We evaluated the incidence, progression and the dynamic risk stratification in differentiated thyroid cancer (DTC) under follow-up in a high-resolution clinic (HRC).
MethodsThis was a retrospective observational study on incident cases in the tumor registry from 2002 to 2017 and their evolution under follow-up in HRC.
ResultsA total of 444 patients (78.5% women, 52.1±14.9 mean years old) were DTC diagnosed from 2002 to 2017. The incidence rate of DTC increased from 5.2 to 25.7×105 habitants/year in women and from 2.3 to 7.1×105 habitants/year in men (P<0.0001). This increased incidence was not associated with an increment in the incidental papillary microcarcinoma diagnosed (from 29.4% to 32%).
In those patients undergoing follow-up at the HRC (84% papillary carcinomas), 65.7% were classified as being at a low risk of recurrence compared to 14.5% at high risk. Of those, 88.8% classified as making an excellent response at diagnosis remained disease-free at the final follow-up visit. However, those patients with an indeterminate or structurally incomplete response at diagnosis evolved to an excellent response in 55.8% and 42.9% of the cases, respectively, compared to 14.8% of those with a biochemically incomplete response (P<0.001)
ConclusionsThe increased incidence of DTC is similar to results published previously in other countries. Dynamic risk stratification systems adequately classify DTC patients and assess diagnostic and treatment procedures, especially in low-risk subgroups.
Evaluar la progresión de la incidencia del carcinoma de tiroides (CDT), así como la evolución de los pacientes en seguimiento en una unidad de alta resolución de tiroides (UART) mediante la estadificación dinámica del riesgo.
MétodosEstudio observacional retrospectivo sobre los casos incidentes en el registro de tumores del periodo 2002–2017 y la evolución de los pacientes en seguimiento en la UART.
ResultadosUn total de 444 pacientes (78,5% mujeres, con una edad media de 52,1±14,9 años) fueron diagnosticados durante el periodo 2002–2017. La tasa de incidencia de CDT aumentó de 5,2 a 25,7×105 habitantes/año en mujeres y de 2,3 a 7,1×105 habitantes/año en varones (p<0,0001). El aumento de la incidencia no se asoció a un incremento en el número de microcarcinomas papilares incidentales diagnosticados (de 29,4% a 32%).
Al evaluar a los pacientes en seguimiento en la UART (84% carcinomas papilares), el 65,7% presentaban un riesgo bajo de recurrencia, frente a un 14,5% con riesgo elevado. El 88,8% de los pacientes con respuesta excelente al diagnóstico se mantenían libres de enfermedad en la última visita de seguimiento. Aquellos pacientes con respuesta indeterminada o estructural incompleta al diagnóstico evolucionaban a respuesta excelente en un 55,8% y 42,9% de los casos, respectivamente; frente al 14,8% en aquellos con respuesta bioquímica incompleta (p<0,001).
ConclusionesEl aumento de la incidencia de CDT sigue una tendencia semejante a las publicadas en nuestro medio. Los sistemas de estadificación dinámica del riesgo clasifican adecuadamente a los pacientes y permiten adecuar las herramientas de diagnóstico y tratamiento, especialmente en los subgrupos de bajo riesgo.
Thyroid cancer is the most common type of endocrine cancer and the leading cause of mortality due to endocrine tumours. However, its prevalence in the Spanish population is less than 1%, and it primarily affects middle-aged women.1
Recent decades have witnessed a gradual increase in the incidence of this type of tumour, with the diagnosis of 2–20 cases per 100,000 population per year, in particular cases of papillary microcarcinoma of thyroid follicular cells.2–4 This increase has not been seen to be accompanied by an increase in mortality; hence, at present, there is debate as to whether the increase in incidence corresponds to overdiagnosis or a genuine finding.5
In this context, international clinical guidelines amended their protocols for diagnosis, treatment and follow-up of thyroid nodules and differentiated thyroid carcinoma (DTC) in favour of less aggressive monitoring measures.6,7 Some authors recently reported stabilisation in the incidence of DTC diagnosis in relation to the implementation of the new protocols and the prohibition of widespread thyroid ultrasound screening.8,9
In parallel, predictive models of DTC survival based exclusively on initial diagnosis factors have proven insufficient in long-term assessment, given their static nature.10 The development of dynamic risk staging models in DTC enables assessment of the stage of the patient's disease at any point in its course as these models incorporate treatment response and the biological characteristics of the tumour and they are adapted to the minimum initial treatment required, probable response, mortality and risk of eventual recurrence.11
The objective of this study was to evaluate the progression of the incidence of DTC in the period from 2002 to 2017 and the characterisation of patients in follow-up for DTC on a high-resolution thyroid unit (HRTU) in endocrinology by means of dynamic risk staging and calculation of risk of recurrence.
