The receptor of parathyroid hormone and parathyroid hormone-related-protein (PTH/PTHrp) is located in the cell membrane of target tissues – kidney and osteoblasts. It is a G protein-coupled-receptor whose Gsα subunit is encoded by the GNAS gene. Our aim was to study whether the single nucleotide polymorphism (SNP) T393C of the GNAS gene is associated with renal stones, bone mineral density (BMD), or bone remodelling markers in primary hyperparathyroidism (PHPT).
MethodsAn analysis was made of clinical and biochemical parameters and densitometric values in three areas and their relationship with the T393C SNP of the GNAS gene in 261 patients with primary hyperparathyroidism and in 328 healthy controls. Genotyping was performed using the Custom Taqman® SNP Genotyping assay.
ResultsThe genotype frequencies of GNAS T/C 393 were similar in the control and PHPT groups. No association was found between genotypes and clinical expression of PHPT (renal stones and bone fractures). A nonstatistically significant trend was seen to lower BMD in the lumbar spine, femoral neck, and total hip in both PHPT and control C homozygote subjects.
ConclusionGenetic susceptibility to PHPT related to the GNAS T393C polymorphism or a major influence in its development and clinical expression were found. A C allele-related susceptibility to lower BMD in trabecular bone in both PHPT and control subjects is not sufficient to suggest a more severe clinical expression of PHPT. This trend may be considered as a basis for further studies with larger sample sizes and complementary functional evaluation.
El receptor de la hormona paratiroidea y de la proteína relacionada con la hormona paratiroidea (PTH/PTHrp) está situado en la membrana celular de sus tejidos diana: riñón y osteoblastos. Se trata de un receptor unido a proteina G cuya subunidad Gsα está codificada por el gen GNAS. Nuestro objetivo fue estudiar si el polimorfismo de un sólo nucleótido (SNP) T393C del gen GNAS se asociaba con litiasis renal, densidad mineral ósea (DMO) o marcadores de remodelado óseo en el hiperparatiroidismo primario (HPTP).
MétodosAnalizamos parámetros clínicos, bioquímicos y densitométricos en 3 zonas y su relación con el SNP T393C del gen GNAS en 261 pacientes con HPTP y en 328 controles sanos. El genotipado se realizó utilizando el ensayo Custom Taqman®.
ResultadosLas frecuencias genotípicas del SNP T/C 393 del GNAS fueron similares en ambos grupos control y HPTP. No encontramos ninguna asociación entre los genotipos y la expresión clínica del HPTP (litiasis renal y fracturas óseas). Encontramos una tendencia no estadísticamente significativa hacia una menor DMO en columna lumbar, cuello femoral y cadera en los sujetos control y HPTP portadores del alelo C.
ConclusionesNo encontramos susceptibilidad genética para el desarrollo de PHPT relacionada con el polimorfismo T393C del gen GNAS ni influencia en su expression clínica. Sí hallamos una tendencia hacia niveles menores de DMO en el hueso trabecular relacionada con el alelo C en pacientes con PHPT y en sujetos control sin ser suficiente para sugerir una expresión clínica más grave. Estos resultados pueden ser considerados como un punto de partida para futuros estudios con mayor tamaño muestral y con evaluación funcional complementaria.
Primary hyperparathyroidism (PHPT) is mainly defined by hypercalcemia, high PTH concentrations, low bone mineral density (BMD) and renal lithiasis. Parathyroid adenomas are the main cause or PHPT in which a mutation confers enhanced survival or proliferative rate to a clone of cells. The parathyroid hormone/parathyroid related protein receptor (PTH/PTHrp) is located in the plasma membrane of target tissues, such as kidney cells and bone osteoblasts. PTH and PTHrp bind this receptor in osteoblasts to increase M-CSF and RANKL (RANK ligand) and to decrease osteoprotegerin (OPG) production leading to enhanced osteoclast proliferation and activity. PTH/PTHrp receptor is a G protein coupled receptor. The GNAS gene, located at 20q13.2–13.3, encodes the stimulatory G protein alpha-subunit (Gsα) of heterotrimeric G proteins (subunits α, β, γ).1,2 The main research conducted on GNAS gene is based on its relationship with pseudohypoparathyroidism which is characterized by Gsα mutation, PTH resistance and low bone mineral density (BMD).3–8 The T393C single nucleotide polymorphysm (SNP) causes a silent T>C substitution; however, it is believed to change the Gsα mRNA folding and therefore its expression. It has been found that the TT genotype is associated with increased Gsα mRNA expression in different tissues.9 Furthermore, different in vitro experiments suggest that increased expression of Gsα is associated with enhanced apoptosis.10–12 In previous studies, the TT genotype of the T393C SNP (rs7121) in the gene GNAS has been related to better clinical outcomes in patients with carcinomas of the urinary bladder, kidney, colorectum and oro/hypopharynx.9,13–15 This relationship is supposed to be mediated by an increase in the apoptotic rate in the TT genotype.
