Therapeutic education is an essential part in the management of type 2 diabetes mellitus (T2D). Implementing a therapeutic education program with the participation of a diabetes specialist nurse (DSN) addressed to patients with T2D using more than 2 insulin injections and sub-optimal metabolic control in primary care (PC) could improve health care and clinical outcomes. Our purpose was to evaluate the clinical, educational and patient satisfaction outcomes of this program.
Material and methodsA prospective, longitudinal study was performed with an evaluation before and after the intervention. The program had a duration of 6 months and included individual on-site, phone and group visits.
Results184 subjects were included and 161 were finally evaluated. 89.4% were included due to sub-optimal metabolic control and 10.6% because of repeated hypoglycemia. In the first group, the mean reduction in HbA1c was −1.34%±1.45% without any increase in hypoglycemia episodes. In the second group, a significant reduction in hypoglycemia episodes/week was observed (2.52±1.66 vs. 0.53±1.06; p<0.05) without any increase in HbA1c. Learning skills, lifestyle, adherence to care, and the perception of quality of life had significantly improved at 6 months (p<0.05). The overall program was positively evaluated by patients, the role of DSN being considered essential by 98% of the responders.
ConclusionA structured therapeutic education program, including a DSN, addressed to insulin treated T2D patients attending primary care facilities and with sub-optimal metabolic control is associated with beneficial effects in terms of clinical, educational and patient satisfaction endpoints.
La educación terapéutica es clave en el tratamiento de la diabetes mellitus tipo 2 (DT2). La participación de una enfermera de práctica avanzada (EPA) en diabetes en un Programa de Atención y Educación Terapéutica (PAET) en Atención Primaria (AP) podría mejorar la atención y los resultados de salud en pacientes en tratamiento con 2 o más dosis de insulina con mal control metabólico. Nuestro objetivo fue evaluar resultados clínicos, educativos y de satisfacción de este programa.
Material y métodosEstudio prospectivo, longitudinal, de intervención y valoración antes y después del programa, con una duración de 6 meses con visitas presenciales, telefónicas e intervención grupal.
ResultadosSe incluyó a 184 pacientes, de los cuales 161 fueron analizados. El 89,4% de los sujetos fueron incluidos por mal control metabólico (GMCM) y el 10,6% por hipoglucemias de repetición (GMCH). En el GMCM el descenso promedio de la hemoglobina glucosilada (HbA1c) fue de –1,34±1,45%, sin aumento de hipoglucemias. En el GMCH, disminuyeron las hipoglucemias/semana (2,52±1,66 vs. 0,53±1,06; p<0,05), sin cambio en la HbA1c final (7,4±0,8%). Las competencias educativas, el estilo de vida, la adhesión al cuidado y la percepción de calidad de vida mejoraron a los 6 meses (p<0,05). Al finalizar el programa, los pacientes consideraron imprescindible el papel de la EPA (98%).
ConclusionesLa implementación de un PAET con la incorporación de una EPA en diabetes en AP permite en su conjunto la mejora de resultados clínicos, educativos y de satisfacción en pacientes con DT2 tratados con ≥ 2 dosis de insulina y control subóptimo.
Diabetes mellitus is one of the biggest problems in public health due to its high prevalence, morbidity and mortality and the substantial associated healthcare costs.1 In Spain, according to the di@betes study, the prevalence of type 2 diabetes mellitus (T2DM) is 13.8% and the estimated prevalence of unknown diabetes is 6%.2 The risk of having the disease increases with age, obesity and sedentary lifestyle, and it is associated with hypertension and dyslipidaemia, thus carrying a high cardiovascular risk.3 There is a direct relationship between the duration of the disease and the percentage of subjects treated with injectable drugs, mainly insulin. Poorly controlled T2DM is associated with a higher prevalence of chronic complications, worse quality of life, lower treatment satisfaction and higher healthcare expenditure.4 Treatment intensification includes the use of several doses of insulin. In some cases, it is pursued late, in advanced stages of the disease, when patients with T2DM are referred for specialised care.
At present, treatment education is part and parcel of comprehensive care for individuals with diabetes. Programmes give structure to care and treatment education, and are necessary to empower patients in matters of lifestyle changes, drug management and prevention and treatment of complications. Structured educational interventions yield improved clinical outcomes, adherence and quality of life; better use of healthcare services and less use of emergency services and hospitalisation due to acute decompensation.5–8 In addition, programmes should be adapted to specific populations, with consideration for type of diabetes and ethnic, social, cognitive, literacy and cultural factors. Access to these programmes remains one of the most significant barriers around the world.9 In this context, advanced practice nurses (APNs) in diabetes in specialised care in the primary care (PC) setting were incorporated into such programmes. These professionals are responsible for designing, implementing and evaluating a structured programmed intended for patients with T2DM. The International Council of Nurses defines an APN as a “nurse who has acquired…the expert knowledge base, complex decision-making skills and clinical competencies”.