Material and methodsThis was a retrospective cohort study of all patients diagnosed with thyroid cancer in follow-up on the HRTU of the Endocrinology Department at a tertiary hospital, as well as incident cases of thyroid cancer in the reference area since 2002. The study was approved by the Independent Ethics Committee at the hospital.
The following clinical data were collected: age at diagnosis; year of cancer diagnosis; duration of clinical course in months from the diagnosis of the disease to the last visit; and type and characteristics of cancer diagnosed: size in centimetres, multifocality, capsular invasion, vascular and extrathyroid spread, and TNM staging. In addition, the following were recorded: risk of recurrence following diagnosis (excluding molecular markers) and dynamic staging of risk at 12 months of follow-up after determining initial treatment response and in the last monitoring visit, according to the criteria of the 2015 American Thyroid Association (ATA) guidelines, in patients who underwent surgery with total thyroidectomy and ablation with radioactive iodine6 and modified dynamic risk staging in patients with total thyroidectomy or lobectomy without ablation with radioactive iodine.12
Histology was classified according to the World Health Organization classification13 into two main categories: papillary carcinoma or follicular carcinoma. The latter includes both minimally and widely invasive variants, the clear cell variant and Hürthle cell carcinoma (or the oncocytic variant). Histological variants of papillary carcinoma were grouped into three categories: classic papillary carcinoma, the follicular variant of papillary carcinoma and the more aggressive histological variants (diffuse sclerosing, solid/trabecular, tall cell and columnar cell subtypes). Tumours were classified at diagnosis according to the eighth edition of the TNM classification of the American Joint Committee of Cancer (AJCC).14 Non-metastatic intrathyroid tumours less than or equal to 1cm in diameter with no lymph node involvement were classified as microcarcinoma.
Statistical analysisThe results are expressed in terms of mean and standard deviation (SD). The normal distribution of the variables was analysed using the Kolmogorov–Smirnov test. Quantitative variables with a normal distribution were analysed with a bilateral Student's t test. Non-parametric variables were evaluated using the Mann–Whitney U test. Qualitative variables were expressed in terms of percentages and were analysed using the chi-squared test (with Fisher's correction when necessary). The SPSS statistical software package, version 17.0 (SPSS Inc., Chicago, IL, United States), was used for analysis.
The specific incidence of thyroid cancer was calculated based on data from the Pathology Department registry and the reference population in the health area published by the Regional Ministry of Health. The standardised incidence rates by sex of the European population were calculated by multiplying the specific incidence rates by age of the European standard population and were expressed in terms of 100,000 population per year.
For the statistical calculation of the trend analysis, the Joinpoint Trend Analysis software programme from the United States National Institutes of Health (NIH) was used. Regression analysis of the standardised incidence rates was used to estimate the annual percentage change in trends in the incidence of thyroid cancer over time. The accepted level of statistical significance was 5% (p<0.05).