The main cellular disturbance underlying parathyroid adenomas is enhanced proliferative rate or survival, and it is known that T393C SNP of the GNAS gene may influence the apoptotic cellular rate. Besides, mutations on Gsα can confere PTH resistance and low BMD as seen in hypoparathyroidism. This is the rationale of the current work and our aim was to study the association between the T393C SNP of the GNAS and the clinical expression of PHPT.
Material and methodsThe study population consisted of 261 Caucasian patients with sporadic PHPT. Every high level of ionic and/or total calcium and/or PTH was studied to confirm the PHPT diagnosis. Cases were defined by chronic hypercalcemia (high concentrations of total and/or ionic calcium in 2 or more biochemical test in 12 months time or more) plus high levels of PTH, or plus inappropriately normal PTH levels which means hypercalcemia and PTH values at the upper limit of the normal range. We excluded subjects with chronic renal failure, normocalcemic hyperparathyroidism cases, familial hypocalciuric hypercalcemia, and the few cases of PHPT that were part of a Multiple Endocrine Neoplasia Syndrome. After cautiously reviewing the family clinical history, we also excluded cases of possible hereditary isolated PHPT.
In order to study the allelic distribution in PHPT versus healthy population, 328 volunteers were enrolled through face to face and written requests from hospital workers, and through civic associations, religious groups and geriatric residences. The presence of PHPT was excluded in all of the control subjects by measuring albumin corrected total calcium and PTH.
Written informed consent was obtained from every participant. This study was approved by the Ethics Committee of our hospital (University Hospital Marqués de Valdecilla, Santander, Spain).
Subjects younger than 18 years old, subjects with non-Spanish ancestry, subjects with history of chronic renal failure or diseases known to affect skeletal homeostasis, or subjects who were taking drugs which interfere with bone metabolism (biphosphonates, strontium ranelate, corticosteroids, anti-epileptics, estrogens, thiazides, hormonal replacement therapy with estrogens in women beyond 55 years old) were excluded.
Fracture condition was defined by any traumatic or spontaneous fracture at any location except the nose, toe, head, jaw, skull and hands. The renal lithiasis condition was defined by any detected lithiasis by abdominal ultrasonography with or without symptoms.
Measurement of BMDBMD was quantified using X-ray absorptiometry (DXA, Hologic, Walthan, MA, USA) in the lumbar spine (L2–4), femoral neck, total hip and radius. The BMD measurement variation coefficient was 1.2% in lumbar spine, total hip locations and radius projection, and 1.4% in the femoral neck.
DNA analysisGenomic DNA was extracted from venous blood (buffy coat) by the Qiagen method (Hilden, Germany) and stored at −40°C until analyzed. Polymerase chain reaction (PCR) products were amplified in a 5μl reaction following the instructions of the manufacturer in an Applied Thermal Cycler 9700 (Applied Biosystems). Cycling conditions consisted of an initial denaturation step at 95°C for 10min and 48 cycles of denaturation at 92°C for 30s and annealing for 1min at 60°C. After amplification, end-point fluorescence reading and allele identification were carried out with an ABI 7300 sequence detector (Applied Biosystems). Random samples (8% of the total samples) were analyzed twice for quality control.
The SNP to be analyzed was rs7121 or 393T/C of the GNAS gene. Genotyping was performed with Custom Taqman® SNP Genotyping assays (Assay-by-Design) (Applied Biosystems, Warrington, Cheshire, UK) using allele discrimination-specific Taqman probes, labelled with VIC and FAM. The following primer sequence was used: CCCGAGAACCAGTTCAGAGT (forward) and GGAAGTCAAAGTCAGGCACGTT (reverse).