Our objective was to evaluate the effectiveness of a structured programme intended for patients with T2DM being treated with ≥2 doses of insulin with poor metabolic control: the Programa de Atención y Educación Terapéutica de Optimización dirigido a personas con Diabetes Tipo 2 [Healthcare and Treatment Education Optimisation Programme intended for people with Type 2 Diabetes Mellitus] (T2DM Optimisation HTEP), which included an APN in diabetes in specialised endocrinology care in PC.
Material and methodsThis was a prospective, longitudinal, pre/post-intervention, uncontrolled study with a duration of 18 months. Clinical and educational data were evaluated at baseline and after six months. The study was approved by the Independent Ethics Committee (HCB/2018/0075). Subjects with T2DM being treated with ≥2 doses of insulin with poor metabolic control from nine Centres d’Atenció Primària (CAPs) from the Àrea Integral de Salut Barcelona Esquerra [Barcelona Esquerra Comprehensive Health Area] (AISBE) that form part of the area of influence of Hospital Clínic de Barcelona [Clinical Hospital of Barcelona] (CAPs: Montnegre, Numància, Comte Borrell, Casanova, Roger, La Marina, Les Corts, Les Hortes and Doctor Carles Ribas) were included between October 2016 and February 2018. At these centres, specialised care in Endocrinology is provided from the Reordenació de l’Atenció Especialitzada [Reorganisation of Specialised Care] (RAE) by endocrinology and nutrition specialists (ENSs) from Hospital Clínic de Barcelona. With the consensus of the different healthcare providers in the region and the Diabetes Unit of the Endocrinology and Nutrition Department of this hospital, the project of incorporating the role of the RAE diabetes APN (RAEDAPN) into the PC setting was set in motion. One of the objectives of the RAEDAPN was the implementation of an HTEP intended for subjects with T2DM being treated with ≥2 doses of insulin with suboptimal metabolic control requiring a referral to an ENS.
Suboptimal metabolic control was arbitrarily defined as: glycosylated haemoglobin (HbA1c) >7.5% in subjects under 75 years of age with no chronic complications or serious comorbidities; in subjects over 75 years of age, HbA1c >8.0% or chronic complications or serious comorbidities; or subjects who, without exceeding these HbA1c levels, had frequent episodes of hypoglycaemia (capillary blood glucose <70mg/dl more than twice weekly) or serious ones (episodes of hypoglycaemia requiring third-party intervention) and were referred to the endocrinology RAE to improve their control.
Those with cognitive impairment who had no family support and those who were not candidates for the intervention due to associated comorbidity or in the opinion of the endocrinology team, as well as those who did not wish to be followed up by specialised care, were excluded.
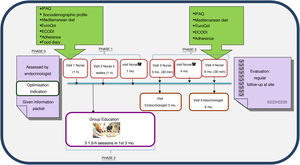
Characteristics of the T2DM optimisation HTEP structured programmeThe programme was agreed upon within the chronic disease group for diabetes of the AISBE with the support of PC and Hospital Clínic management. The programme has a duration of six months and is structured in individual visits with the ENS and the RAEDAPN, telephone visits and group education. The RAEDAPN agrees upon schedules for visits, resources, cadences, contents and educational materials as well as the methodology used in the individual visits and group intervention.10 The PC teams (the patients’ primary family medicine and nursing teams) were informed of the subject’s inclusion in the programme.
