a consequence of the target organ affected the gut. Celiac disease and IBD share many genetic risk loci and many clinical symptoms, yet IBD patients are not routinely screened for celiac disease.37 Moreover, in a case–control study, patients with concurrent diagnoses of celiac disease and UC were more likely to have pancolitis compared to the non-celiac IBD controls.38 The authors, however, were not sure whether having the two diseases simultaneously lead to worse outcomes. Although celiac disease was not more common in those with CD or UC, co-existing IBD seems to occur more commonly in patients with celiac disease.38 In fact, the prevalence of IBD in celiac patients has been reported to be 5–10 times higher than in the general population. Non-celiac gluten sensitivity (NCGS) is a condition characterized by symptoms due to the ingestion of gluten-containing food in the absence of CD or wheat allergy. Patients with CD and self-reported NCGS seem to be more significantly affected by joint pains compared to UC patients, while in CD patients, a higher percentage of fatigue and headache was evident after intake of gluten.39 In their conclusion, the authors suggestted that a gluten free diet (GFD) might be more useful in CD than in UC. Indeed, the study by Herfarth et al.,40 showed that there was substantial use of a GFD among IBD patients, of whom the majority described an improvement in their gastrointestinal symptoms and disease course. According to the authors of the study, testing GFD in clinical practice in patients with significant intestinal symptoms, which are not solely explained by the degree of intestinal inflammation, has the potential to be a safe and highly efficient therapeutic approach after appropriate testing for celiac disease.40 Moreover, gluten restriction may also be beneficial for patients with symptoms of IBS.41 In summary, no consistent association between total carbohydrate intake and IBD risk was found. However, patients with IBD often experience symptoms after carbohydrate intake, and therefore, specific diets have been developed, such as the low-FODMAP diet, the SCD and the IBD-AID. Scientific literature has yet to accurately ascertain how patients can best benefit from these diets. Consequently, patients are advised to follow such diets only after medical assessment, with appropriate supervision, by a trained nutritionist. IBD and celiac disease do not seem to be more strongly correlated with each other than with other immune-related disorders. However, research on the presence of celiac disease is desirable in all patients with IBD in order to assess whether a GDF is required. Low FODMAP diet, SCD and IBD-AID, as well as making adjustments to carbohydrate intake (3 portions a day) can be recommended as an effective treatment option for some patients with IBD.
Fruits and vegetablesThis research has been carried out based on the keywords: “vegetables” OR “fruit” OR “fruit extracts” OR “vegetable extracts” AND “colitis” OR “crohn's disease” OR “ulcerative colitis” OR “IBD”. As shown in Table 3, fifteen articles were sourced: four reviews, five animal model studies, two clinical trials, a meta-analysis, a report, a population-based study and a cross-sectional study.
Fiber, micronutrients (such as vitamins C and E and folate), and phytochemicals (such as carotenoids, phenolics, isoflavones, and indoles) are abundant in fruit and vegetables. A previous meta-analysis by Li et al.,42 demonstrated inverse associations between intake of vegetables and the risk of UC, and intake of fruit and the risk of UC and CD. The main findings from that study were that: (1) butyrate, the major anion produced by the bacterial fermentation of dietary fiber in the colon, reduced mucosal inflammation by suppreessing the production of nuclear factor-kappa B (NF-κB) in colon cells; (2) microbial translocation across the gut mucosa was influend by dietary fiber; and (3) vitamin A was required for the expression of surface markers such as α4β7 and chemokine receptor type (CCR)9, which can correct homing of the cells to the gut. In addition, the authors proposed that flavonoids might be involved in the maintenance of the intercellular junctional integrity, which is one of the major determinants of the intestinal barrier function, and impairment in intestinal barrier function has been associated with IBD.42 Previous work by Yamamoto et al.,43 has indicated some dietary factors that correlate with the increased incidence of IBD, such as refined sugar, fat and fast food, while fruits and vegetables decreased the risk of CD and UC and fiber decreasing the risk of CD. In contrast to fats, a diet high in fruits and vegetables seems to be associated with a reduced risk of CD more than UC.44 Therefore “Mediterranean” and vegetarian diets are known for their anti-inflammatory effects and could prevent dysbiosis and subsequent IBD.45 The specific consumption of citrus fruit, fruit juices, and vegetables could lower the risk of development of both diseases, and there is an inverse relationship between consumption of bran and the onset of CD. The protective effects of fruit and vegetables can be attributed to their fiber content as well as their micronutrient content.46 Daily fruit intake is protective against UC, irrespective of fiber intake. In fact, the intake of citrus has been shown to be negatively associated with UC and CD risk. There is biological evidence for a positive effect of fruit and vegetable intake on the intestinal detoxifying enzymes, such as glutathione S-transferases, through enhancement and expression of these enzymes. Fiber alone has, in some studies, been shown to be protective against IBD.47 Fruits and vegetables include diverse items, and it is difficult to generalize the impact of these food groups on patients’ symptoms. For example, only bananas are more commonly reported to improve symptoms, whereas leafy and non-leafy vegetables, tomatoes, fruits, nuts, high-fiber foods, seeds, corn and beans were more frequently reported to worsen symptoms.48 Moreover, another observational study in New Zealand categorized foods in relation to their effects on symptoms of CD.49 Melon as a beneficial food has received a limited number of adverse reports. Conversly, grapefruit intake was correlated with a major risk of CD symptoms. Moreover, a small group of root vegetables (boiled potatoes, pumpkin and kumara or sweet potato) mainly showed beneficial effects, with significant numbers claiming symptom reduction and only few reports of adverse effects. Moreover, carrots, yams and parsnips were reported to be nearly as good in terms of beneficial effects. In contrast, no considerable reports on symptom reduction with corn were found, although approximately 50% of subjects reported worsening of symptoms. Among the vegetables, evidence of benefit was lowest for gherkins with broccoli and cauliflower showing contrasting effects.49 The study by Walton et al.,50 supports the idea that fruit and vegetable consumption is recommended, depending on the degree of cooking and fiber content. In fact, low consumption of high-fiber cooked fruit and vegetables, without skins, rather than that of raw and uncooked high-fiber fruit and vegetables is suggested.50 Moreover, extracts from fruits that contain different types of polyphenols, including flavonoids, that could potentially affect the gut ecosystem may be beneficial in symptom control. There is strong evidence that polyphenols from wild blueberries exert a prebiotic effect on Bifidobacterium spp., whereas the inhibition of Clostridiales was reported in high-fat/high-sucrose-fed mice treated with a cranberry extract and with a concord grape extract sorbed to a protein matrix.51 Furthermore, ellagitannins and ellagic acid (EA) found in pomegranate (Punica granatum L.) have been reported to exert numerous biological activites, such as anti-inflammatory and antioxidant properties. Additionaly, Boussenna et al.,52 found in an animal model study that polyphenol-rich red grape pomace extracts consumption exerted protective effects against dextran sodium sulfate (DSS)-induced colitis, and further suggested potential beneficial effects of anthocyanins. Moreover, it has been reported that ginseng berry estract has beneficial therapeutic effects in inhibiting DSS-induced colitis and suppressing immune activation.53
Larrosa et al.,54 evaluated the anti-inflammatory activity of pomegranate extract (PE) supplementation for the treatment of IBD in colitis-induced rat model. The rats were fed with 250mg/kg/day of PE or 15mg/kg/day of microbiota-derived metabolite urolithin-A (UroA) for 25 days prior to inducing DSS-colitis. The anti-inflammatory activity exerted by UroA was relatively stronger than that produced by the PE, whereas PE decreased oxidative stress in plasma and colon mucosa suggesting that the ellagitannin and EA-enriched PE together with some minor amount of UroA could act as a synergic anti-inflammatory cocktail. Also the findings of Rosillo et al.,55 demonstrated, for the first time, that dietary PE and EA reduce the severity and extension of chronic colonic damage in rats. They suggested that inhibition of mitogen-activated protein kinases (MAPKs) and NF-κB signaling pathways by dietary EA and EA-enriched PE could explain the reduced cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) immune signals in colonic mucosa, thereby reducing the development of chronic experimental colitis. The authors of the same study also concluded that dietary supplementation of EA contributed significantly to the beneficial effects observed, thus representing a novel pharmacological strategy to prevent inflammatory responses. The radical-scavenging and anti-inflammatory properties of the flavonoids present in bergamot juice extract (Bje) have raised interest in using the compounds as therapeutic agents in treating IBD. The anti-inflammatory activity of BJe was demonstrated in an experimental model of IBD.56 Moreover, the authors showed that treatment with BJe decreased the incidence of diarrhea, body weight loss and positively modulated colon inflammation.
In conclusion, considering the high contents of fiber, micronutrients and phytochemicals, five portions of tolerated fruit and vegetables can be eaten every day. Moreover, low consumption of skinless cooked fruit and vegetables rather than that of raw and uncooked high-fiber fruit and vegetables is also suggested.
Olive oilThis research has been carried out based on the keywords: “olive oil” or “extravirgin olive oil” AND “colitis” AND “Crohn's disease” AND “ulcerative colitis” AND “IBD” AND “inflammatory bowel disease”. As shown in Table 4, 17 articles were sourced: one review, one survey study, two in vitro studies, eleven animal model studies (one of these was a case–control study) and two human studies, a food survey and a case–control study.