Biochemical analysisFasting venous blood samples, for general biochemical analysis and specific determinations were obtained after 30min of supine rest from an antecubital vein. Samples were centrifuged immediately and serum was stored at −40°C in multiple aliquots until assayed to avoid freeze cycles. Total calcium (in serum and urine) and alkaline phosphatase were measured by automated methods in an ADVIA 2000 (Siemens Corp., Tarrytown, NY, USA); intra- and inter-assay variations were <2%. Serum total calcium was corrected by albumin. Ionized calcium was measured by calcium-selective electrodes automated in a Ciba Corning 634 Ca++/pH Analyzer (Ciba Corning Diag. Corp, Medfield, Massachusetts, USA). Intact PTH was determined by automated immunoassay in a Liason (DiaSorin, Stillwater, Minnesota, USA), the sensitivity of the assay was 5pg/mL, and intra- and inter-assay variabilities were <5 and <8%, respectively. 25-hydroxy-vitamin D3 (25-OH-vitamin D) was measured by radioimmunoassay after extraction (DiaSorin, Stillwater, Minnesota, USA); the minimum detectable concentration was estimated to be 1.5ng/mL and intra- and inter-assay precision were 9.4 and 10.8%, respectively. Bone alkaline phosphatase was determined by immunoassay (Alkphase B kit, Metra Biosystems, Mountain View, CA, USA), and the minimum detectable dose was 0.7U/L, intra- and inter-assay variabilities were 3.5 and 6.2%, respectively and specificity: bone: 100%; liver: 3–8% and intestine: 0.4%. Serum osteocalcin was measured by immunoradiometric assay (IRMA) (OSTEO-RIACT kit, CIS Bio international, Gif-sur-Yvette, France), and the sensitivity was 0.4ng/mL, and intra- and inter-assay variabilities were 2.0 and 4.4%, respectively. Collagen type I N-terminal propeptide levels were measured by RIA (Orion Diagnostica, Espoo, Finland), and the sensitivity was 2μg/L and intra- and inter-assay variabilities were 8.75 and 5%, respectively. Crosslaps were measured by ELISA (Nordic Bioscience Diagnostics, Herlev Hovedgade, Denmark), and the sensitivity of assay was 0.010ng/mL and intra- and inter-assay variabilities were 5.1 and 6.6%, respectively. All biochemical and DXA procedures were performed at time of diagnosis and before any specific treatment was initiated.
Statistical analysisThe results were processed with the computer package SPSS (Statistical Package for Social Sciences, Chicago, IL, USA). Normal or non-normal distribution of the variables under study was analyzed with the Kolmogorov–Smirnov test. All data were expressed as mean±SD (standard deviation of the mean), or the median and range for non-normal variables. Allele frequencies were estimated by counting, and the χ2 test was used to identify significant departure from the Hardy–Weinberg equilibrium and for allele frequencies related to control and PHPT patients as well. In order to test association between the SNP studied and PHPT, an odds ratio between cases and controls was calculated in addition. Fracture and nephrolithiasis frequency in the different genotype groups was tested by odds ratio. Differences in age, body mass index, bone mineral density (BMD) and bone remodelling markers between genotype groups were examined using the student-t test or U Mann–Whitney test adjusted for age, BMI and gender after grouping heterozygote and TT homozygote subjects.
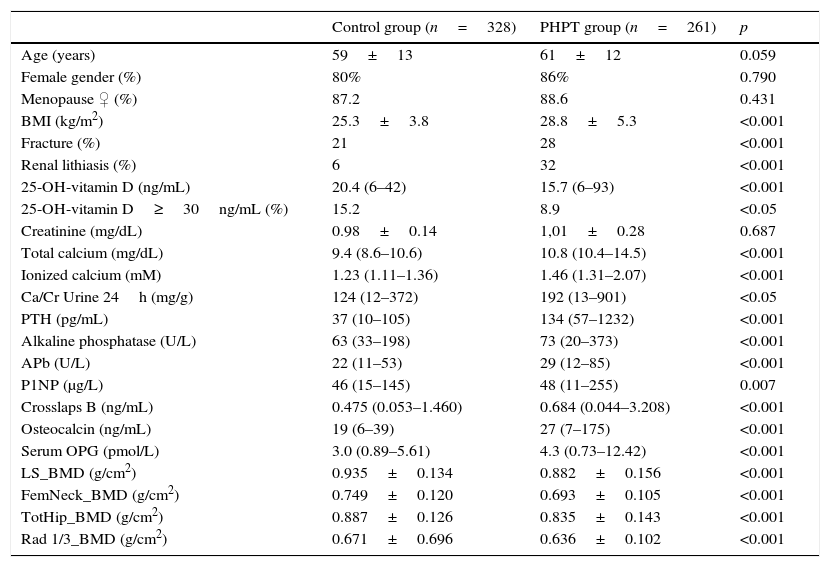
ResultsGeneral characteristics, bone biochemical parameters and BMD measurements of control and PHPT subjects are shown in Table 1. PHPT subjects had higher BMI and lower levels of 25-OH-vitamin D than control subjects.