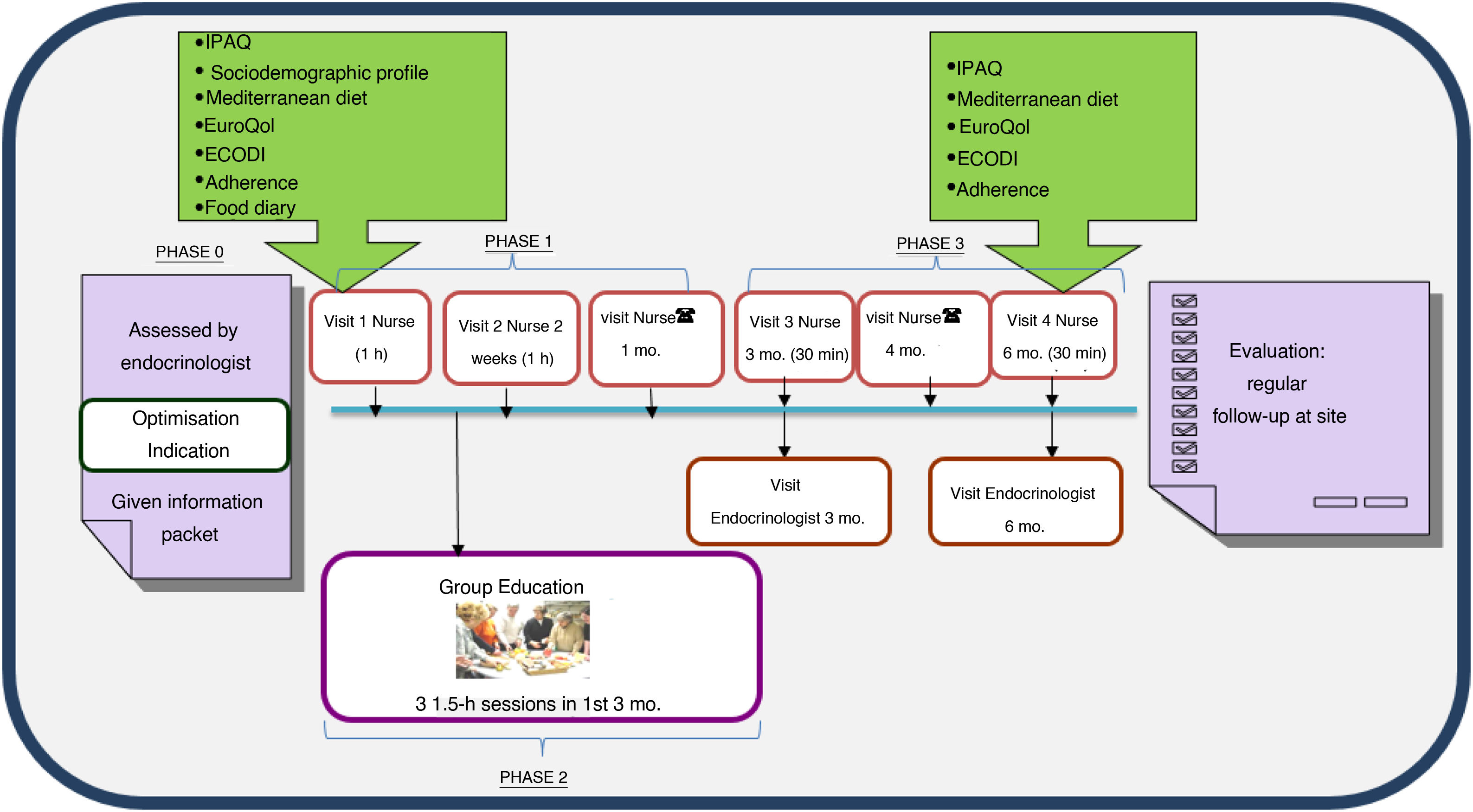
The programme consists of three phases (Fig. 1).
Phase 1, personalised education: this includes an joint initial visit in which the subject is included in the programme at the discretion of the ENS. The programme information sheet is provided and questionnaires (see below) are administered to determine the subject’s needs, and the intervention is adapted with personalised objectives. After two weeks, another visit is made with the aim of assessing adherence to and effectiveness of the changes in treatments and lifestyle and the educational recommendations agreed upon with the subject or the family. A month after inclusion, a telephone visit is made with the RAEDAPN to answer questions, assess control targets and rule out acute complications and possible adverse effects.
Phase 2, standardised group education: this consists of three sessions per subject or family of 1.5h per session, in groups of up to 10 people, to be conducted within three months of inclusion. Standardised materials and educational content centred on three themes — diabetes and strategies for improving control, healthy eating and adaptation, and acute/chronic complications and abilities in insulin therapy use — are used.
Phase 3, follow-up: this consists of joint visits three and six months after inclusion and a telephone visit four months after inclusion. The need for intermediate visits with the ENS or the RAEDAPN is assessed. When the programme ends, regular follow-up by the ENS and the patient’s PC team continues. Should the patient exhibit criteria for re-entry into the programme, follow-up with the RAEDAPN will resume.
Variables analysedSociodemographic variables: age, gender, country of origin, language barrier and occupational status.
Clinical variables: family history of diabetes, cardiovascular risk factors (blood pressure, weight, height, body mass index and waist circumference) and metabolic control (HbA1c, lipid panel, creatinine and albumin-to-creatinine ratio). Types of drug treatment and non-drug treatment of diabetes were recorded. The presence of chronic complications (retinopathy, nephropathy, ischaemic heart disease, cerebrovascular disease, vascular disease and peripheral neuropathy) was evaluated.