Extra virgin olive oil (EVOO) contains an abundance of phenolic antioxidants including simple phenols (hydroxytyrosol, tyrosol), aldehydic secoiridoids, flavonoids and lignans (acetoxypinoresinol, pinoresinol).57 All of these phenolic substances are potent inhibitors of reactive oxygen species (ROS). The colonic mucosa of cancer patients and those suffering from predisposing inflammatory conditions, such as UC and CD, generates very higher quantities of ROS compared with normal tissue.57 Owen et al.,58 demonstrated that the antioxidant phenolic compounds present in olive oil are potent inhibitors of free radical generation by the fecal matrix. The authors of the same study also demonstrated that combined treatment of hydroxytyrosol, oleic acid and omega-3 fatty acids exhibit huge therapeutic benefits in colitis.58 Another in vitro study on blood and intestinal T cells from IBD patients and healthy subjects, showed that unsaponifiable fraction modulates the activity and the gut homing capacity of T cells, and might therefore be considered as a dietary complement with an anti-inflammatory role in IBD patients.59 Sánchez-Fidalgo et al.,60 in an animal models study, confirmed that hydroxytyrosol may improve chronic colitis through iNOS downregulation plus its antioxidant capacity.60 In particular, hydroxytyrosyl acetate could be used as a supplement in order to prevent UC through its anti-inflammatory effects.61 It has also been demonstrated that bioactive components present in the unsaponifiable fraction of EVOO have favorable properties in colitis in mice.62 In a series of studies, Spanish researchers from the University of Seville observed a positive effect of EVOO on colitis. In their first study, Sánchez-Fidalgo et al.,63 confirmed that EVOO has protective/preventive effects in UC-associated colorectal cancer, with a better disease activity index (DAI), a minor number of dysplastic lesions, a lower β-catenin immunoreactivity, a reduction in proinflammatory cytokine levels, a non-modification of p53 expression and, COX-2 and iNOS reduction in the colonic tissue. This same group followed that study with another study designed to evaluate the protective effect of dietary EVOO polyphenol extract supplementation in a chronic DSS-induced colitis model.65 The authors demonstrated that EVOO polyphenol extract supplementation possessed marked protective effects on experimental colitis through peroxisome proliferator-activated receptor-γ (PPARγ) up-regulation and NF-κB and MAPK signaling pathway inhibition, thus decreasing the inflammatory cascade.64 De Coffee et al.,65 explained that not all high-fat diets aggravate colitis, as evidenced by the reduced susceptibility of infected, olive oil-fed mice to acute colitis. Another study examined the effect of different dietary oils on the severity of chronic colitis, development of colitis-associated premalignant changes, and colonic expression of COX-2 in interleukin-10 knockout (IL-10−/−) mice.66 The authors of that study suggested that olive oil inhibits COX-2 immunostaining and decreases the risk of neoplasia associated with chronic colitis. Furthermore, Takashima et al.,67 reported that chronic feeding of 5% EVOO inhibited chronic inflammation, attenuated cell proliferation and recovered apoptosis in a DSS-induced colitis mouse model. They explained that EVOO was able to attenuate the expression of signal transducer and activator of transcription–3 (STAT3), phosphorylated STAT3, COX-2 and iNOS. In addition, EVOO attenuates increases in cell proliferation caused by DSS and recoveres decreases in apoptosis (cleaved caspase-3).67 Camuesco et al.,68 suggested that administration of the flavonoid and the use of olive oil and omega-3 polyunsaturated fatty acid (PUFA) to diet in rats could be used for the treatment of these intestinal inflammatory disorders. The same authors confirmed that the anti-inflammatory effect of olive oil was enhanced after omega-3 PUFA incorporation into the olive oil-based diet, thus modifying the omega-6/omega-3 PUFA ratio in colonic tissue. This dietary combination could result in a synergistic effect in the management of IBD because other proinflammatory mediators such as LTB4 and TNF-α are also downregulated.69 There is only one article which gave abnormal results: here, partial replacement of soybean oil with olive oil had unfavorable effects on the incidence and severity of experimental UC.70 The study of D'Souza et al.,71 suggested that specific dietary patterns could be associated with higher or lower risks for CD in children. In particular, consumption of olive oil, with other beneficial elements such as vegetables, fruits, nuts, fish and grain, was inversely associated with decreasing the risk of developing CD in both genders.71 Conversely, another study by Octoratou et al.,72 showed that increased consumption of olive oil was associated with an increased risk of developing CD. A possible explanation for their observation is the increased consumption of fried food and the decreased consumption of vegetables that has antioxidaant properties.72 In summary, several studies have demonstrated that dietary polyphenols possess protective and therapeutic effects in the management of IBD mediated via down-regulation of inflammatory cytokines and enzymes, enhancing antioxidant defense, and suppressing inflammatory pathways and their cellular signaling mechanisms.73
In conclusion, considering its high antioxidant content, EVOO is recommended to be consumed raw 2 times a day in average doses of 20–40g for optimum health benefits.
Yogurt and fermented milkThis research has been carried out based on the keywords: “yogurt” OR “fermented milk” AND “colitis” AND “Crohn's disease” AND “ulcerative colitis” AND “IBD” AND “inflammatory bowel disease”.
As shown in Table 5, a total of 16 articles were sourced: one article and one review, two in vitro studies, five animal model studies and seven humans studies (two of which were food surveys, plus four clinical trials and two randomized placebo controlled trials).
Gut microbiota has a primary role in priming and regulating mucosal and systemic immunity and the immune system also contributes to host control over microbiota composition and is evident in CD and UC.74 Deviation of the normal intestinal microbiota composition, dysbiosis, is commonly observed in CD or UC patients. This condition is often characterized by an increased relative abundance of facultative anaerobic bacteria (e.g., Enterobacteriaceae, Bacilli) and a reduction of obligate anaerobic bacteria of the classes Bacteroidia and Clostridia. Until now, it is unclear whether dysbiosis is a cause or a consequence of IBD.74 The intestinal microbiota interacts with human health and its modulation by dietary constituents, and in particular probiotics and prebiotics, is an interesting way to prevent or treat some diseases, such as IBD.75 There are various foods that are associated with a lower risk of CD development. One of these are represented by milk and yogurt.72
Yogurt is a fermented milk made with a starter culture consisting of different probiotics that could be colonized the intestine.76 Sheikhi et al.,76 in an in vitro study conducted on peripheral blood mononuclear cells (PBMC) from UC patients treated with Bifidobacterium lactis BB-12 and Lactobacillus acidophilus LA-5, showed that both probiotics have a pivotal impact on immune system of UC patients because they modulate cytokine secretion. Similarly, Imaoka et al.,77 demonstrated how probiotic Bifidobacterium strains in Bifidobacteria-fermented milk (BFM) enhances interleukin (IL)-10 production in PBMC and inhibits IL-8 secretion in intestinal epithelial cells, suggesting that BFM has anti-inflammatory effects against UC.77 In a murine model, Veiga et al.,78 analyzed the effects on the microbiota following the ingestion of BFM. They found that there was a decrease in cecal pH and modifications in short chain in the fatty acids profile, in addition to an increase in the abundance of select lactate-consuming and butyrate-producing bacteria. These metabolic changes created a non-permissive environment for Enterobacteriaceae in the UC mouse model.78
Also in mice, yogurt exerted a beneficial effect on acute intestinal inflammation in UC and CD by regulating T-cell expansion and modulating the expression of Toll-like receptors (TLRs), with a decrease of TLR4(+) and increase of TLR9(+) cells.79 Yogurt administration during the remission phase prevented the recurrence of inflammation without producing undesirable side effects. The yogurt effect may be mediated by increased IL-10 production and changes in intestinal microbiota, as demonstrated by Chaves et al.,79 in a mouse model. Moreover, Gobbato et al.,80 demonstrated the anti-inflammatory effect of yogurt with Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus in an experimental mouse model of IBD induced by trinitrobenzene sulfonic acid (TNBS). The effect was mediated by an increase in the number of the imunoglobulin (Ig)A+ cells, a decrease in CD8+ population and by the induction of apoptosis of the infiltrative cells in the large intestine. Fermented-milk also represented a good substrate due to its inflammation lowering properties, as indicated by Saraiva et al.81 The authors of that study demonstrated that 15-lipoxygenase-1 (15-LOX-1) producing Lactococcus lactis was effective in the prevention of intestinal damage associated with IBD in a murine model. They also confirmed previous reports showing that fermented milk is an effective form of administration of recombinant lactic acid bacteria expressing beneficial molecules.81 Mice that received milk fermented by L. lactis strains producing IL-10 in the cytoplasm or secreted to the product showed lower damage scores in their large intestines, decreased interferon (IFN)-γ levels in their intestinal fluids and lower microbial translocation to the liver, compared to mice receiving milk fermented by the wild-type strain or those not receiving any treatment. Del Carmen et al.,82 showed that the employment of fermented milks as a new form of administration of IL-10-producing L. lactis is effective in the prevention of IBD in a murine model of CD.82
In light of these interesting in vitro and animal model studies, much research has been performed in humans with yogurt or fermented milk. Probiotic yogurt intake was associated with significant anti-inflammatory effects that paralleled the expansion of peripheral pool of putative Treg cells in IBD patients compared to few effects in controls, as reported by Boroja et al.83 In their study, 20 healthy controls and 20 subjects with IBD (15 with CD and 5 with UC) received probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 supplemented yogurt for 30 days. In another clinical trial, 500ml of a fermented milk product (Cultura) containing live Lactobacilli (La-5) and Bifidobacteriae (Bb-12) was given daily for 4 weeks to 51 UC patients and 10 patients with familial adenomatouspolyposis, operated on with ileal-pouch-anal-anastomosis (IPAA), and six UC patients operated on for IRA. The median endoscopic score of inflammation was significantly decreased during intervention in the UC/IPAA patients.84 Furthermore, a randomized controlled study examined the effects of a synbiotic in UC patients that was made up of live Bifidobacterium breve strain in BFM and galacto-oligosaccharide (GOS). The authors reported that after one year of treatment, the clinical status of the UC patients, assessed by colonoscopy, had significantly improved.85 The same authors underlined that supplementation with BFM product was successful in maintaining remission and had possible preventive effects on the relapse of UC. They reported exacerbation of symptoms in 3 out of 11 subjects in the BFM group and in 9 out of 10 in the control group. Moreover, the analysis of microflora and the organic acids in feces showed a significant reduction in the relative proportion of Bacteroides vulgatus in Bacteroidaceae and butyrate concentration, respectively, after supplementation with BFM, in comparison with before.86 Likewise, a randomized placebo-controlled trial of BFM supplementation as a dietary adjunct was conducted to treat active UC in 20 patients. Clinical and endoscopic activity indices and histological scores were similar in the two groups before treatment. Although improvements were significant in both groups, the clinical activity index was significantly lower in the BFM than in the placebo group after treatment. Furthermore, the post-treatment endoscopic activity index and histological scores were significantly reduced in the BFM, but not the placebo group. The authors reported increases in fecal butyrate, propionate and short-chain fatty acid concentrations in the BFM group.87
In summary, several in vitro and animal model studies have demonstrated that yogurt and fermented milk are rich sources of bioactives with immunomodulatory properties that are useful in preventing or treating IBD. Further, clinical research in subjects affected by IBD confirmed that yogurt and fermented milk have a pivotal impact on the immune system and, consequently, on maintaining remission and reducing the clinical activity index of IBD patients. Daily intake of two servings of yogurt or fermented milk (125ml), as breakfast or as a snack, is beneficial for patients with IBD. In addition, yogurt and fermented milk are lactose free and rich in calcium, which is essential in preventing loss of bone mass that is frequent in patients suffering from IBD due to low calcium intake (see section on dietary supplementation).
In conclusion, considering that yogurt and fermented milk are rich sources of calcium and bioactives with immunomodulatory properties, daily intake of two servings of yogurt or fermented milk (125ml) is reccomeded.
FishThis research has been carried out based on the keywords: “fish” or “long-chain n-3 PUFA” AND “colitis” or “crohn's disease” or “ulcerative colitis” or “IBD” or “inflammatory bowel disease”. As shown in Table 6, seven articles were sourced: one in vitro–vivo study, two animal model studies, and four humans studies (three case controls and one prospective study).
A high intake of dietary long-chain n-3 PUFA, the fat of fish, may be associated with a reduced risk of UC.88 A case control study performed by Rashvand et al.,89 reported that higher consumption of total fats, oleic acid, saturated fatty acids, total PUFAs, trans fat, monounsaturated fatty acids (MUFAs), and linoleic acid were significantly associated with increased risk of UC. However, no statistically significant associations were detected between the risk of disease and omega-3 PUFAs and cholesterol intake.89 It is obvious that a diet rich in MUFAs, saturated fatty acids, and omega-3 PUFAs, with limited omega-6 PUFAs is recommended to maintain defensive inflammatory and mucosal responses to enteric infection while mitigating the damaging effects of inflammation.65 Okada et al.,90 in their study examined the effects of diet containing large amounts of trans fatty acids (TFAs) on mice with induced colitis and showed that TFA diet significantly elevated IL-6, IL-12p40, IL-23p19 and retinoic acid-related orphan receptor (ROR)γt mRNA levels in the colons of DSS-treated animals. Moreover, IL-17A mRNA levels were elevated significantly by the TFA diet, with or without DSS treatment. They also examined the expression of proinflammatory cytokines in lipopolysaccharide (LPS)-stimulated RAW264.7 cells and peritoneal macrophages, that were up-regulated.90 Similarly, a group of mice were fed on a mixture of soybean oil, and either a low fish oil diet or a high fish oil diet for 24 days. The results showed that diets with an omega-6/omega-3 PUFA ratio of 2:1 or 4:1 regulates the helper T cells (Th)/regulatory T cells (Treg) balance and attenuates inflammatory mediator production in colitis.91
In the case of CD, there is a synergism between fat intake and single nucleotide polymorphisms in apoptotic genes in modulating disease activity; both are known to affect the rate of apoptosis in inflammatory cells which is known to be a defect in the development of CD.92 In the study of Eaton et al.,93 patients with primary sclerosing cholangitis, regardless of IBD status, were less likely to consume fish. A diet rich in fish has been associated with decreased risk of immune mediated conditions, probably for high concentration of omega-3 fatty acids.93
In conclusion, considering the content in omega 3, the intake of 4 portions of fish weekly is reccomended.