General features of the 2 study groups: control and PHPT subjects.
| Control group (n=328) | PHPT group (n=261) | p | |
|---|---|---|---|
| Age (years) | 59±13 | 61±12 | 0.059 |
| Female gender (%) | 80% | 86% | 0.790 |
| Menopause ♀ (%) | 87.2 | 88.6 | 0.431 |
| BMI (kg/m2) | 25.3±3.8 | 28.8±5.3 | <0.001 |
| Fracture (%) | 21 | 28 | <0.001 |
| Renal lithiasis (%) | 6 | 32 | <0.001 |
| 25-OH-vitamin D (ng/mL) | 20.4 (6–42) | 15.7 (6–93) | <0.001 |
| 25-OH-vitamin D≥30ng/mL (%) | 15.2 | 8.9 | <0.05 |
| Creatinine (mg/dL) | 0.98±0.14 | 1,01±0.28 | 0.687 |
| Total calcium (mg/dL) | 9.4 (8.6–10.6) | 10.8 (10.4–14.5) | <0.001 |
| Ionized calcium (mM) | 1.23 (1.11–1.36) | 1.46 (1.31–2.07) | <0.001 |
| Ca/Cr Urine 24h (mg/g) | 124 (12–372) | 192 (13–901) | <0.05 |
| PTH (pg/mL) | 37 (10–105) | 134 (57–1232) | <0.001 |
| Alkaline phosphatase (U/L) | 63 (33–198) | 73 (20–373) | <0.001 |
| APb (U/L) | 22 (11–53) | 29 (12–85) | <0.001 |
| P1NP (μg/L) | 46 (15–145) | 48 (11–255) | 0.007 |
| Crosslaps B (ng/mL) | 0.475 (0.053–1.460) | 0.684 (0.044–3.208) | <0.001 |
| Osteocalcin (ng/mL) | 19 (6–39) | 27 (7–175) | <0.001 |
| Serum OPG (pmol/L) | 3.0 (0.89–5.61) | 4.3 (0.73–12.42) | <0.001 |
| LS_BMD (g/cm2) | 0.935±0.134 | 0.882±0.156 | <0.001 |
| FemNeck_BMD (g/cm2) | 0.749±0.120 | 0.693±0.105 | <0.001 |
| TotHip_BMD (g/cm2) | 0.887±0.126 | 0.835±0.143 | <0.001 |
| Rad 1/3_BMD (g/cm2) | 0.671±0.696 | 0.636±0.102 | <0.001 |
PHPT: primary hyperparathyroidism. Data shown as mean±SD or median and range for non-normal variables. BMI: body mass index, Ca/Cr: calcium/creatinine, PTH: parathormone, APb: bone alkaline phosphatase, OPG: osteoprotegerin, LS: lumbar spine, fem. neck: femoral neck, tot hip: total hip, 1/3 Rad: third distal radius, and BMD: bone mineral density.
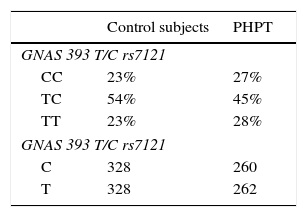
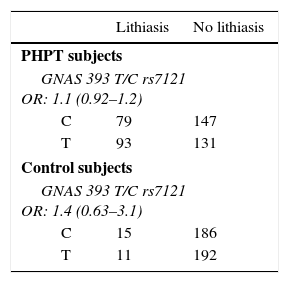
Allelic frequencies did not deviate from the Hardy–Weinberg equilibrium. Genotype frequencies for both controls and primary hyperparathyroidism subjects were similar (Table 2). We did not find any difference in nephrolithiasis or fracture rates between genotypes in the PHPT group nor in control subjects (Table 3).