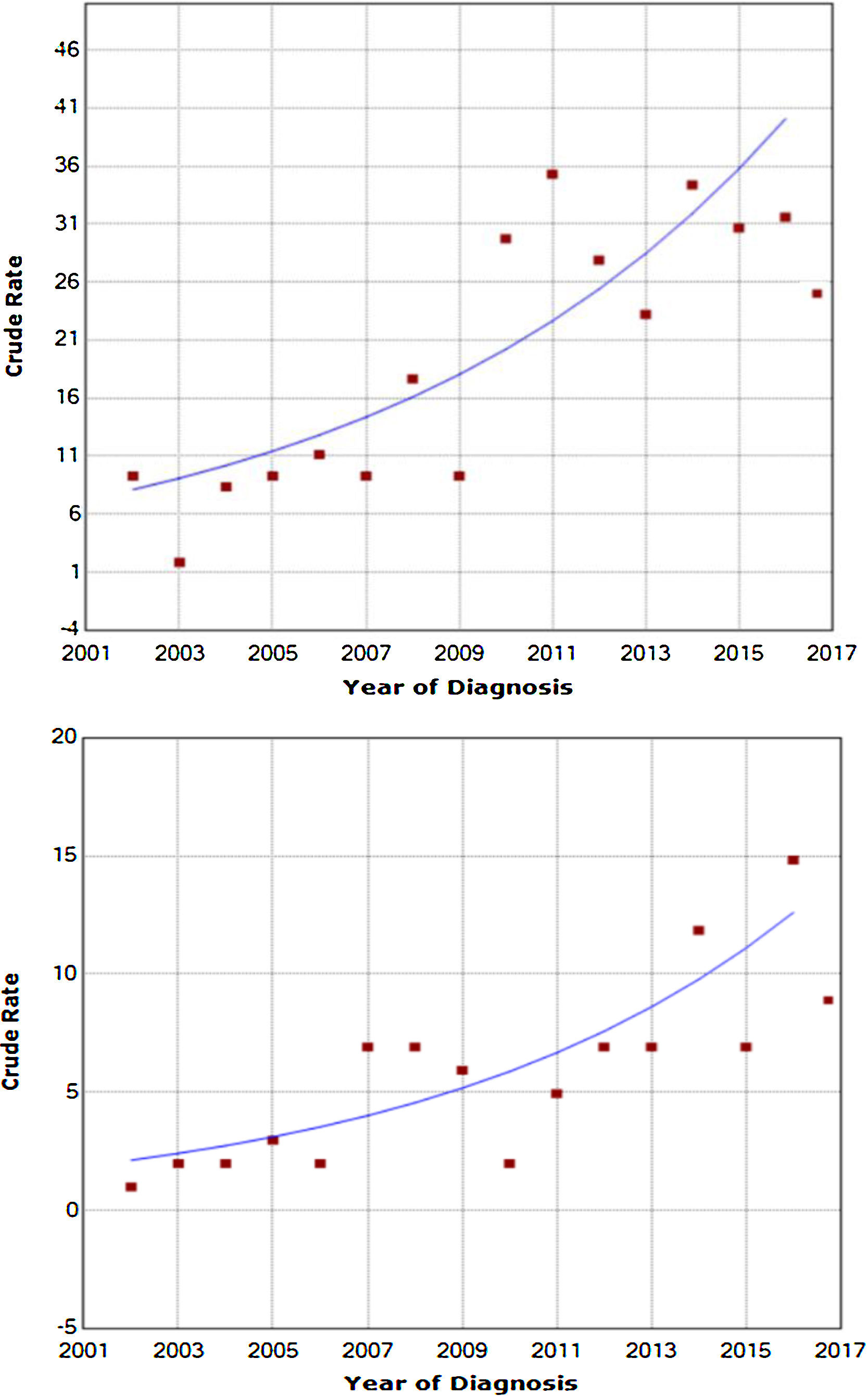
ResultsBetween 2002 and 2017, thyroid cancer was diagnosed in 444 patients; 78.5% were women, and their mean age was 52.1±14.9 years. During this period, the incidence of thyroid cancer was seen to be increased both in women (from 5.2 to 25.7×105 population per year) and in men (from 2.3 to 7.1×105 population per year); this increase was statistically significant (p< 0.0001) (Fig. 1). The increase in the recorded incidence of DTC was not associated with a significant increase in the number of incidental diagnoses of papillary microcarcinoma (29.4% in 2002; 32% in 2017). The annual percentage change in the incidence in men was 13.57%, whereas in women it was 12.08% (p<0.0001).