LegumesThis research has been carried out based on the keywords: “legumes” OR “beans” OR “pea” OR “cowpea” AND “colitis” OR “crohn's disease” OR “ulcerative colitis” OR “IBD”.
As shown in Table 7, five articles were sourced: three mice model studies, a clinical trial and an in vitro study.
Common legumes are rich in dietary fiber, starch, protein and phenolic compounds with demonstrated antioxidant and anti-inflammatory potential, which may help alleviate intestinal diseases, including IBD.
Triggs et al.,49 evaluated different types of legumes in order to demonstrate their beneficial or adverse effects on symptoms of IBD. Their results showed that the effects ranged from approximately 15% reports of symptom enhancement but ca. 9% reports of symptom reductions for green beans, to 40% of individuals claiming symptom enhancement and only 5% reporting that they can tolerate baked beans. Moreover, an animal model study showed the preventive effects of two pea (Pisum sativum) seed albumin extracts, either in the presence or absence of soluble polysaccharides, in DSS-induced colitis in mice. The intestinal anti-inflammatory effect of two pea seed albumin extracts (PSE and AF-PSE) ameliorates DSS-induced damage to mice. This beneficial effect was demonstrated histologically by an amelioration in the intestinal mucosa and preservation of the epithelial integrity, and by a clear improvement of the gene expression of many of the inflammatory markers evaluated, including proinflammatory cytokines, inducible enzymes, chemokines, and adhesion molecules, all of them clearly involved in the pathogenesis of IBD.94
In addition, another study investigated the effect of cooked whole-bean flours, with various phenolic compounds, in a mouse model of acute colitis. The authors of that study reported that bean-containing diets exerted both beneficial and adverse effects during experimental colitis through the reduction of inflammatory biomarkers both locally and systemically while aggravating colonic mucosal damage. Currently, there is no evidence to suggest that cooked bean-supplemented diets would induce toxic or adverse effects in the colon. Although raw beans are known to contain a number of anti-nutritional components (i.e., lectins and protease inhibitors) that may adversely affect gut health.95 Results from an in vitro study support evidence of the anti-inflammatory potency of cowpea (Vigna unguiculata L. Walp.) polyphenolics in modulating the level of inflammatory markers relevant to colon inflammation. Overall, cowpea polyphenolics extracts inhibit the generation of ROS and inflammation.96
Finally, during colitis, it seems that bean consumption reduces disease severity and colonic histological damage, increases gene expression of barrier function promoting genes (Muc1-3, Relmβ, andReg3γ) and reduces colonic and circulating inflammatory cytokines (IL-1β, IL-6, IFNγ and TNF-α). Therefore, prior to disease induction, bean supplementation enhances multiple concurrent gut health promoting parameters that translates into reduced colitis severity. These data demonstrate a proof-of-concept regarding the gut-priming potential of beans in colitis, which could be extended to mitigate the severity of other gut barrier-associated pathologies.97
In summery, even if in animal model an increased bean consumption may represent a dietary strategy to improve gut health during times of disease remission and to reduce the severity of relapse in mucosal damage-associated pathologies, in humans the effects of legumes intake range from approximately 15% reports of symptom enhancement but ca. 9% reports of symptom reductions for green beans, to 40% of individuals claiming symptom enhancement and only 5% reporting that they can tolerate baked beans. Consequently, further research is required to understand the mechanisms through which beans exert their effects on colonic inflammation and the impact on colitis severity in human subjects.
In conclusion, the advice is to evaluate whether legumes are tolerated, considering various types of cooking methods and the possible consumption of dehulled or smoothies legumes, if they are tolerated, can be consumed 2–3 times a week.
EggsThis research has been carried out based on the keywords: “eggs” AND “colitis” or “Crohn's disease” or “ulcerative colitis” or “IBD” or “inflammatory bowel disease”.
As shown in Table 8, eight articles were sourced: one review, three animal studies and four human studies (of which one was a prospective study, one clinical study and two double-blind randomized placebo controlled trials).
Eggs are actually recognized as a functional food and contain a variety of bioactive compounds that can influence pro- and anti-inflammatory pathways (e.g., egg phospholipids, cholesterol, the carotenoids lutein and zeaxanthin, and bioactive proteins).98 Shi et al.,99 in in vitro and in vivo studies showed that eggshell membrane is a well-known natural bioactive material and could be used in the treatment of IBD, because it attenuates the severity of intestinal inflammation via down-regulation of the anti-inflammatory cytokine, IL-10.
In her review, Andersen underlines that individuals with UC have lower levels of phosphatidylcholine in the gastrointestinal mucus layer, and supplementation of phosphatidylcholine has positive clinical outcomes and in a porcine model of DSS-induced colitis, hen egg lysozyme supplementation reduced intestinal gene expression of pro-inflammatory cytokines (TNF-α, IL-6, IFN, IL-8, IL-17) while increasing expression of anti-inflammatory IL-4 and transforming growth factor β(TGF-β). Ovotransferrin, an iron-binding glycoprotein with antibacterial activity, has additionally been shown to reduce inflammatory colitis pathology in a DSS-induced mouse model of colitis. Oral administration of egg ovotransferrin reduced inflammatory cytokines, while additionally mitigating clinical markers of colitis, including weight loss and histological scores of the colon.98,99
Phosphatidylcholine has an anti-inflammatory effect in ulcerative colitis.100 The study of Stremmel et al. demonstrates that retarded release oral phosphatidylcholine is effective in alleviating inflammatory activity caused by UC in patients with chronic active, non steroid dependent, UC, with an improvement of clinical activity index (CAI) of 90%.100 A low amount of protective mucus phosphatidylcholine is a characteristic feature in UC and explains an increased susceptibility to luminal contents and phosphatidylcholine reduced corticosteroid dependence more than placebo in patients with chronic steroid-refractory UC.101,102
In the study of Kobayashi et al.,103 in mice, a supplementation of 50 or 250mg/kg body weight of ovotransferrin significantly reduced clinical signs, weight loss, shortening of the colon, and inflammatory cytokine markers of colitis. In mice with induced colitis, freeze-dried egg yolk can be used as a source of exogenous antisecretory factor (AF), that has anti-inflammatory properties: as results, AF-treated mice showed milder colonic damage compared with the vehicle group and thus, administration of AF-rich egg yolk has a therapeutic effect in the late phases of TNBS colitis in Balb/c mice.104 Considering the role of foods in the etiology of IBD in a large prospective cohort of French middle-aged women67,105 high consumption of eggs was not suspected as a risk factor.106 In summary, because all the studies on animal models with induced colitis agree that there is an advantage, thanks to various anti-inflammatory mechanisms, in consuming eggshell membrane or phosphatidylcholine by egg101,102 or hen egg lysozyme98 or ovotransferrin98,103 and because has been shown in a large cohort of middle-aged women that taking the eggs is not a risk of developing IBD, eggs can be eaten freely (4–6 eggs/week).
In conclusion, 4–6 eggs/week, preferably soft boiled or boiled or omelet baked, never fried are to eat.
MeatThis research has been carried out based on the keywords: “meat”, or “saturated fatty acids” AND “colitis” or “crohn's disease” or “ulcerative colitis” or “IBD” or “inflammatory bowel disease”.
As shown in Table 9, eleven articles were sourced: one animal study, one meta-analysis, one review, one systematic review, seven human studies of which two were cross-sectional studies, two case–control, and three prospective studies.
A meta-analysis that included nine studies, published in 2015, revealed that meat consumption may increase the risk of IBD and that this association is greater for red meat than white meat or processed meat. The authors underline that there was a significant association between total meat intake and UC, but not with CD, probably because of the heterogeneity among studies.107 The explanation of this phenomenon could be high temperature, which creates chemical products that influence digestive tracts, or heme iron, in red meat, which promotes the formation of N-nitroso compounds, which subsequently influences cell proliferation in the digestive tract. Nitrites, which are mainly found in processed meats, create nitrosylating agents that react with amines or amides under certain conditions. Finally, the authors of the meta-analysis underline the great amount of fat and omega-6 fatty acids presented in these foods.107
Intestinal microbiota could be changed from different cooking processes, as demonstrated by Eaton et al.93 The authors reported that cooking steaks or burgers so they are more well done was associated with primary sclerosing cholangitis (PSC) and that this cooking practice has been shown to increase the presence of dietary advanced glycoxidation end products (AGEs). Stimulation of receptors for AGE (RAGE) can lead to production of pro-inflammatory cytokines, vascular adhesion molecules, and increase vascular permeability. Furthermore, AGE has also been shown to stimulate RAGE gene expression, exacerbate oxidative stress and increase hepatic fibrosis.93 The authors also observed a negative association between grilled or barbecued meat (chicken, steak or burger).93 Yet the frequency of chicken, steak or burger consumption was not associated with PSC unless the method of food preparation was taken into account.93
Curiously, co-consumption of resistant starch and red meat seems to be beneficial in mice, as demonstrated by the study of Le Leu et al.108 probably because of the change in microbiota. Moreover, Vidarsdottir et al.109 reported that people affected by CD or UC avoided some foods, including processed meat and meat, whereas foods that seem to have positive effects on symptoms were fish, non-processed foods, and chicken. However, patients who reduced meat intake had lower blood values of ferritin compared to others (but iron intake was not significantly different between patients who restricted meat and meat products in comparison with those who did not).109
These animal products are rich in fats. High dietary intakes of total fats, PUFAs, omega-6 fatty acids, and meat were associated with an increased risk of CD and UC.27 In particular, a cohort study, performed by Jantchou et al.,106 demonstrated that a high intake of meat and fish, but not eggs or dairy products, increased risk of IBD in women. This was also confirmed by Andersen et al.,110 who noted a high risk of relapses. Similarly, Tasson et al.,111 in their study found a positive association between meat intake and disease relapse, in remission patients. The work by Jowett et al.,112 had specified the kind of meat that was associated with high risk of IBD, mainly red meat and processed meat increased the likelihood of relapse compared with the bottom tertile of intake. In this cohort study, the authors discussed about meat in general, but they underlined the role of red and processed meats, but not white meat, probably because these foods are rich sources of sulfur and sulfate which increase the concentration of fecal hydrogen sulfide that is toxic to the colonocyte.112 However, not all studies are consistent. A recent study observed that processed meats are not associated with an increased risk of flare.113 White meat is inserted in the group of “prudent” food by Maconi et al.,114 highlighting how it is not as dangerous as red or processed meat, but at the same time, it is not a healthy food.
In summary, in regard to the risk of IBD, the literature agrees that a Western diet, including meat consumption, seem to be involved in the increased occurrence of the disease.