Lifestyle and treatment education variables: at baseline and after six months, the following were evaluated using validated questionnaires: knowledge of diabetes (Escala de Conocimientos sobre la Diabetes (Diabetes Knowledge Scale) [ECODI], 0–14),11 adherence to treatment self-management (Self-Care Inventory-Revised [SCI-R]),12 adherence to the Mediterranean diet (0–14)13 and physical activity (International Physical Activity Questionnaire [IPAQ], 1–3),14 and subjective perception of health status (European Quality of Life-5 Dimensions [EuroQoL-5D], 0–10).15
Variables in relation to patient experience and satisfaction with the HTEP: these were evaluated using an anonymous (ad hoc) survey at the end of the programme.
Organisation/management variables: effectiveness and compliance with the structure of the programme measured by number of subjects included, attendance to group education, number of visits made per ENS and RAEDAPN, telephone visits, hospital admissions and emergency visits for diabetes-related reasons.
Statistical analysisResults are expressed in terms of mean±standard deviation or proportions. Intergroup comparisons were made using Student’s t test for unpaired and paired data, or using the Mann–Whitney U test. Proportions were compared using Fisher’s exact test. Strengths of association between two variables were calculated using Pearson's correlation coefficient. A p value <0.05 was considered statistically significant. Data analysis was performed with the SPSS software programme, version 20.0 (SPSS Inc., Chicago, IL, United States).
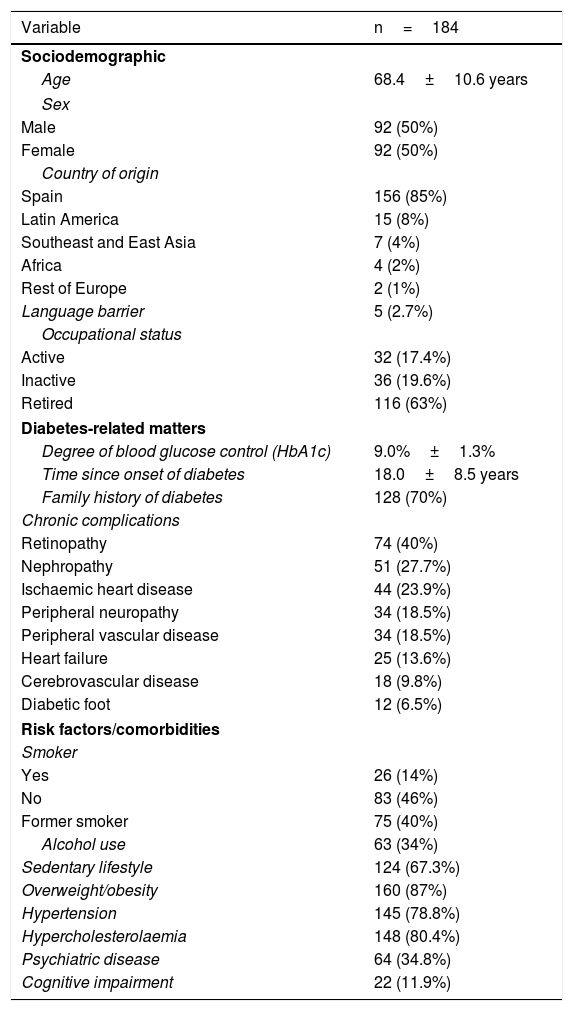
ResultsParticipant characteristicsA total of 184 patients with T2DM being treated with ≥2 doses of insulin were included. Table 1 shows their clinical characteristics. Of the subjects, 89.4% (n=144) were included in the programme due to poor metabolic control (poor metabolic control group [PMCG]) and 10.6% (n=17) were included due to repeat episodes of hypoglycaemia (poor control due to episodes of hypoglycaemia group [PCHG]).
Sociodemographic profile of the subjects included.
| Variable | n=184 |
|---|---|
| Sociodemographic | |
| Age | 68.4±10.6 years |
| Sex | |
| Male | 92 (50%) |
| Female | 92 (50%) |
| Country of origin | |
| Spain | 156 (85%) |
| Latin America | 15 (8%) |
| Southeast and East Asia | 7 (4%) |
| Africa | 4 (2%) |
| Rest of Europe | 2 (1%) |
| Language barrier | 5 (2.7%) |
| Occupational status | |
| Active | 32 (17.4%) |
| Inactive | 36 (19.6%) |
| Retired | 116 (63%) |
| Diabetes-related matters | |
| Degree of blood glucose control (HbA1c) | 9.0%±1.3% |
| Time since onset of diabetes | 18.0±8.5 years |
| Family history of diabetes | 128 (70%) |
| Chronic complications | |
| Retinopathy | 74 (40%) |
| Nephropathy | 51 (27.7%) |
| Ischaemic heart disease | 44 (23.9%) |
| Peripheral neuropathy | 34 (18.5%) |
| Peripheral vascular disease | 34 (18.5%) |
| Heart failure | 25 (13.6%) |
| Cerebrovascular disease | 18 (9.8%) |
| Diabetic foot | 12 (6.5%) |
| Risk factors/comorbidities | |
| Smoker | |
| Yes | 26 (14%) |
| No | 83 (46%) |
| Former smoker | 75 (40%) |
| Alcohol use | 63 (34%) |
| Sedentary lifestyle | 124 (67.3%) |
| Overweight/obesity | 160 (87%) |
| Hypertension | 145 (78.8%) |
| Hypercholesterolaemia | 148 (80.4%) |
| Psychiatric disease | 64 (34.8%) |
| Cognitive impairment | 22 (11.9%) |
Data are presented in terms of mean±SD and number (%).