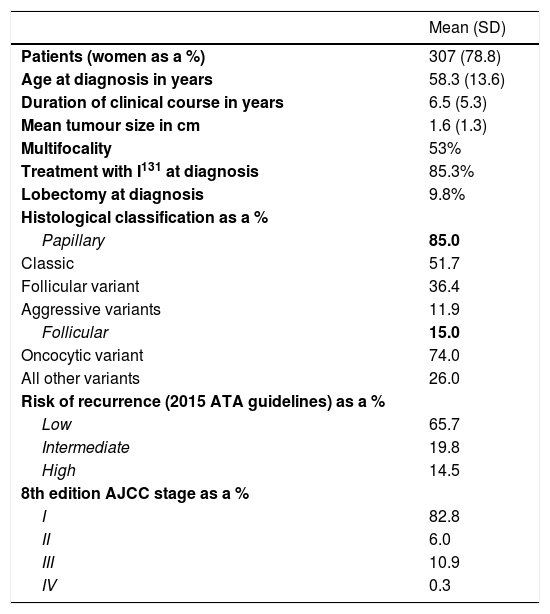
As all patients in follow-up for DTC on the HRTU were analysed, a total of 307 patients were evaluated. Their mean age was 58.3±13.6 years, and their mean follow-up since diagnosis was 6.5±5.3 years. Their mean tumour size was 1.6±1.3cm in diameter. Of the patients, 14.7% did not meet criteria for treatment with I131, and 9.8% were treated with initial lobectomy with curative intent. The predominant histology was papillary carcinoma (51.7% classic papillary carcinoma, 36.4% follicular variant and 11.9% aggressive subtypes), followed by follicular carcinoma (74.0% oncocytic variant). Evaluation of the risk of recurrence at diagnosis in patients with DTC found that 65.7% were at low risk and that 14.5% were at high risk (Table 1).
Characteristics of patients in follow-up for DTC on HRTU.
| Mean (SD) | |
|---|---|
| Patients (women as a %) | 307 (78.8) |
| Age at diagnosis in years | 58.3 (13.6) |
| Duration of clinical course in years | 6.5 (5.3) |
| Mean tumour size in cm | 1.6 (1.3) |
| Multifocality | 53% |
| Treatment with I131 at diagnosis | 85.3% |
| Lobectomy at diagnosis | 9.8% |
| Histological classification as a % | |
| Papillary | 85.0 |
| Classic | 51.7 |