In conclusion, white meat should be 3 portions per week, while the intake of red meat and processed meat should be an exception. The cooking method is also important: more delicate cooking (e.g., steam) is preferable.
Dairy productsThis research has been carried out based on the keywords: “dairy products””, or “milk” or “cheese” AND “colitis” or “crohn's disease” or “ulcerative colitis” or “IBD” or “inflammatory bowel disease”.
As shown in Table 10, sixteen articles were sourced: one review, one systematic review and meta-analysis, one in vitro study, one animal study, one dietary survey, eleven human studies (of which two case–control, three clinical studies, one cross-sectional, three cohort studies and two clinical trials).
Dairy products are common components of a Western diet and are being increasingly consumed in developing nations.115 The scientific opinion is contradictory. An European Prospective Cohort Investigation was performed by Opstelten et al.; a total 401,326 participants were enrolled and those who consumed milk had significantly reduced odds of CD, compared to people who did not consume it. However, the clear dose-response relationship was not well established. The study also found non-significant reduced odds of UC.115
Milk fat globule–epidermal growth factor 8 (MFG-E8) plays an important role in maintaining intestinal barrier homeostasis and accelerating intestinal restitution. MFG-E8 mRNA and protein expression was lower in ulcerative colitis patients than in controls. MFG-E8 expression was inversely correlated with mucosal inflammatory activity and clinical disease activity in patients. Apoptosis induction was also detected in the intestinal epithelium of ulcerative colitis patients by terminal-deoxynucleoitidyl transferase mediated nick-end labeling assay.116 Different dairy components, such as fats or proteins may exert disparate effects, emphasizing that the exact mechanisms underlying these associations are still unclear.115
Generally, dairy products contain different bioactive nutrients that can influence inflammatory status.117 Unlike saturated fatty acids (FAs) that activate proinflammatory markers, protein and amino acid composition and magnesium may have beneficial effects on inflammatory profile; on the contrary, studies on vitamin D demonstrate conflicting results.117 Milk fat contains short-chain SFA (C4:0 to C8:0, 3–7% of total FA) and medium-chain SFA (C10:0 to C14:0, 11% of total FA). In human primary adipocytes, Lauric Acid had no effect on IL-6, TNF-α, and MCP-1 levels and did not activate the TLR-2 and TLR-4 in cells, but in other study lauric and myristic acids (MA, 14:0) increased MCP-1 gene expression in 3T3-L1 adipose cells. Overall, short- and medium-chain FA may improve or at least have no effects on inflammatory profile.117
On the other hand, some studies explain the negative role of milk on IBD, because of the change in microbial profile that can perturb immune homeostasis and increase incidence of colitis as explained by Devkota et al.: they performed a study in mice in which consumption of a diet high in saturated (milk derived)-fat (MF), but not polyunsaturated (safflower oil)-fat (PUFA), changes the conditions for microbial assemblage and promotes expansion of a low abundance, sulfite-reducing pathobiont, Bilophila wadsworthia.118 In association it occurred a pro-inflammatory TH1 immune response and increased incidence of colitis in genetically susceptible mice because of MF-promoted taurine-conjugation of hepatic bile acids, which increased the availability of organic sulfur used by sulfite-reducing microbes like B. wadsworthia.118
However, the restriction of cow's milk and dairy products (or the substitution with soy milk) is common in IBD patients, probably because of the exacerbation or onset of symptoms, but another explanation could be the development of lactose intolerance.119 However, the same authors underline that the frequency of gastrointestinal symptoms was higher among the CD patients who restricted dairy products compared with those with no restrictions, but the same result was not observed in UC patients.119 Some factors must be evaluated before the patient restricts dairy products, such as the fat present in these foods, the amount of lactose consumed, the residual activity of intestinal lactase, the ability of the colonic flora to ferment lactose and individual sensitivity to the products of lactose fermentation.119
In Canadian IBD patients, up to 15% of them avoided foods on the basis of professional advice, with milk and milk products being the item most typically avoided based on a health professional's advice.120 Some authors suggest supplementation with whey protein as an alternative to soy protein in CD patients, in order to reduce body fat and thus could contribute to reduction of inflammation.121
Curiously, cow's milk allergy (CMA) in infants is a risk factor for contracting pediatric IBD and asthma for Crohn's disease, as underlined by Virta et al.122 The same author confirms this result in another study, underlining the possibility that in genetically predisposed infants, dietary modification due to symptoms suggestive of CMA may influence gut microbiome and immune responses and favor the development of IBD.122
In adults, there is a significant relationship between UC and intake of cow milk, cow milk UHT and casein, measuring levels of anti-body against dairy protein, because food allergy is one of the problems that can arise with ulcerative colitis.123 In UC patients allergic to dairy products, the use these products can increase the severity of UC.123 However, Strisciuglio et al. have proposed a cow's milk protein elimination diet for children with UC, but the result did not support this proposal/theory.124
In China, milk and foods containing cow milk are associated with increased risk of IBD, because of common cow's milk protein allergy (IgE-mediated) may induce the abnormal immune response of digestive tract that increases the relative risk of IBD.125
While lactose intolerance may occur as a result of small intestinal inflammation, causing loss of brush-border lactase activity, there is no specific information on why lactose avoidance was recommended or which type of health professional was providing this guidance.120 Lactose sensitivity occurred in a much higher proportion of patients, (approximately 70%), with IBD than previously thought.126 In a recent systematic review and meta-analysis, IBD rates were found to be different between populations of Canada and New Zealand, because Indigenous people are predominantly lactose non persistent (LNP) and Caucasians are predominantly lactose persistent (LP). However, there is no certain relationship between IBD and dairy foods, but their restriction has a negative impact: nutritional consequences of dairy restrictions might adversely impact bones.127 In the study of Eadala et al., patients were in remission, so the lactose sensitivity cannot be attributed to any ongoing major inflammation. Overall, the authors suggest the “bacterial metabolic toxin” hypothesis for which Archea could be implicated in the pathogenesis through activation of immune responses, movement of microbial cells along nerve axons or generation of peptide and metabolic toxins.126 In humans, the study performed by Nolan-Clark et al. revealed that some dairy products (cream, ice cream and cheese) that have a high fat content were most frequently reported to worsen perceived CD symptoms, as opposed to others foods such as butter, standard cow's milk and reduced fat's cow milk. However, the lactose content of dairy products did not influence self-reported CD symptoms for the majority of patients.128 But authors emphasize that there is a highly individual nature in these conclusions.128
MAP has long been suspected as an aetiological agent of Crohn's disease in human.129 Common methods of pasteurization are not enough to kill all MAP present in the milk and the bacterium has been isolated from raw milk, pasteurized milk and cheese samples.130 The addition of a starter culture could restrict the persistence of Map in ultra-filtered white cheese.129 Numerous studies on MAP and IDB risk are currently underway.
In conclusion, it is always useful to evaluate the presence of lactose intolerance (diagnosed by breath test) and allergy to milk proteins; in the presence of lactose intolerance, patients may take seasoned cheeses, which do not contain lactose. In case of allergy to milk proteins, milk and derivatives must be excluded from the diet and it is therefore necessary to supplement the diet with adequate amounts of calcium.
Nuts and dried fruitsThis research has been carried out based on the keywords: “dried fruit””, or “hazelnuts” or “pistachios” or “almonds” or “nuts” or “walnuts” or “peanuts” or “cashew” AND “colitis” or “crohn's disease” or “ulcerative colitis” or “IBD” or “inflammatory bowel disease”.
As shown in Table 11, eleven articles were sourced: four animal studies, two review, one review and meta-analysis, two dietary survey and two studies in humans (one cohort and one prospective study).
Dried fruit is a group of foods that includes nuts, almonds, pistachios, hazelnuts, walnuts, peanuts and cashews.
An important and recent study was made by Gholami et al, and evaluated the effects of Pistacia atlantica (P. atlantica), butyrate, Lactobacillus casei (L. casei), and especially their combination therapy on mice with colitis. As a result, even if a single treatment with each compound demonstrated efficacy in the reduction of oxidative stress, the combination therapy was still better, with significant alleviation of colitis in terms of pathological scores and reduction of myeloperoxidase (MPO) activity (55%, P=0.0009) and improvement of edema, necrosis and neutrophil infiltration.131 The same food was used by Tanideh et al. for their study on rats: high doses of P. atlantica fruit oil extract, administered orally and rectally, can improve colitis physiologically and pathologically in a rat model, and may be efficient for ulcerative colitis.132 Another study in rats used a particular type of dried fruit, called Terminalia chebula (TCE). TCE indicated the presence of active principles with proven antioxidants, anti-inflammatory, immunomodulatory, and free radical scavenging and healing properties.133 Mandalari et al. have investigated the effect of natural almond skin (NS) powder in mice with to experimental colitis,134 as a result, there was a reduction in the occurrence of diarrhea and body weight loss. This was associated with a significant reduction in colonic MPO activity. NS powder also reduced NF-κB and p-JNK activation, the pro-inflammatory cytokines release, the appearance of i-NOS, nitrotyrosine and PARP in the colon and reduced the up-regulation of ICAM-1 and the expression of P-selectin.134
However, despite these promising studies in animal models, in humans, considering self-reported food-related gastrointestinal symptoms in IBD, nuts are important triggers of GI symptoms48,49,120 as Octuratou demonstrated in a prospective study that nuts significantly decrease the risk for development of Crohn's disease.72
Nuts were frequently reported to worsen GI symptoms, probably due to the high fiber content.135
In conclusion, nuts represent a symptom-provoking food in IBD patients; however, since the oily nuts are rich in monounsaturated fatty acids (in particular hazenuts), antioxidant and mitigating chronic pro-inflammatory processes bioactive substances, like extravirgin olive oil136,137 as demonstrated by studies in animal models131–134 it might be interesting to review the intake of oils derived therefrom, such as hazelnut or pistachio or almond oil (2, 3 times a week, as a cold dressing).
SpicesThis research has been carried out based on the keywords: “IBD” or “colitis” or “crohn's disease” or “ulcerative colitis” AND “spieces” or “thyme” or “ginger” or “curcumin” or “glabridrin” or “Malva sylvestris” or “piperine” or “cumin” or “garlic oil” or “Rosmarinus officinalis” or “saffron” or “botanicals”.
As shown in Table 12, thirty-six articles were sourced: eight review, sixteen animal studies, one paper, one in vitro study, ten humans study of which four open-label studies, four clinical trials, one case–control and one prospective trial.