Ultimately, 87.5% (n=161) of the subjects completed the six months of follow-up and 12.5% (n=23) did not (8% died, 21.7% were admitted to community health centres/home care/hospital and 69.5% left follow-up for unknown reasons). The characteristics of the patients who completed the programme were similar to the initial sample.
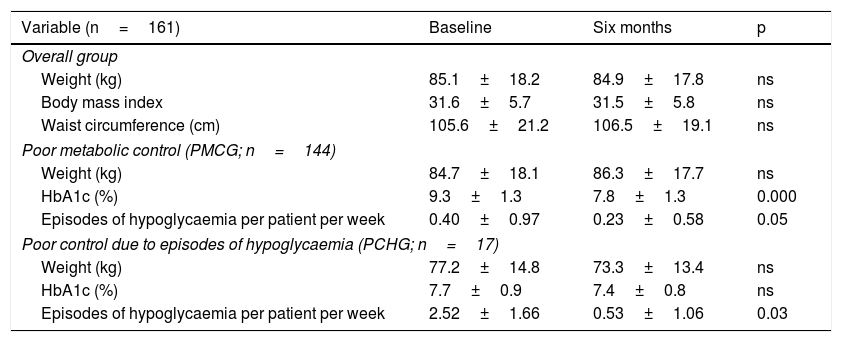
Clinical, anthropometric and treatment resultsA statistically significant reduction in HbA1c was seen in the overall group six months (9.0%±1.3% versus 7.8%±1.3%; p=0.000) from the start of the programme, associated with a reduction in the number of episodes of mild hypoglycaemia per patient per week (0.62±1.20 versus 0.26±0.65; p=0.001). There were no significant differences in weight, body mass index or waist circumference compared to baseline. Initially, just 3.1% of the subjects in the overall group had an HbA1c <7%, 8.7% had an HbA1c <7.5% and 14.3% had an HbA1c <8%. After six months, these percentages improved significantly to 20%, 42% and 62%, respectively (p<0.001). These results were similar in the PMCG, while the PCHG showed a reduction in the number of episodes of hypoglycaemia after six months, with no significant changes in HbA1c or weight (Table 2). The subjects included in the PMCG were younger (67.6±10.5 versus 75.1±9.6 years of age; p=0.007) and had higher HbA1c levels at baseline (9.3%±1.3% versus 7.7%±0.9%; p=0.000) than those included in the PCHG. The reduction in HbA1c was greater in the PMCG than in the PCHG (–1.34%±1.45% versus –0.35%±0.65%; p=0.000); comparable HbA1c levels were achieved at the end of follow-up in the two groups.
Results by reason for inclusion.
| Variable (n=161) | Baseline | Six months | p |
|---|---|---|---|
| Overall group | |||
| Weight (kg) | 85.1±18.2 | 84.9±17.8 | ns |
| Body mass index | 31.6±5.7 | 31.5±5.8 | ns |
| Waist circumference (cm) | 105.6±21.2 | 106.5±19.1 | ns |
| Poor metabolic control (PMCG; n=144) | |||
| Weight (kg) | 84.7±18.1 | 86.3±17.7 | ns |
| HbA1c (%) | 9.3±1.3 | 7.8±1.3 | 0.000 |
| Episodes of hypoglycaemia per patient per week | 0.40±0.97 | 0.23±0.58 | 0.05 |
| Poor control due to episodes of hypoglycaemia (PCHG; n=17) | |||
| Weight (kg) | 77.2±14.8 | 73.3±13.4 | ns |
| HbA1c (%) | 7.7±0.9 | 7.4±0.8 | ns |
| Episodes of hypoglycaemia per patient per week | 2.52±1.66 | 0.53±1.06 | 0.03 |
Data are presented in terms of mean±SD and p.
Over the course of follow-up, there was one episode of serious hypoglycaemia in a context of excessive alcohol use and an error in the insulin dose administered.