| Follicular variant | 36.4 |
| Aggressive variants | 11.9 |
| Follicular | 15.0 |
| Oncocytic variant | 74.0 |
| All other variants | 26.0 |
| Risk of recurrence (2015 ATA guidelines) as a % | |
| Low | 65.7 |
| Intermediate | 19.8 |
| High | 14.5 |
| 8th edition AJCC stage as a % | |
| I | 82.8 |
| II | 6.0 |
| III | 10.9 |
| IV | 0.3 |
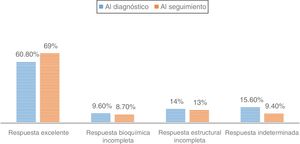
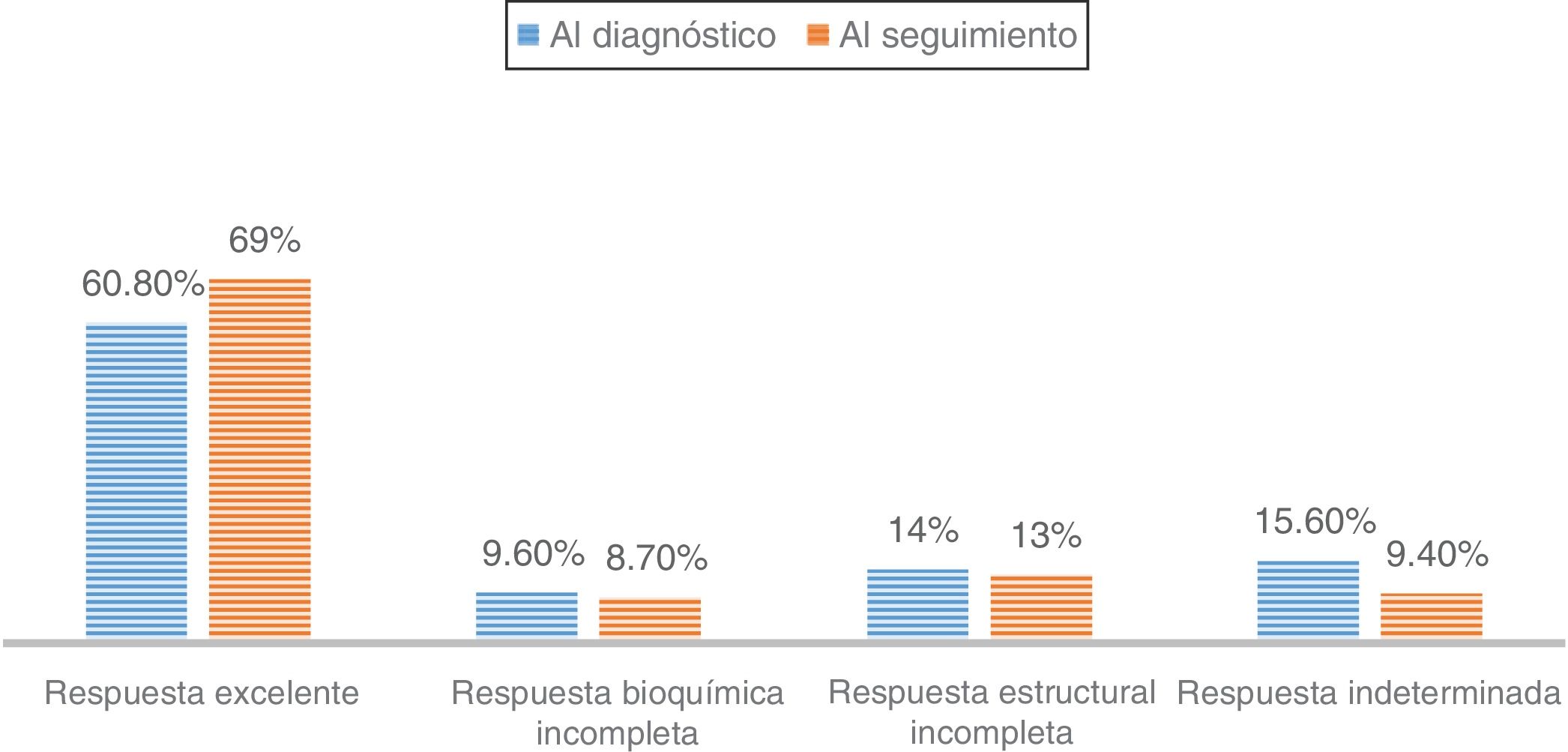
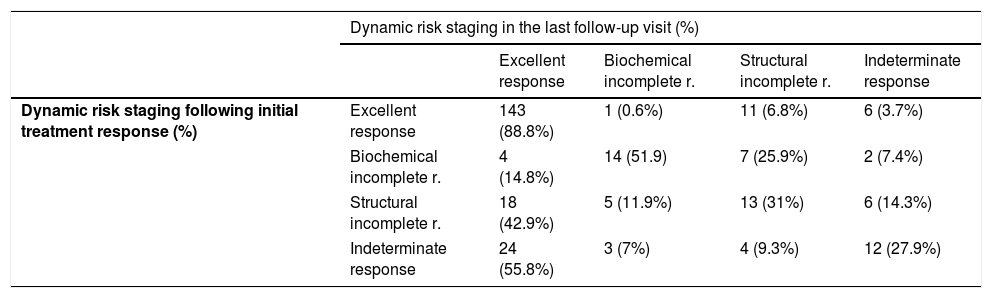
Finally, the percentage of patients with DTC and more than one year of follow-up according to dynamic risk staging at diagnosis and in the last monitoring visit was evaluated (Fig. 2), as was the clinical course of the patients with DTC according to their initial classification (Table 2). Among patients classified as "excellent response" at diagnosis, 88.8% remained disease-free in their last follow up visit, while 6.8% were reclassified as “structural incomplete response”. However, patients with “indeterminate response” or “structural incomplete response” at diagnosis progressed to “excellent response” in 55.8% and 42.9% of cases, respectively, compared to 14.8% of patients with “biochemical incomplete response” (p<0.001) (Table 2).