All reviews on pre-clinical studies agree to show that spices, in particular ginger, curcumin, rosmarine officinalis, saffron, cumin, garlic, piperine, and botanicals, such as Boswellia serrata, Andrographis paniculata, Plantago lanceolata, Tormentilla erecta-Rosaceae, possess beneficial effects in preventing/ameliorating IBD.138–142 Botanical therapies exert their therapeutic benefit by different mechanisms, including immune regulation, antioxidant activity, inhibition of leukotriene B4 and NF-κB, and antiplatelet activity.138
However, there are studies showing no positive results. The reasons are probably related to poor design of the studies, the small number of patients included, the variety of substances tested, the inadequate amount of botanicals and the not adequate analysis and description of the results.143,144
Several studies demonstrate that the rhizome of Ginger has biological activities such as cytotoxic, antioxidant, and anti-inflammatory effects.145 Ginger extracts improve colitis thanks to a reduction in the levels of NF-κB and IL-1b.140 Ginger extracts showed anti-inflammatory and anti-oxidant properties in rats, improving malondialdehyde (MDA), protein carbonyl (PCO), and reduced glutathione (GSH) with the catalase (CAT) and superoxide dismutase (SOD) activity, myeloperoxidase (MPO), TNF-α, and prostaglandin E2 (PGE2).145 Another study demonstrated that Ginger volatile oil reduced the colon weight/length ratio in rats and could effectively reduce symptoms of experimental colitis.146 Zerumbone (ZER), significantly lowers the levels of IL-1beta, TNF-α, and PGE2 and suppressed DSS-induced colitis in mice.147 Administration of 6-gingerol significantly reversed the DSS-mediated reduction in body weight, diarrhea, rectal bleeding, and colon shrinkage to near normal levels, significantly suppressed the circulating concentrations of IL-1β and tumor necrosis factor alpha, and restored the colonic nitric oxide concentration and myeloperoxidase activity to normal in mice. 6-gingerol suppressed the induction of ulcerative colitis in mice via antioxidant and anti-inflammatory activities.148
Finally, preclinical studies have shown that pretreatment with ginger extract ameliorated acetic acid-induced edematous inflammation in the colon by significantly attenuating the extent and severity of edema, necrosis, and inflammatory cell infiltration in the mucosa. The activity of colonic MPO and levels of lipid peroxides, protein carbonyl content, TNF-α, and PGE2 were also decreased. Ginger administration restored the levels of GSH, catalase (CAT), and SOD. The protective effect of the highest dose of ginger was comparable to that of the standard sulfasalazine.145
Isik et al. conducted a study in rats in order to evaluate the effects of cumin. As result, Black cumin oil, by preventing inflammatory status in the blood, partly protected colonic tissue against experimental ulcerative colitis, because it decreased the proinflammatory cytokines, lactate dehydrogenase, triglyceride, and cholesterol, which were increased in colitis.149
Regarding spice, Rosmarine officinalis could also help in the treatment of colitis in rats.150
Saffron was tested by Hamid et al. The authors affirmed that the degree of colitis caused by administration of TNBS is significantly attenuated by crocetin. The anti-inflammatory effects of crocetin are associated with a reduction in (i) upregulation of proinflammatory TH1 cytokine response leading to the suppression of iNOS and attenuation of the recruitment of neutrophils, (ii) lipid peroxidation and (iii) ultimately, tissue injury. Being a relatively nontoxic natural product, combined with its excellent anti-inflammatory activity via reducing inflammatory cytokines, iNOS and NFκB downregulation, crocetin could be useful in human IBD as a supplement therapy.151 With respect to saffron's effectiveness in UC, studies have shown that oral administration of crocetin to mice (25–100mg/kg b.wt. per day) for 8 days significantly ameliorated TNBS-induced UC thanks to a reduction of NO, neutrophil infiltration, and lipid peroxidation in the inflamed colon, favorable expression of TH1 and TH2 cytokines, and down-regulation of NF-κB.152
The anti-inflammatory actions of curcumin on colitis may involve an inhibition of the TLR4/NF-κB signaling pathway and IL-27 expression.140 In rats, it was used in enemas with curcumin that improved the inflammation of the colonic mucosa, reduced the inflammatory grade and decreased the tissue content of MPO in colon segments without a fecal stream.153 Curcumin in mice alters the enzyme activities of paraoxonase (PON), carbonic anhydrase (CA), glucose-6-phosphate dehydrogenase (G6PD) and cytosolic β-glucosidase.153 Gopu et al. evaluated the effect of flunixin and curcumin in experimentally induced ulcerative colitis in rats: as results, curcumin and flunixin are able to decrease serum LDH, ALP, IL-1β, and tumor necrosis factor-α levels, as well as colonic MPO and lipid peroxide levels, whereas increased colonic prostaglandin E2 and IL-10 concentrations were observe.154 In humans, specifically in pediatric subjects with UC or Crohn's disease, curcumin may be used as co-therapy with conventional medicine or as alternative therapeutic choice without clinically significant side effects or adverse events.155 In adults, Curcumin seems to be a promising and safe medication for maintaining remission in patients with quiescent UC.156 Overall, an open lab study with patients with ulcerative proctitis and with Crohn's disease demonstrated that curcumin is able to improve symptoms in all proctitis patients and to lower CDAI scores in Crohn's disease patients.157
Piperine has an anti-inflammatory effect at colorectal sites that is due to down-regulations of the production and expression of inflammatory mediators, and it also reduces FFA-induced TLR4 mediated inflammation in mice.158
Rats fed garlic (0.25g/kg b. wt.) orally for 4 weeks and 3 days during acetic acid–induced colitis showed a significant reduction in colon weight. Garlic administration restored the levels of GSH and antioxidant enzymes with a concomitant decrease in lipid peroxidation levels, as compared to placebo-treated colitis groups. Also, garlic treatment in the presence of the amino acid l-arginine (625mg/kg b. wt.) mitigated the changes in both colon weight and colon tissue contents of lipid peroxidation and GSH.159
Interesting data on the effects of B. serrata extracts (BSE) and its active components, boswellic acids, resulted from preclinical studies on animals models,160–162 and clinical studies163,164 in patients with chronic and ulcerative colitis. However, pharmacokinetics studies revealed low systemic absorption of boswellic acids in animals and human.165 In order to improve the low bioavailability of BSE, a lecithin-based delivery form of standardized BSE (Casperome®) has been designed.165–167 Casperome, used as supplementation in 22 subjects, attenuates symptoms associated with mild UC in remission.168
Plantago ovata inhibits the protein kinase C, as it down-regulates the expression of intercellular adhesion molecule-1 and inhibits the inflammation produced from 5-hydroxy-6,8,11,14-eicosatetraenoic acid and leukotriene B4. The enzymatic dissolution of the seeds of Plantago ovata results in the production of short chain fatty acids that have favorable effects in patients with patients with UC. In an open clinical study, 105 patients with UC in remission were randomized to receive either Plantago ovata seeds (10g b.i.d.), mesalazine (500mg t.i.d.), or Plantago ovata seeds with mesalazine in the same doses. The rate of recurrence after 6 months did not differ in the three groups (40% vs. 35% vs. 30%).169
A. paniculata inhibits in vitro production of TNF-α, IL-1β and NF-κB. Other herbal preparations such as GBF have prebiotic characteristics that could increase luminal butyrate production by modulating the microfloral distribution.170 A recent randomized, double-blind, placebo-controlled study compared the extract of A. paniculata with placebo in 224 adult patients with mild to moderately active UC. Treatment in a dose of 1800mg per day resulted in a statistically significant better clinical response compared to placebo (60% vs. 40%; P=0.018), although the proportion of remission after 8 weeks did not differ in the two groups.170
The second study was also a randomized, double-blind, multicenter study of an 8-week duration with parallel groups. The study showed similar effectiveness with mesalazine in patients with mild to moderate UC. In this study, there was no difference in the proportion of endoscopic remission in the two groups after 8 weeks (28% vs. 24%).171
Tormentil extracts (T. erecta-Rosaceae) have antioxidative properties. In a relevant study, 16 patients with active UC received Tormentil extracts in escalating doses and with 2400mg Tormentil extracts per day, the median clinical activity index and CRP improved from 8 (6–10.75) and 8 (3–17.75)mg/l at baseline to 4.5 (1.75–6) and 3 (3–6)mg/l, respectively. During therapy, the clinical activity index decreased in all patients, whereas it increased during the washout period 172. Tormentil extracts appeared safe up to 3000mg/d.172
In summary, all reviews on pre-clinical studies and some human clinical trials carried out recently show that certain spices, in particular ginger, curcumin, rosmarine officinalis, saffron, garlic, cumin, piperine, and specific botanicals, such as B. serrata, A. paniculata, P. lanceolata, and T. erecta-Rosaceae, reduced the inflammatory activity in experimental colitis, decreased the levels of many inflammatory indices, including serum cytokines and indices of oxidative stress and improves clinical symptons.
In conclusion, a regular daily consumption of ginger, curcumin, rosmarine officinalis, saffron, cumin, garlic, and piperine possess beneficial effects in preventing/ameliorating IBD; concerning botanicals, several herbal remedies, such as B. serrata, A. paniculata, P. lanceolata, T. erecta-Rosaceae, could represent an alternative approach for the management of very mild UC or UC during stabilized remission phase.
Sweetener and sugarThis research has been carried out based on the keywords: “sweetener” OR “sugar” OR “artificial sweetener” OR “sucralose” OR “polyols” OR “saccharin” AND “colitis” OR “crohn's disease” OR “ulcerative colitis” OR “IBD.
As shown in Table 13, nine articles were sourced: four reviews, a letter to the editor, a clinical study, a multicenter hospital-based case–control study, a comprehensive study, and a randomized, double-blinded placebo-controlled cross-over study.
The presence of artificial sweetener in food is shown to have a strong correlation to microbial changes that can be influential in IBD development.173
Sakamoto et al.,174 used a validated semi-quantitative food frequency questionnaire (FFQ) to identify that in the pre-illness diet of patients with IBD, there was an increased consumption of sugars, sweeteners, and sweets and that these were positively associated with an increased risk of CD and UC. Supporting this link was the introduction of the artificial sweetener sucralose, which was closely followed by a spike in the incidence of IBD. Sucralose has demonstrated bacteriostatic effects and limits the ability of bacteria to process normal sugar through inhibition of 2 key enzymes: invertase and sucrose permease.175
There is other evidence that suggests sucralose may be linked to IBD through a similar mechanism as saccharin; it may be a key causative factor for IBD, through its inhibition on gut bacteria and the resultant impaired inactivation of digestive proteases and over digestion of the mucus layer and gut barrier.
In addition to saccharin, many other chemicals are used as food additives in modern society. Some of them may also be capable of inhibiting gut bacteria.176,177
Finally, sorbitol, mannitol, maltitol, and xylitol are sugar alcohols. They are poorly absorbed in the small intestine, consequently entering the colon, where they are subject to anaerobic fermentation. Compared with healthy individuals, patients with IBS report an increased and discordant absorption of mannitol and sorbitol.28 Moreover, increased and discordant absorption of mannitol and sorbitol occurs in patients with IBS compared to that in healthy controls. Polyols induced gastrointestinal symptoms in patients with IBS independently of their absorptive patterns, suggesting that the dietary restriction of polyols may be efficacious.178
Other data on increased sugar and refined carbohydrate intake and the progress of IBD are intriguing but currently less clear, and there is a striking difference between epidemiological data and experimental data.179
Concerning sugar, sugar intake has also been identified as a factor in the pathogenesis in IBD in studies across Europe and the United States, although reductions in the incidence of IBD are not observed with restriction of sugar intake. Importantly, however, sugars in the context of these studies included artificial sweeteners, which may have an effect on gut bacteria that promotes the development of IBD.120 However, others data on increased sugar and refined carbohydrate intake and the progress of IBD are intriguing but currently less clear, and there is a striking difference between epidemiological data and experimental data.179
In conclusion, the intake of sugar and sweeteners must be excluded from the diet.
Alcoholic beveragesThis research has been carried out based on the keywords: “alcoholic beverages” or “alcohol” AND “colitis” AND “Crohn's disease” AND “ulcerative colitis” AND “IBD” AND “inflammatory bowel disease”.
As shown in Table 14, five articles were sourced: one review, one clinical study, one case-report, one cross-over and one cross-sectional study.