Odds ratio for lithiasis and fracture condition in primary hyperparathyroidism and control subjects.
| Lithiasis | No lithiasis | |
|---|---|---|
| PHPT subjects | ||
| GNAS 393 T/C rs7121 OR: 1.1 (0.92–1.2) | ||
| C | 79 | 147 |
| T | 93 | 131 |
| Control subjects | ||
| GNAS 393 T/C rs7121 OR: 1.4 (0.63–3.1) | ||
| C | 15 | 186 |
| T | 11 | 192 |
| Fractures | No fractures | |
|---|---|---|
| PHPT subjects | ||
| GNAS 393 T/C rs7121 OR: 1.4 (0.84–1.46) | ||
| C | 45 | 153 |
| T | 49 | 167 |
| Control subjects | ||
| GNAS 393 T/C rs7121 OR: 1.08 (0.48–2.4) | ||
| C | 13 | 152 |
| T | 13 | 164 |
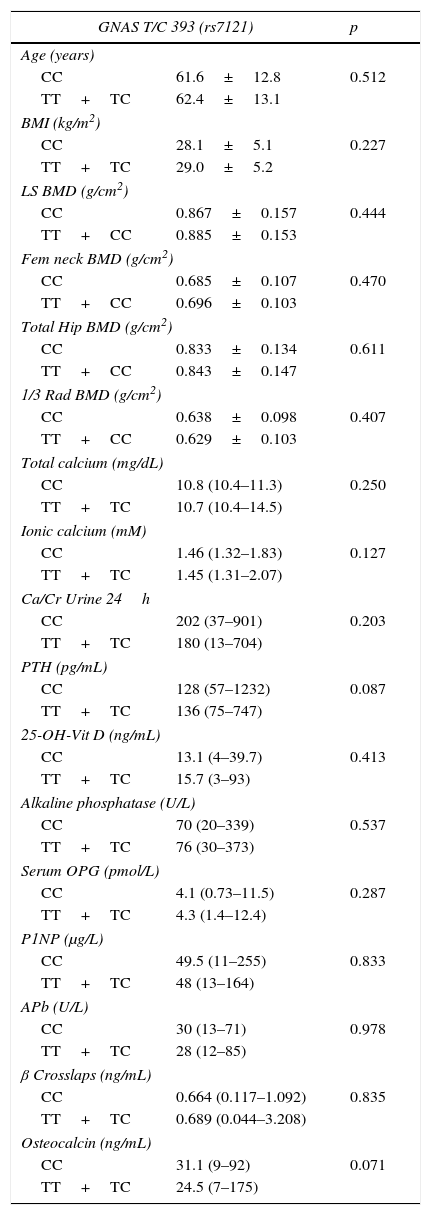
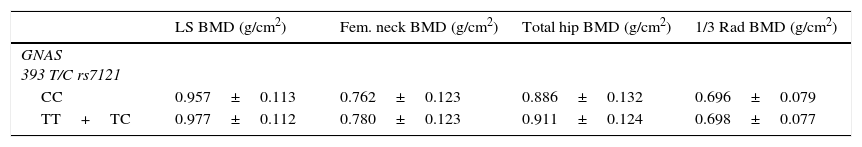
BMD at all sites and bone remodelling markers were similar in the two groups (CC vs. TC+TT) (Table 4) after adjusting for gender, age and BMI in PHPT patients, although a non-statistically significant trend towards lower BMD in lumbar spine, femoral neck and total hip was observed in the CC group. Control subjects showed the same non-statistically significant trend towards lower BMD in lumbar spine, femoral neck and total hip in CC group (Table 5). These results in both PHPT and control groups were not different in the subgroup of women after adjusting for their menopausal status.
Comparison between genotypes of GNAS T/C 393 (rs7121) in primary hyperparathyroidism subjects.