Changes over time in dynamic risk staging following determination of initial treatment response and in the last DTC follow-up visit (p<0.001).
| Dynamic risk staging in the last follow-up visit (%) | |||||
|---|---|---|---|---|---|
| Excellent response | Biochemical incomplete r. | Structural incomplete r. | Indeterminate response | ||
| Dynamic risk staging following initial treatment response (%) | Excellent response | 143 (88.8%) | 1 (0.6%) | 11 (6.8%) | 6 (3.7%) |
| Biochemical incomplete r. | 4 (14.8%) | 14 (51.9) | 7 (25.9%) | 2 (7.4%) | |
| Structural incomplete r. | 18 (42.9%) | 5 (11.9%) | 13 (31%) | 6 (14.3%) | |
| Indeterminate response | 24 (55.8%) | 3 (7%) | 4 (9.3%) | 12 (27.9%) | |
DTC is by far the most common endocrine neoplasm, and its incidence has been gradually increasing in recent decades in a number of countries (European countries, the United States, South Korea and others). This study is among the first in Spain to evaluate the progression of the incidence of DTC over an extensive, recent period of time, spanning 15 years (2002–2017). The results obtained demonstrate a significant increase in incidence rates adjusted for the European population, total incidence rates and incidence rates by sex, and they are similar to those obtained in other national and international series.2–4
However, this increase in DTC incidence has not escaped debate.15 Multiple epidemiology studies have attempted to evaluate the root cause of this increase, without clearly demonstrating an increase in associated mortality. Some authors have even reported a decrease therein.16 For its part, this increase in DTC subtypes with better prognoses has brought about changes in diagnosis and treatment protocols for DTC and nodular thyroid disease with the goal of developing less aggressive strategies for the patient.6,7
Most of the studies conducted to date have attributed the increase in DTC incidence to indolent subtypes. More specifically, this increase has often been linked to diagnosis of the papillary variant of DTC and, in particular, to forms measuring less than a centimetre detected as incidental findings in supplementary tests performed for another reason or in the evaluation of thyroidectomy procedures.3,9 In fact, there is doubt as to whether this increase in incidence is real or due to overdiagnosis secondary to improvements in imaging techniques (especially in high-resolution ultrasound) and to implementation of screening protocols for thyroid nodule disease, with the associated consequent morbidity and mortality.8,15
In our results, the clear increase in DTC incidence in both sexes was not accompanied by an increase in the diagnosis of forms measuring less than a centimetre or incidentalomas, which remained stable at around 30% over the course of 15-year follow-up period, contrary to the majority opinion to date. However, the most recent published studies with DTC incidence data in the last decade have backed our results and shown an increase in DTC incidence unrelated to an increase in diagnosis of forms measuring less than a centimetre.17 Recent studies have also supported the stabilisation of the increase in DTC incidence, in part as a result of the new criteria for diagnosis and fine needle aspiration of thyroid nodules in the last three years.9 Indeed, this study reflects a stabilisation in recent years in the increase in incidence (especially in women) coinciding with the publication of the new criteria for monitoring and the implementation of the HRTU in 2014; this response will have to be evaluated in future studies.