Chronic alcohol exposure has several deleterious effects on the intestinal mucosa and can favor and sustain local inflammation.180 The immune system could be modified by heavy acute and chronic alcohol consumption that could therefore play an important role in IBD. Acutely, alcohol consumption decreases T cell activity and IL-12 levels in healthy controls.181 Chronically, alcohol increases liver Kupffer cell activity and is associated with increased generation of proinflammatory mediators such as TNF-α, IL-1, and IL-6 in patients with liver disease. Alcohol has also been shown to acutely disrupt gut barrier function, and can increase intestinal permeability in human subjects, to which patients with IBD are particularly susceptible.181 Alcoholic beverages (hard liquor, wine, or beer) can lead to very severe diarrhea, depending upon the amounts consumed, probably caused by their sugar content.182 Food avoidance among IBD is prevalent for alcohol and other foods,120 taking into account that histamine-releasing food items and foods rich in biogenic amines, such as alcoholic beverages, are important triggers of GI symptoms. Hey et al. have evaluated the effect of 5 different alcoholic drinks (red wine, white wine, Smirnoff Ice, Elephant Beer and pure ethanol) on abdominal discomfort in patients with CD: the authors noted that patients with CD who consumed Smirnoff Ice and Elephant beer have an increased effect on self-reported abdominal pain, probably because of the high sugar content in these drinks.183 Interestingly, Ballo et al.,180 reported a case of a woman with a history of alcohol abuse and suspected carcinoid syndrome, but specific clinical examinations showed non-specific inflammatory bowel disease with severe colic wall thickening, suggesting the intriguing possibility that alcohol bring to an acute IBD mimicking carcinoid syndrome. Regarding the rate and pattern of alcohol consumption, Swanson et al.,181 affirmed that inactive IBD patients consume alcohol in a similar quantity and pattern as the general US population (62%). However, inactive IBD patients reported worsening of their GI symptoms with alcohol consumption when compared to patients with IBS. This finding may be due to a leaky gut and increase luminal immune activity by increasing intestinal permeability and antigen exposure, or could be a consequence of an osmotic diarrhea due to the high sugar content of most alcoholic drinks.
In conclusion, alcohol intake, particularly if associated with high sugar content (as in beer) should be avoided.
Vitamin DThis research has been carried out based on the keywords: “vitamin D” OR “serum vitamin D levels” OR “vitamin D deficiency” OR “vitamin D supplementation” AND “colitis” OR “crohn's disease” OR “ulcerative colitis” OR “IBD”.
As shown in Table 15, seven articles were sourced: two reviews, a systematic review and meta-analysis, an observational multicenter study, a physician-blinded prospective study, a retrospective study and a longitudinal study.
Vitamin D plays a pivotal role in several immune-mediated diseases, and its association with IBD is debated.184 Vitamin D deficiency is significantly higher in IBD patients and this condition may negatively affect the gut barrier and immune system functions, thus potentially impacting IBD onset and progression.185
This is common especially in CD, and it is associated with increased disease activity, a relapsing disease course, and higher inflammatory activity. However, the importance of vitamin D supplementation as an anti-inflammatory therapeutic agent has yet to be investigated.186 Moreover, vitamin D deficiency is associated with higher morbidity and disease severity. IBD patients with low mean vitamin D levels have worse disease activity, worse pain, higher need for biologics, steroids and narcotics, and increased health care utilization, while Vitamin D supplementation also appears to be correlated with improvement in health-care utilization, which is reflective of improved overall health.187
Ghaly et al. sustain the opinion that vitamin D deficiency increases the risk of developing IBD in areas of low UV exposure and is associated with more active disease and worse outcomes such as hospitalization and surgery. Vitamin D deficiency is also related with reduced muscle bulk in IBD and may play an important role in maintaining balance and fall prevention.188
Finally, a physician-blinded prospective study determined that low serum levels of vitamin D (≤35ng/ml) increase the risk of UC relapse during periods of clinical remission because of it is a nontoxic supplement that may have protective effects in the maintenance of clinical remission in patients with ulcerative colitis.189
Hlavary et al.,190 showed that vitamin D serum concentration correlated with health-related quality of life in UC and CD patients during the winter/spring period. IBD patients who are vitamin D insufficient might benefit in fact from an adequate dose of vitamin D supplementation to increase their vitamin D serum concentration to around 50ng/ml.
In summary, there are data in animal studies, epidemiologic and small cohort studies that demonstrating that vitamin D may influence IBD disease activity and progression due to its well-established immunomodulatory and anti-inflammatory activity. Moreover there are data that vitamin D deficiency occurs frequently in IBD patients (secondary to increased sun avoidance while taking thiopurines, small bowel inflammation causing relative malabsorption, ileal resection affecting bile salt resorption and increased stool excretion).
In conclusion, it is mandatory that vitamin D levels in the blood in IBD patients is controlled and constantly monitored. Individuals who are vitamin D insufficient (< 30ng/ml) should benefit from an adequate dose of vitamin D oral supplementation to increase their vitamin D serum concentration to around 50ng/ml.
Bone mineral density, calciumThis research has been carried out based on the keywords: “Calcium” OR “serum calcium levels” OR “calcium deficiency” OR “calcium supplementation” AND “colitis” OR “crohn's disease” OR “ulcerative colitis” OR “IBD”.
As shown in Table 16, eight articles were sourced: three clinical trials and an animal model study, three reviews and a gastroenterological guideline.
Decrease in bone density is a relatively common complication of IBD, occurring in about 20–50% of patients,191 and poor bone density may be caused by malabsorption of calcium and vitamin D due to steroid therapy, malnutrition, and an unbalanced diet.192Moreover, reduction in bone mineral mass is caused not only by inflammatory processes in the bowel, as osteoporosis occurs in very young IBD patients and in newly diagnosed subjects who have not yet undergone any pharmacological treatment,192 so it has been suggested that another cause of loss in mineral mass may be genetic variants of genes encoding for RANKL/RANK/OPG signaling pathway molecules. This author demonstrated that different molecular background of osteoporosis is associated with CD and UC diseases.193
Finally, dysregulated Ca2+ homeostasis likely contributes to the etiology of IBD-associated loss of bone mineral density (BMD). The end result of the process is decreased renal Ca2+ reabsorption and urinary Ca2+ wasting. This mechanism, along with the previously implicated changes in intestinal Ca2+ absorption, likely contributes to the systemic imbalance of Ca2+ homeostasis and to IBD-associated loss of BMD.194
In patients with IBD and celiac disease, serum calcium level, corrected for albumin, should be measured at diagnosis and monitored at least annually, as suggested by the American Gastroenterological Association Medical Position Statement: Guidelines on Osteoporosis in Gastrointestinal Diseases.195
Vernia et al. demonstrated that diet in IBD patients contained significantly less calcium than in healthy controls. Self-reported lactose intolerance, leading to dietary restrictions, is the single major determinant of low calcium intake. According to study results, inadequate calcium intake is present in one third of IBD patients and represents a reversible risk factor for osteoporosis, suggesting the need for tailored nutritional advice in IBD.196
A study by Lim et al. demonstrated that in 41 IBD patients, 21 classified into the normal group and 20 into the malnourished group, the lower bone density subjects were more in the malnourished group; in this group the intake of calcium was lower.197
In summary, it is mandatory that in patients with IBD, bone mineral density should be monitored annually through the use of the computerized bone mineralometry (MOC), and specific biochemical analysis (such as calcium corrected for albumin, ionized calcium, vitamin D, parathyroid hormone, bone isoenzyme of alkaline phosphatase), in particular if there is lower back pain. In the case of osteopenia, dietary supplementation with specific nutrients useful for bone health, such as vitamin K, essential aminoacids,198 should be recommended. In the event of an osteoporosis diagnosis, a personalized pharmacological therapy should be prescribed in addition to dietary supplementation.
In conclusion, bone mineral density should be monitored annually and dietary supplementation with specific nutrients useful for bone health other than vitamin D, such as vitamin K and essential aminoacids, should be recommended for IBD patients with osteopenia or osteoporosis.
Omega-3This research has been carried out based on the keywords: “IBD” OR “ulcerative colitis” OR “Crohn's disease” AND “omega-3” OR “PUFA”.
As shown in Table 17, seventeen articles were sourced: one review, five animal studies, eleven human studies of which four were randomized, double blind placebo controlled studies, one double blind placebo controlled trial, two multicenter trials, three clinical studies and one open-label.
Literature is rich in studies considering omega-3 fatty acids in different types of animal models with intestinal inflammation. Nieto et al.,199 emphasized that the use of balanced diets containing (omega-3) long chain LC-PUFA with adequate amounts of antioxidants improve the histology of the colon, decreasing inflammation in specific tissue and could ameliorate the inflammation and mucosal damage in UC rats, probably not only to their inhibition of PGE2 and LTB4 synthesis, but perhaps also to modulation of pro-inflammatory cytokines. Fats rich in omega-6 PUFA enhance IL-1 production and tissue responsiveness to cytokines, whereas fats rich in omega-3 PUFA have the opposite effect. Fish oil supplementation down-regulates pro-inflammatory cytokines, such as TNF-α, IL-1, and IL-6, which may in turn lessen activation of leukocytes and, therefore, oxidative damage.199
In a study on rats by Reddy and Naidu, the results demostrated that an unbalanced omega-6/omega-3 PUFA diet systemic arachidonic acid/docosahexaenoic acid ratio and attenuates colon inflammation in young adult rats.105 A similar study performed by Huang et al, demonstrated that compared with the omega-6/omega-3 PUFA ratio of 4:1, the ratio of 2:1 was more effective in reducing inflammatory reactions in DSS-induced colitis in rats.89 The same authors, in another study, suggested that diets enriched with fish oil upregulated peroxisome proliferator-activated receptors (PPAR-γ) and decreased NF-κB activation that may consequently reduce luminal inflammatory mediator production.200
Hillier et al.,201 demonstrated that colonic lipids, prostaglandin and thromboxane synthesis can be readily altered by dietary supplementation with fish oil. The extent of incorporation of the fatty acids present in oils is dependent upon the individual fatty acid profile. In addition to fish oil containing omega-3 fatty acids of animal origin, omega-3 fatty acids of vegetable origin have also been studied. Reifen et al.,202 demonstrated the beneficial role of diet enriched in plant-derived oil rich in α-linolenic acid (ALA) in rats with intestinal inflammation. Furthermore, their findings present a possible advantage of plant-derived oil rich in ALA compared with fish oil supplementation, particularly in patients who take anticoagulant medications and/or suffer from conditions that involve bleeding events, such as active IBD. Taken together, the outcomes of this study suggest that ALA alone has potential as an anti-inflammatory agent that is not necessarily dependent on its conversion to the longer (omega-3) PUFAs, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). In vitro, ALA supplementation was shown to be effective at inhibiting inflammation induced by IL-1β by down-regulating mRNA level of pro-inflammatory genes including IL-8, COX-2 and iNOS compared to naïve cell as well as fish oil- and oleic acid-treated cells.202
In humans, changes have been demonstrated in the serum fatty acid pattern of patients with CD, with significant depletion of omega-3 PUFAs, but it is unclear whether the low omega-3 PUFAs value is a result of a decrease in dietary intake.203
Subsequently, Pearl et al.,204 studied colonic mucosa biopsies from 69 UC patients and found that inflamed mucosa had higher levels of arachidonic acid, docosapentaenoic acid and DHA, and lower levels of linoleic, ALA and EPA compared with the control group. The severity of inflammation was positively associated with the levels of arachidonic acid, docosapentaenoic and DHA and negatively associated with levels of linoleic, ALA and EPA, suggesting that there are modifications in fatty acid metabolism in the inflamed gut mucosa. This is probably due to local alterations in the PUFA biosynthetic pathway.