Six months after the start of the programme, improvements were seen in levels of total cholesterol (176±45mg/dl versus 163±41mg/dl; p=0.000), triglycerides (200±172mg/dl versus 172±121mg/dl; p=0.001) and LDL cholesterol (94±42mg/dl versus 84±28mg/dl; p=0.008). Figures for systolic blood pressure (139±19mmHg versus 137±16mmHg), diastolic blood pressure (75±15mmHg versus 75±10mmHg), albuminuria-to-creatinine ratio (130±358mg/g versus 92±219mg/g) and 10-year cardiovascular risk estimate using Registre Gironí del Cor [Girona Heart Registry] (REGICOR) tables (7.7±7.9 versus 6.7±3.6) showed no significant differences at the end of follow-up.
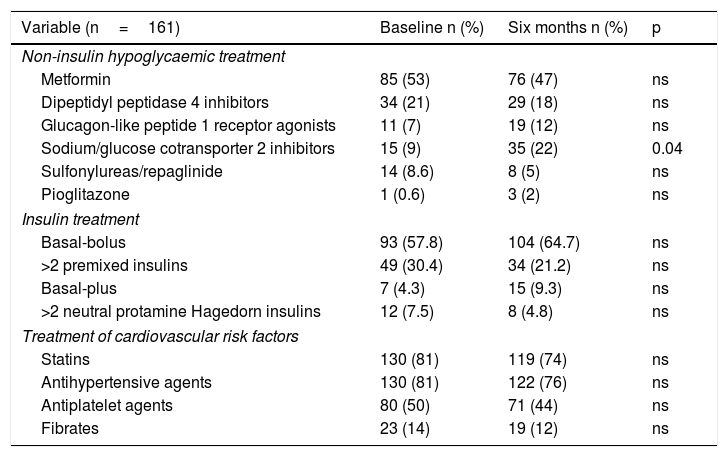
Regarding drug treatment, there were no changes with respect to treatment with non-insulin hypoglycaemic agents, apart from an increase in the percentage of subjects who were taking sodium/glucose cotransporter 2 (SGLT2) inhibitors after six months (9% versus 22%; p=0.04). These subjects were younger (65.4±8.4 versus 69.8±10.6; p=0.015), had suffered from diabetes for more years (15.4±5.8 versus 19.0±9.0; p=0.014), had a higher body mass index (33.8±5.8 versus 30.4±5.2; p=0.000) and were receiving more total daily insulin units compared to those not on SGLT2 inhibitors (88.8±43.7 versus 68.9±42.6; p=0.023). Treatment with secretagogues (sulfonylureas/repaglinide) was stopped in six of the 14 subjects who were taking them at the start of the programme.
As for insulin therapy, there were no significant differences in treatment type after six months. Glargine insulin was used as basal insulin by 58.4% (n=94) and insulin was self administered three or more times per day by 68.3% (n=110). At the end of follow-up, 23.6% (n=38) increased their number of injections per day and also increased their basal insulin units (31.7±22.6 versus 36.9±26.5; p=0.000), but not their dose of rapid-acting insulin or their total dose.
Regarding the rate of use of drug treatment of other cardiovascular risk factors, there were no significant differences at the start and end of the programme (Table 3).
Changes in treatment of diabetes and cardiovascular risk factors.
| Variable (n=161) | Baseline n (%) | Six months n (%) | p |
|---|---|---|---|
| Non-insulin hypoglycaemic treatment | |||
| Metformin | 85 (53) | 76 (47) | ns |
| Dipeptidyl peptidase 4 inhibitors | 34 (21) | 29 (18) | ns |
| Glucagon-like peptide 1 receptor agonists | 11 (7) | 19 (12) | ns |
| Sodium/glucose cotransporter 2 inhibitors | 15 (9) | 35 (22) | 0.04 |
| Sulfonylureas/repaglinide | 14 (8.6) | 8 (5) | ns |
| Pioglitazone | 1 (0.6) | 3 (2) | ns |
| Insulin treatment | |||
| Basal-bolus | 93 (57.8) | 104 (64.7) | ns |
| >2 premixed insulins | 49 (30.4) | 34 (21.2) | ns |
| Basal-plus | 7 (4.3) | 15 (9.3) | ns |
| >2 neutral protamine Hagedorn insulins | 12 (7.5) | 8 (4.8) | ns |
| Treatment of cardiovascular risk factors | |||
| Statins | 130 (81) | 119 (74) | ns |
| Antihypertensive agents | 130 (81) | 122 (76) | ns |
| Antiplatelet agents | 80 (50) | 71 (44) | ns |
| Fibrates | 23 (14) | 19 (12) | ns |
Data are presented in terms of number (%) and p.