Particularly significant are the very high percentage of patients, even in specialised DTC follow-up units, with a low risk of recurrence (65.7%) or stage I at diagnosis (82.8%), as reflected in this study. In this context of high incidence and good prognoses, endocrinologist-led HRTUs are being formed as a standardised, efficient way to use resources.18 Therefore, the use of dynamic risk staging is of particular interest, as it enables dynamic evaluation of the clinical course of the patient and the need for added follow-up and treatment at all times.6
This study shows how initial dynamic risk staging influences prognosis even years after diagnosis. It is particularly significant that the percentage of patients labelled in each category at diagnosis and after a mean of 6.5 years of follow-up remained virtually unchanged over time, and only increased slightly in the “excellent response” subgroup (Fig. 2).
Specifically, patients classified at diagnosis as “excellent response” rarely showed substantial changes in their staging during follow-up (11.2%) (Table 2). This reclassification also largely corresponded to suspicious findings on imaging (neck ultrasound), without clear pathological significance, as recently demonstrated.19 In fact, more and more experts are recommending that neck ultrasound be avoided as a monitoring method in patients in this subgroup, given their good prognosis, the risk of morbidity associated with this imaging technique due to discovery of false positive suspicious imaging and the risk of pursuing unnecessary diagnosis and treatment behaviours.20
Ultimately, in the majority group of patients labelled as “excellent response”, the low risk of recurrence would require less intensive follow-up, with the resulting benefit in terms of morbidity and efficient use of resources, though it has not yet been standardised in clinical guidelines.21
By contrast, patients initially classified in any group with active disease or risk group (“biochemical incomplete response”, “structural incomplete response” or “indeterminate response”) are less commonly labelled “excellent response” in the course of follow-up. From this point of view, our results show that more than half of patients initially included in “biochemical incomplete response” remained there during follow-up and 25% were reclassified in the “structural incomplete response” group.22
As for the “indeterminate response” category, different studies have reported findings of lesions during follow-up in 15% of cases; this rate is similar to our rate of around 10%.6
Finally, patients in the “structural incomplete response” category — that is, patients who presented macroscopic disease despite additional treatments — remained in the same dynamic staging in 31% of cases over the course of follow-up. In addition, the “structural incomplete response” category determined the greatest increase in disease-specific mortality. These results are similar to those published by other groups.23
This study represents the daily clinical activity of a HRTU where virtually all follow-up, diagnosis and treatment of nodular and malignant thyroid disease in the reference area are performed. This means that it is a good reflection of the healthcare activity of a highly complex specialised unit in the standardised diagnosis and follow-up of thyroid cancer.
As fundamental limitations of this study, we could point out the relatively limited number of mean years of follow-up (6.5) in a disease that progresses slowly such as DTC, especially its indolent forms. Similarly, the loss to follow-up on HRTUs of a proportion of the patients who underwent surgery (incident cases) in the health area may have been due to multiple reasons unrelated to DTC (home transfer, follow-up at another hospital, death due to an unrelated cause, poor compliance with follow-up, etc.); this limitation is common to all studies of complex diseases with long clinical courses. However, it is not possible to rule out greater loss to follow-up in patients with "excellent response" for years and, therefore, the possibility that mild forms were less represented in this study.
In conclusion, the diagnosis and follow-up of DTC constitute real challenges in a disease with a significant increase in incidence in recent decades, although the majority of patients have good prognoses and high treatment response rates, essentially thanks to initial dynamic risk classification. Dynamic risk staging is a fundamental tool in the management of the patient with DTC as it enables adaptation of follow-up and treatment tools, especially in low-risk subgroups.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Díaz-Soto G, Torres BT, López JJ, García S, Quiñones MÁ, de Luis D. Incidencia y estadificación dinámica del riesgo del carcinoma diferenciado de tiroides en una unidad de alta resolución. periodo 2002–2017. Endocrinol Diabetes Nutr. 2021;68:636–641.






![Increase in DTC incidence in women (annual percentage change [APC] 12.08) and in men (APC 13.57%), respectively (p<0.0001). Increase in DTC incidence in women (annual percentage change [APC] 12.08) and in men (APC 13.57%), respectively (p<0.0001).](https://static.elsevier.es/multimedia/25300180/0000006800000009/v1_202112120659/S2530018021001281/v1_202112120659/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)