Given this background, numerous studies have been performed in order to evaluate the efficacy of omega-3 fatty acids (EPA and DHA) supplementation in IDB patients, even if not all studies are in agreement with each other. These controversies over the omega-3 fatty acid effects on IBD are due to the enormous variability in the size of samples, the amount of omega-3 fatty acid administered, and the methodology employed in the studies.205
The study of Trebble et al.,206 analyzed the supplementation of fish oil (2.7g of EPA and DHA/day) with antioxidants in CD patients without the use of corticosteroid. The study showed that dietary supplementation was associated with modified cytokines from circulating (peripheral blood) PBMC that are responsible of some manifestations of CD, and lower production of PGE2 and IFN-γ by circulating monocytes or macrophage.
Yasueda et al.,207 demonstrated that an omega-3 emulsifying formulation is safe and useful for maintaining remission in patients with CD. This was confirmed by the study of Geerling et al.,208 which investigated the effects of supplementation with liquid formula containing either antioxidants or omega-3 fatty acids, plus antioxidants on the antioxidant status and fatty acid profile in patients with CD in remission. The authors showed that omega-3 fatty acids and antioxidants decreased the proportion of arachidonic acid, increased the proportion of EPA and DHA, and changed the eicosanoid precursor profile.
Another study performed by Belluzzi et al.,209 demonstrated that supplementation with a particular formulation of fish oil (2.7g of omega-3/day) in CD patients in remission was useful in reducing the rate of relapse as a result of its anti-inflammatory properties. However, in a similar study, treatment with omega-3 free fatty acids (4g/day of omega-3) was not effective in the prevention of relapse in CD210 or extending the remission in CD with 5g/day of omega-3.211
In UC patients, fish oil supplementation (2,7 EPA of day) declined mean disease activity, even though it was not significantly associated with a reduction in mucosal leukotriene B4 production, in which levels are generally increased in UC.212 Stenson et al.,213 also performed a similar study using a higher dosage of EPA (3.24g/day) but for less time, and the result was a decrease in rectal dialysate levels of Leukotriene B4.
In summary, the studies on animal models demonstrated there is evidence that the gastrointestinal mucosa is highly responsive to omega-3 LC-PUFA. This may be related to the reduction in intestinal production of the precursor of pro-inflammatory cytokines (leukotrienes and prostaglandins) of odd series and to the reduction of the protein expression of intestinal NFκB p65 related to apoptotic cells. In humans, changes have been demonstrated in the serum fatty acid pattern of patients with CD, with significant depletion of omega-3 PUFAs, however it is unclear whether the low omega-3 PUFAs value is a result of a decrease in dietary intake. Also in UC patients, it has been shown that inflamed mucosa had higher levels of arachidonic, docosapentaenoic and DHA and lower levels of linoleic, ALA and EPA compared with the control group.
In conclusion, the supplementation omega-3 fatty acids can be helpful in the treatment of UC and CD as it can alleviate the symptoms and help the recovery of the mucosal due to its anti-inflammatory properties. Further, it is extremely important that the patient is adequately monitored for identification of the personalized daily amount recommended to help prevent or induce remission of IBD, also according to the different states of disease.
Other micronutrientsThis research has been carried out based on the keywords: “IBD” OR “crohn's disease” OR “ulcerative colitis” AND “vitamins” or “minerals” or “micronutrients”.
As shown in Table 18, 56 articles were sourced: three animal studies, two meta-analysis, three systematic reviews, thirteen reviews, two reviews and meta-analysis, one case control study, one ECCO guideline, one open label study, two randomized controlled trial, seven cross-sectional studies, ten cohort studies and eleven clinical studies.
Micronutrient deficiencies occur in more than half of patients with IBD, as underlined in the review of Weisshof and Chermesh.214 Most common are deficiencies of iron, B12, vitamin D, vitamin K, folic acid, selenium, zinc, vitamin B6, and vitamin B1. Deficiencies are more common in Crohn's disease than in ulcerative colitis, and more in active disease than at times of remission. Micronutrient deficiency is associated with prolonged and complicated course of disease.214 These deficits may originate from very different causes, such as long-term inflammation in the gut mucosa and decreased oral intake,215 and they cause a wide range of symptoms and complications, which makes diagnosis and management difficult. Good clinical care of patients with CD should include surveillance for micronutrient deficiencies and in respective cases a consequent treatment and supplementation.216
Ascorbic acidAscorbic acid could be used to prevent and treat IBD, because of its anti-oxidant and anti-inflammatory properties, decreasing iNOS and COX-2 expression through inhibiting NF-κB pathway activation, as demonstrated by the study of Yan et al. on mice with induced-colitis.217 Overall, ascorbic acid treatment markedly attenuated the increased DAI score in mice and ascorbic acid-treated mice exhibited less inflammatory cell infiltration and only mild evidence of crypt distortion.217 A particular study, performed by Shaghaghi et al. emphasized that a genetic variant in the ascorbate transporter locus is associated with an increased risk of CD in a Canadian IBD cohort, but the mechanism is still not clear.218
It's most likely that patients with inactive or mildly active CD can be oxidatively stressed and have increased requirement in antioxidant vitamins, such as Vitamin C and E (supplementation with vitamins E (800IU) and C (1000mg)).219
Vitamin APatients with CD have higher prevalence of vitamin A blood deficiency, as shown by the study of Soraes-Mota.220 It is still unclear why Vitamin A is involved in the pathophysiology of CD, but Fransen et al. hypothesized a polymorphism.221 Children and young adults with IBD have low serum levels of vitamin A, specifically in CD patients who have active disease, and the level correlates with alpha-tocopherol levels, but are independent from the supplementation. For this, the severity of disease activity is better than nutritional status in order to predict the risk of hypovitaminosis.222
However, low serum retinol level (SRL) was not related to vitamin A quantitative intake, so new studies are necessary to explain the mechanisms behind the development of vitamin A deficiency in patients with IBD.220 A recent study confirmed that serum Vitamin A levels and disease activity do not correlate with FOXP3 and IL-23 receptor (IL-23R) expression in colonic mucosa of UC patients, both in remission and in active disease, even after an established role of Vitamin A in inhibiting Th17 responses in mice models and humans.223 Retinoic acid may be related to the production of the transforming growth factor-b (TGF-b) and IL-10, helping in the resolution of the inflammation condition.224 Vitamin A and zinc serum deficiency are common in patients newly diagnosed as having IBD, and levels should be assessed at the time of diagnosis so repletion can commence.215
Vitamin KPatients with IBD often exhibit vitamin K deficiency that correlatesto inflammatory status because of the inhibition of IL-6 in murine model.225 Also, vitamin K serum deficiency was highly prevalent in pediatric IBD.226 O’Connor et al. performed a study in which patients with CD were supplemented with phylloquinone, in order to evaluate the vitamin K status and bone health. Their result was that supplementation with 1000mg of phylloquinone daily (with Ca and vitamin D3) had no effect on the indices of bone health in adult CD patients in clinical remission with likely vitamin K insufficiency.227 However, a more recent study demonstrates that plasma concentrations of phylloquinone (PK), menaquinone-7 MK-7 were quite low in patients with IBD, especially CD, despite apparently sufficient intake of these vitamins.228 Impaired intestinal absorption of these fat-soluble vitamins is likely to be associated with vitamin K deficiency, because low plasma concentrations of vitamin K (and 25OH-D) were independent risk factors for low bone mineral density (BMD) and they were associated with the patients’ fat intake, but not with their intake of these vitamins.228
Vitamin B6Selhub et al. demonstrated that histological and molecular features of colonic inflammation are significantly attenuated by both mild B6 inadequacy and moderate B6 supplementation in the IL10−/− mouse model of IBD.229 In humans with IBD, low vitamin B6 plasma levels are an independent risk factor for thrombosis, especially those with active disease.230 In light of this, low vitamin B6 plasma levels in IBD are probably a consequence of disease activity and hypercatabolism induced by systemic inflammation, even if other relationships could not be excluded (as dietary intake, malabsorption, drug interference).230
ThiamineA mild thiamine deficiency could generate fatigue related to ulcerative colitis or Crohn's disease and in humans the administration of large quantities of thiamine (dosage ranged from 600mg/day to 1500mg/day depending on body weight) increases the concentration in the blood to levels in which the passive transport restores the normal glucose metabolism in all cells, and leads to a complete regression of fatigue, even if the authors of the study underline possible collateral effects. This study was performed in patient with UC in remission and CD in quiescent phase.231
Folate – Vitamin B12 – IronCD commonly involves the small intestine, which is the site of vitamin B12 and folate absorption.232 A significant proportion of patients with CD suffer from serum vitamin B12 and/or folate deficiency, and associated macrocytic anemia.232,233 The prevalence of vitamin B12 and folate deficiency in serum was respectively 15.6% and 22.2% in CD but it was only 2.8% and 4.3% in ulcerative colitis.232 This was confirmed by Huang et al.: the serum deficiency of vitamin B12 and folate were higher in CD patients than UC and control group.234 Prior ileal or ileocolonic resection is a risk factor of serum vitamin B12 abnormalities, and a disease duration within 5 years, is a risk factor of low serum folate levels in CD patients.234 Vitamin B12 and folate deficiency are rare in children newly diagnosed with IBD.215 A previous study, performed by Heyman et al., showed a higher red blood cell folate (RBCF) and whole-blood folate (WBF) in children with newly diagnosed IBD than in controls. Higher concentrations of RBCF and WBC in whites compared to other ethnic groups were found, probably for different genetic background.235
Some authors suggest the possibility that the rise in IBD in Canada was due, in part, to the adoption of processed flour (0.15mg of folate per 100g of flour) in the early 1900s, a change that was partly reversed with the addition of folate to flour in 1997/1998.236 Other authors suggest that disease activity is a risk factor for folate deficiency.232
In CD patients, prior small intestinal surgery is an independent risk factor for having a low serum vitamin B12 level.232,233 Management of vitamin B12 (cobalamin, Cbl) deficiency in inflammatory bowel disease (IBD) is often not evidenced based because of uncertainty on whether it causes enough malabsorption to result in clinical disease.237 However, Battat et al., in their study, concluded that only ileal resections greater than 20cm in CD predispose to deficiency and warrant treatment.237 The evaluation of B12 status in patients with CD could be assess by Holotranscobalamin (holoTC) combined with methylmalonic acid (MMA) that identifies impaired B12 status in patients otherwise considered replete with traditional serum testing. The holoTC and MMA also exclude B12 deficiency in patients otherwise considered deficient using serum Testing.238 Genetically, individuals carrying the haplotype (GG) formed by transcobalamin gene polymorphisms (TCN2) seem to be at higher risk of developing CD, but this study was performed only with Chinese patients.239
A recent meta-analysis, performed by Pan et al., identified a significant relationship between low serum folate concentration and IBD, but not between vitamin B12 and IBD, partially in opposition with previous studies, but suggested a supplementation of folate and vitamin B12 in IBD patients in order to prevent other disease. The authors suggest that low serum concentrations of folic acid may be an important risk factor for IBD patients.240
Hyperhomocysteinemia is a common condition in IBD patients, as IBD significantly increased the risk of hyperhomocysteinemia, especially in patients in active phase of disease, without correlation with thromboembolic event.241,242 Patients with active disease have lower serum levels of folate, cobalamin and pyridoxine, compared to those in the quiescient phase.241 According to this, a meta-analysis suggests that deficient folate status is associated with a higher impact of MTHFR C677T polymorphism on IBD risk,242 but another meta-analyses suggests that only the variant MTR A2756G indicated an association with the risk of IBD for the allele contrast and the dominant model.243