At the start of inclusion in the programme, 69.9% (n=112) of the subjects had errors in their insulin administration technique (71.7% did not rotate puncture sites, 43% had lipodystrophy and 79% reused needles more than three times) and 28% (n=45) made errors in performing capillary blood glucose checks. Educational reinforcement was required in the eating plan (87.3%; n=140), physical activity recommendations (68%; n=109) and hypoglycaemia prevention/treatment measures (>90%; n=145, <33% had glucagon). After six months, 59.6% (n=96) of the subjects had improved their insulin administration and capillary blood glucose self-check techniques, and 52% (n=84) had improved their adherence to the eating plan.
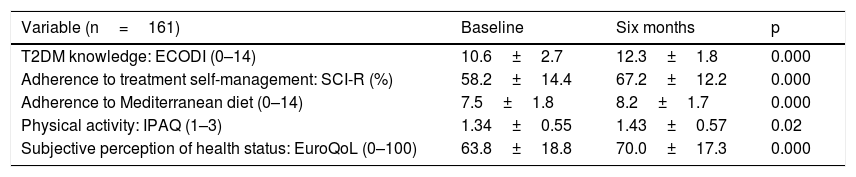
As Table 4 shows, the scores on the different questionnaires had improved significantly at the end of the programme. Comparison of those who completed personalised education and those who participated in the group intervention revealed significant differences only in adherence to treatment self-management (SCI-R), which improved in those who attended the group visit (58.0±27.9 versus 33.9±33.7; p=0.000).
Results of questionnaires at baseline and after six months.
| Variable (n=161) | Baseline | Six months | p |
|---|---|---|---|
| T2DM knowledge: ECODI (0–14) | 10.6±2.7 | 12.3±1.8 | 0.000 |
| Adherence to treatment self-management: SCI-R (%) | 58.2±14.4 | 67.2±12.2 | 0.000 |
| Adherence to Mediterranean diet (0–14) | 7.5±1.8 | 8.2±1.7 | 0.000 |
| Physical activity: IPAQ (1–3) | 1.34±0.55 | 1.43±0.57 | 0.02 |
| Subjective perception of health status: EuroQoL (0–100) | 63.8±18.8 | 70.0±17.3 | 0.000 |
Data are presented in terms of mean±SD, ranges and percentage.
The number of visits planned in the programme design was not changed; in-person and telephone access were offered, and excess additional visits which might compromise PC resources were not made. A total average of 2.6 (0–3) in-person visits with the ENS, 4 (1–6) in-person visits with the RAEDAPN and 1.8 (0–2) telephone visits were made. To optimise resources, some PCCs had combination group sessions. A total of nine sessions were held, with an attendance rate of 44.7% (n=72) of the patients included. None of the patients included were referred to the day hospital at the reference hospital. As mentioned above, just one patient required care from the medical emergency department due to an episode of serious hypoglycaemia.
Patient satisfaction resultsAt the end of the programme, the opinion of the patients included in the T2DM Optimisation HTEP was solicited with an ad hoc questionnaire (n=128). Their assessment of the programme, the information supplied, the coordination between PC and specialised teams and the follow-up performed was positive, with a rating of 4.6/5. Of those surveyed, 98% considered a RAEDAPN necessary, and 92% considered their expectations met at the end of the programme. The opinion of those who attended the group sessions was also evaluated anonymously using an ad hoc questionnaire. Their overall assessment was positive; they considered the information provided and recommendations made useful in improving the management of the disease. Of those who attended group sessions, 97.5% would recommend them to other people with T2DM. They also rated the content as good or excellent (4.8/5).
DiscussionThe results of our study suggest that the HTEP aimed at patients with T2DM being treated with ≥2 doses of insulin with poor metabolic control, featuring the incorporation of an APN into the PC setting, has short- to medium-term clinical and educational benefits. In addition, its assessment by patients was satisfactory.
The T2DM Optimisation HTEP is an educational programme new to our region, set in motion in the PC setting, that managed to demonstrate improvements in metabolic control in subjects with T2DM being treated with ≥2 doses of insulin with suboptimal control, without increasing their weight or number of episodes of hypoglycaemia. The programme was designed based on the evidence obtained in similar programmes already implemented in our area,16,17 with the difference that it was implemented in PC. For that reason it required agreement and approval on the part of the different healthcare providers in the health area who participated in the project.