However, other studies are not agree with this: in Greek patients, for example, hyperhomocysteine is associated with lower levels of folate, but not vitamin B12.244 Similarly, a cross-sectional study asserts that hyperhomocisteinemia is associated with lower, but not necessarily serum deficiency of vitamin B12.245 Another study performed by Mahmood et al. concluded that high serum homocysteine and high folate levels were associated with low vitamin B12 levels in patients attending the IBD clinic.246
Children with IBD have higher plasma tHcy concentrations as a consequence of disease, probably due to folate status, associated with diet or the pathophysiology.247
Anemia is common in patients with IBD and, in Brazilian IBD patients, there is no difference between CD groups and UC patients.248 The same authors underline that the most common etiologies of anemia found in both groups were iron deficiency anemia, followed by the anemia of chronic disease.248 In the majority of cases, however, IBD-associated anemia is a prime example of combined iron deficiency anemia and anemia of chronic disease.249 However, gastroenterologists should keep in mind other causes of anemia as vitamin B12 and folate deficiency, as above.250 In IBD, iron status is more closely related to the quality and quantity of dietary iron intake than in the general healthy population.251 However, there was no evidence of an association between iron deficiency and fatigue in the absence of anemia.252 It could be diagnosed using transferrin receptor-ferritin index (TfR-F) in addition to ferritin.253 A treatment with iron could be useful in patients with inactive or mildly-active IBD, even if some studies declared that advice must be given to patients on modifying their diet to enhance dietary iron.251 A systematic review and meta-analysis, performed by Avni et al. concluded that treatment for anemia in IBD should include intravenous (IV) iron and not oral iron replacement, due to improved hemoglobine (Hb) response, no added toxicity and no negative effect on disease activity.254 Conversely, other studies suggest that there are no differences between intravenous (IV) or oral (PO) iron, but patients who received IV iron had a greater rise in serum ferritin, and were less likely to stop treatment due to adverse events when compared to those who received PO iron.255 With the new generation of IV iron compounds, a safe and highly-efficient treatment has become available and should help to overcome the reservation of gastroenterologists to replenish deficient iron stores in patients with IBD according to existent guidelines.256 Erythropoiesis stimulating agents (ESA) therapy may also be used in order to treat the anemia of chronic disease that usually accompanies iron deficiency in IBD.254 In a systematic review, only 1 study showed oral iron supplementation to worsen disease activity in 2 patients with UC, although quality of life improved significantly in the same group of patients, and intravenous iron supplementation seems to be safe in patients with active IBD.257 Iron therapy can be administered concomitantly with TNF inhibitors, a class of drugs used increasingly in the management of IBD.257 In 2015 guidelines were defined for diagnosis and management of iron deficiency and anemia in IBD.258
Children with IBD have both an absolute iron deficiency (depleted iron stores) and a functional deficiency (caused by chronic inflammation).259
SeleniumAn adequate nutritional Selenium (Se) status is important in IBD patients to minimize the cardiovascular risk associated with increased inflammation biomarkers, especially in undernourished CD patients. It is related to iron status; serum Se concentrations are lower in IBD patients than healthy controls.260 Se, in the form of glutathione peroxidase, contributes to the immunity of the gut (Gut – Associated Lymphoid Tissue GALT).261 Therefore, the cause and effect relationship between selenium deficiency and IBD needs to be examined further.262
Female gender and low serum albumin level were risk factors for selenium deficiency in Korean patients with IBD.263
Children with IBD have lower serum levels of Se and it could be a result of reduced intake, reduced absorption, or increased mobilization for mopping up free radicals that contribute to tissue injury in these conditions.264
ZincThe intake of zinc (9–27mg/day) was inversely associated with risk of CD but not UC. Considerable mechanistic plausibility supports this association, including the effect of zinc on autophagy and microbial clearance, innate immune response and maintenance of the integrity of the epithelial barrier.265 Zn deficiency may result from poor oral intake, decreased absorption, or previous small bowel resection, and is thought to contribute to mucosal inflammation.266 It is associated with an increase of disease-specific outcomes as hospitalizations, surgeries, complications both in UC and CD patients. Normalization of minerals is related to improvement in these outcomes.266
Patients younger than 40 years were at increased risk for zinc deficiency in Korean patients with IBD, even if lower serum levels of zinc in this specific population were similar to that of Western IBD patients.263 A possible explanation is poor zinc absorption and increased intestinal loss of zinc; one important fact to note is that zinc deficiency is not only the consequence of poor dietary intake but also is correlated with the inflammatory process in IBD. This hypothesis is supported by the finding that zinc deficiency was highly prevalent even though sufficient supplements were provided through multivitamin additives to patients with IBD.267
As for Selenium, children with IBD have lower levels of Zn and some authors proposed a link between Zn levels and albumin.264
MagnesiumMagnesium deficiency is frequent in IBD: it has been reported in 13–88% of patients216: it's a frequent complication of IBD, due to decreased oral intake or malabsorption or increased intestinal losses, and a parenteral or oral supplementation are necessary.268
CopperChildren with CD, but not those with UC, presented increased copper levels, which is known to be elevated under inflammatory conditions, indicating a marked increase in ceruloplasmin as previously reported by Ojuawo and Keith.264
In conclusion, because micronutrient deficiencies occur in more than half of patients with IBD (most common are deficiencies of iron, B12, vitamin D, vitamin K, folic acid, selenium, zinc, vitamin B6, and vitamin B1), good clinical care should include surveillance for micronutrient deficiencies, assesssed by blood analysis or evaluation of food intake with food frequency questionnaires and in respective cases, a subsequent personalized supplementation.
ProbioticsThis research has been carried out based on the keywords: “probiotics” AND “IBD” OR “ulcerative colitis” OR “Crohn's disease”. As shown in Table 19, ten articles were sourced: one animal study, one systematic-review, six reviews and two European evidence-based consensus.
With regard to the effectiveness of probiotics intake in IBD patients, two Cochrane reviews agree that there is insufficient evidence to demonstrate that the administration of probiotics can be helpful in maintaining remission in patients with IBD, compared with mesalazine or other pharmacological treatments.269,270 Specifically, Rolfe et al.,269 underlined that there was no statistically significant benefit of Escherichia coli Nissle (EcN) for reducing the risk of relapse compared to placebo or Lactobacillus GG after surgically-induced remission or medically-induced remission. They concluded that there was no statistically-significant benefit of probiotics for reducing the risk of relapse compared to maintenance therapy employing aminosalicylates or azathioprine.
Conversely, the meta-analysis by Fujiya et al.,271 found that probiotics treatment may be a useful therapeutic option for adult and pediatric patients with UC, both in combination with specific therapy that is in the maintenance phase. For both types of patients suffering from CD, this meta-analysis confirms the results of the two previously mentioned Cochrane studies, reporting that there is no evidence of effectiveness.271
However, the argument is still very much in conflict; a more recent review written by Lichtenstein et al. underlines what kind of probiotics and prebiotics are useful for CD.272 The authors underline that probiotics investigated thus far confer little benefit in inducing clinical remission in Crohn's patients, with the possible exception of Saccharomyces boulardii in certain populations (e.g., non-smokers), long-term treatment by probiotic microorganisms shows no benefit in maintaining medically achieved remission in patients with either colonic or ileo-colonic luminal Crohn's disease. Data thus far concerning the ability of probiotics to prevent post-operative clinical or endoscopic recurrence of CD are not convincing.
However, the observation is that S. boulardii could be more successful than other probiotic agents in maintaining medically achieved remission.272 Similarly, another recent review underlines what kind of probiotics and prebiotics are useful in the treatment of UC; studies can be classified into the following two groups: those that investigated induction of remission in active UC, and those that investigated maintenance of remission. Most studies were performed with EcN 1917 or VSL#3, a probiotic preparation containing a mixture of eight different bacterial species.273 The alterations in the gut microbiota observed in IBD patients, in particular in CD and in UC patients with pouchitis, provide the rationale for administering probiotic agents in the medical treatment of those conditions.274 For example, the effect of prophylactic ingestion of EcN 1917 in a murine model of colitis was evaluated by Nicoli et al.,275 in which they observed reduced inflammation, as assessed by reduced levels of neutrophils, eosinophils, chemokines and cytokines. They also reported an increase in the number of regulatory T-cells in Peyer's patches. In their study procedure, germ-free mice received fecal content from control or EcN-treated mice and were then subjected to dextran sulfate sodium-induced colitis. They observed protection from colitis in animals that were colonized with fecal content from EcN-treated mice.275 EcN is a nonpathogenic Gram-negative strain and it is used in many gastrointestinal disorders, including diarrhea, uncomplicated diverticular disease, and UC. It is the only probiotic recommended in the European Chron's and Colitis Organisation (ECCO) guidelines as an effective alternative to mesalazine in maintenance of remission in UC patients.276,277 Regarding UC, ECCO guidelines suggest that no evidence has yet been reported that any other probiotic is effective for maintaining remission in patients with UC.277 ECCO guidelines for CD suggest that there is not enough evidence to suggest that probiotics are beneficial for the maintenance of remission in CD.278
In conclusion, although animal model studies are consistent in demonstrating the efficacy of probiotics in colitis models, the results of human studies are still very conflicting. Currently, EcN is the only probiotic recommended in the ECCO 2012 guidelines as an effective alternative to mesalazine in the maintenance of remission in UC patients.
ConclusionsRecent literature suggests that diet plays a pivotal role in therapy of Inflammatory Bowel Disease, through management of inflammation and oxidative stress. So, considering that a specific diet can be helpful support therapy for patients suffering from IBD, in this narrative we built a food pyramid on this topic, because food pyramid allows patients to easily figure out what to eat. The pyramid shows that carbohydrates should be consumed every day (3 portions), together with tolerated fruits and vegetables (5 portions), yogurt (125ml), and EVOO; weekly, fish (4 portions), white meat (3 portions), eggs (3 portions), pureed legumes (2 portions), seasoned cheeses (2 portions), red or processed meats (once a week). In the top of the pyramid there are two pennants: one red means that subjects with IBD need some personalized supplementation (omega 3, vitamin D and calcium) and one black means that there are some foods that are banned (alcoholic beverages, in particular beer, sugar and sweets, foods with lactose).
Authorship of manuscript and assignment of rightsConceptualization, M.R. and S.P.; Methodology, A.M.; Formal Analysis, S.P.; Investigation, A.M. and D.S.; Resources, M.R.; Data Curation, A.M. and S.L.; Writing-Original Draft Preparation, M.R. and G.I.; Writing-Review & Editing, V.I., TA.A. and G.C.; Visualization, M.F.; Supervision, G.P. and P.A.; Project Administration, S.P and M.R.
All authors approved the submitted version and agreed to be personally accountable for the authors’ own contributions and for ensuring that question related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved.
FundingThis research received no external funding.
Conflicts of interestThe authors declare no conflict of interest.
We thank Dr. Antonio RICCI, MD from Department of Pediatrics, Foundation IRCCS Policlinico San Matteo, University of Pavia, Pavia, 27100, Italy who provided insight and expertise that greatly assisted the research.