The clinical outcomes achieved were highly satisfactory. The overall subject group improved their HbA1c by more than one percentage point, with no increase in their weight, with reduction of the number of episodes of hypoglycaemia and with no significant changes in treatment, thanks to a HTEP carried out by a RAEDAPN. Taken together, this triad of positive results could be considered the gold standard in evaluating any intervention in people with T2DM.18 The DAWN2 study in Spain found that just 59% of patients with T2DM and 21% of family members were aware of having received any type of educational activity. The authors recommended including HTEPs in the portfolio of services offered by hospitals to improve access and availability for family members and patients with T2DM with a view to improving self-care, especially at the onset of the disease.19
The results were also positive when analysed by reason for inclusion; subjects with high numbers of episodes of hypoglycaemia decreased the frequency of these episodes without worsening their HbA1c. Multiple studies have determined the frequency of episodes of hypoglycaemia in subjects with T2DM being treated with insulin, but to our knowledge there are no prior studies with an educational programme intended to reduce their frequency. In our case, healthcare was provided with a standardised educational programme featuring personalised control targets in elderly subjects with multiple comorbidities and risk factors, with consideration for patient preferences and risks prior to intensification of a treatment regimen.20 In this regard, secretagogues were stopped and SGLT2 inhibitors were added, especially in younger and obese subjects, according to the recommendations in the clinical guidelines.
Concerning the educational variables of the programme, the positive effects of the programme on knowledge of diabetes and self-management of the disease, treatment adherence and adherence to healthier eating patterns, with no worsening in patient-perceived health, merit special mention. Reinforcement of self-care through treatment education may be useful for subjects with T2DM who have not achieved their blood glucose targets.21 Furthermore, the content and delivery of the programme were rated as satisfactory by the participants. Incorporation of supplementary group dynamics enables more interaction between individuals with the same disease and allows them to share experiences, clear up uncertainties, gain knowledge and reduce the impact of the disease on their quality of life.22
The training of nursing professionals in diabetes is key to the practical application of the roles of the APN.23 RAEDAPN training is master's-level training in diabetes and treatment self-management; teaching/learning abilities; and management, planning and evaluation of educational programmes, as proposed by various scientific associations, such as the Sociedad Española de Diabetes [Spanish Diabetes Society].24
The main limitation of our study lies in its design. A study of these characteristics without a control group does not allow the results obtained to be reliably attributed to the intervention made. Similarly, we cannot be sure of the duration of the results beyond the six months of the intervention, nor can we be certain that its results can be extrapolated to other health areas. However, this type of study in the real world and routine clinical practice aid in evaluating new initiatives in educational programmes and their overall and ongoing outcomes. It should be noted that, thanks to the results of the study, RAEDAPNs have been indefinitely incorporated into specialised endocrinology care in our health area.
In summary, the implementation of a structured programme aimed at patients with T2DM being treated with ≥2 doses of insulin with poor metabolic control, which includes an APN in diabetes in specialised care in PC, is perceived as satisfactory by patients and is associated with clinical and educational benefits.
Conflicts of interestNone of the authors has any conflicts of interest related to the content of the manuscript.
We would like to thank the institutional members who have supported the programme in the region and invested in its continuity: Dr Belén Enfedaque, healthcare director of Atenció Primària Barcelona Ciutat [City of Barcelona Primary Care] of the Institut Català de la Salut [Catalan Health Institute]; Dr Iskra Liguerre and Mr José Losada, operations director and associate director of the Servei d’Atenció Primària Litoral-Esquerra [Litoral-Esquerra Primary Care Service] of the Institut Català de la Salut; Dr Jaume Benavent and Ms Sílvia Roura, manager and associate manager of the Consorci d’Atenció Primària de Salut Barcelona Esquerra; and Dr Francesc Vila and Mr Daniel Lucena, healthcare director and nursing coordinator of the Equip d’Atenció Primària Poble Sec [Poble Sec Primary Care Team]. Finally, we would like to thank Dr Josep Vidal and Ms Inma Pérez, medical director and nursing director of the Institut Clínic de Malalties Digestives i Metabòliques [Clinical Institute of Gastrointestinal and Metabolic Diseases] (ICMDM) at Hospital Clínic de Barcelona; the strategic management of the Àrea Integral de Salut Barcelona Esquerra; and the CAPs team managers, Ms Mónica Gómez and professionals where the study was conducted.
We would also like to thank the patients for their selfless participation.
We are particularly grateful to Novo Nordisk for its non-profit support for this project.
Both authors contributed equally to the content of the manuscript.
Please cite this article as: Cabré Font C, Colungo Francia C, Vinagre Torres I, Jansà i Morató M, Conget Donlo I. Resultados del programa de educación terapéutica de optimización dirigido a pacientes insulinizados con diabetes tipo 2 desarrollado por enfermería de práctica avanzada en diabetes en el ámbito de atención primaria. Endocrinol Diabetes Nutr. 2021;68:628–635.