| GNAS T/C 393 (rs7121) | p | |
|---|---|---|
| Age (years) | ||
| CC | 61.6±12.8 | 0.512 |
| TT+TC | 62.4±13.1 | |
| BMI (kg/m2) | ||
| CC | 28.1±5.1 | 0.227 |
| TT+TC | 29.0±5.2 | |
| LS BMD (g/cm2) | ||
| CC | 0.867±0.157 | 0.444 |
| TT+CC | 0.885±0.153 | |
| Fem neck BMD (g/cm2) | ||
| CC | 0.685±0.107 | 0.470 |
| TT+CC | 0.696±0.103 | |
| Total Hip BMD (g/cm2) | ||
| CC | 0.833±0.134 | 0.611 |
| TT+CC | 0.843±0.147 | |
| 1/3 Rad BMD (g/cm2) | ||
| CC | 0.638±0.098 | 0.407 |
| TT+CC | 0.629±0.103 | |
| Total calcium (mg/dL) | ||
| CC | 10.8 (10.4–11.3) | 0.250 |
| TT+TC | 10.7 (10.4–14.5) | |
| Ionic calcium (mM) | ||
| CC | 1.46 (1.32–1.83) | 0.127 |
| TT+TC | 1.45 (1.31–2.07) | |
| Ca/Cr Urine 24h | ||
| CC | 202 (37–901) | 0.203 |
| TT+TC | 180 (13–704) | |
| PTH (pg/mL) | ||
| CC | 128 (57–1232) | 0.087 |
| TT+TC | 136 (75–747) | |
| 25-OH-Vit D (ng/mL) | ||
| CC | 13.1 (4–39.7) | 0.413 |
| TT+TC | 15.7 (3–93) | |
| Alkaline phosphatase (U/L) | ||
| CC | 70 (20–339) | 0.537 |
| TT+TC | 76 (30–373) | |
| Serum OPG (pmol/L) | ||
| CC | 4.1 (0.73–11.5) | 0.287 |
| TT+TC | 4.3 (1.4–12.4) | |
| P1NP (μg/L) | ||
| CC | 49.5 (11–255) | 0.833 |
| TT+TC | 48 (13–164) | |
| APb (U/L) | ||
| CC | 30 (13–71) | 0.978 |
| TT+TC | 28 (12–85) | |
| β Crosslaps (ng/mL) | ||
| CC | 0.664 (0.117–1.092) | 0.835 |
| TT+TC | 0.689 (0.044–3.208) | |
| Osteocalcin (ng/mL) | ||
| CC | 31.1 (9–92) | 0.071 |
| TT+TC | 24.5 (7–175) | |
PHPT: primary hyperparathyroidism. Data shown as mean±SD or median and range for non-normal variables. LS: lumbar spine, Fem. neck: femoral neck, 1/3 Rad: third distal radius, BMD: bone mineral density, Ca/Cr: calcium/creatinine, PTH: parathormone, APb: bone alkaline phosphatase, and OPG: osteoprotegerin. Data were adjusted for age, body mass index and sex.
Comparison between genotypes of GNAS 393 T/C in control subjects.
| LS BMD (g/cm2) | Fem. neck BMD (g/cm2) | Total hip BMD (g/cm2) | 1/3 Rad BMD (g/cm2) | |
|---|---|---|---|---|
| GNAS 393 T/C rs7121 | ||||
| CC | 0.957±0.113 | 0.762±0.123 | 0.886±0.132 | 0.696±0.079 |
| TT+TC | 0.977±0.112 | 0.780±0.123 | 0.911±0.124 | 0.698±0.077 |
p: not statistically significant in any site.
Data shown as mean±SD. LS: lumbar spine, Fem. neck: femoral neck, 1/3 Rad: third distal radius, and BMD: bone mineral density.
In the present study we analyzed the role of T393C SNP of the GNAS in the pathogenesis and in the clinical expression of PHPT. To the best of our knowledge, this is the first study assessing the relationship between the T393C SNP of the GNAS gene and clinical expression of PHPT: BMD, bone fractures, nephrolithiasis and biochemical parameters.
We found lower levels of 25-OH vitamin D in PHPT than in control subjects in accordance with previous studies.16,17 Several physiopathological mechanisms have been hypothesized: PTH related conversion of 25-OH-vitamin D to 1,25(OH)2-D, shortening of 25-OH-vitamin D half-life or in the opposite way chronic 25-OH-vitamin D deficiency as a causal factor of parathyroid gland hyperplasia.
PHPT subjects had a median BMI in the overweight range while control subjects had a median normal weight consistent with the results of previous studies.18–21 The cause and effect mechanism could be hypothesized in both directions. Obesity may promote vitamin D deficiency resulting in autonomous stimulation and growing of parathyroid glands. An alternative explanation is that PHPT is the cause of obesity, via adipokines such as leptin and vitamin D,22 being considered as a component of its clinical expression.23
We found a not statistically significant higher frequency of T homozygous genotype in PHPT than in control subjects not being enough to suggest a causal relationship. Genotype distribution was similar than showed in a previous study in Spanish population24 about migraine with a slightly higher frequency of CC homozygosity of T393C SNP of the GNAS in our control versus their control subjects.
C homozygous subjects showed a non statistical significant trend towards lower BMD at lumbar spine and hip (mainly trabecular bone) than TT+TC subjects and higher BMD in distal radius (mainly cortical bone). It is noteworthy that trabecular bone loss is greater in CC subjects and the opposite is observed for cortical bone. Since that trend is similar in PHPT than in control subjects, a genetic susceptibility more than an effect of PHPT could be suggested. C homozygous subjects showed a non statistical trend towards higher PTH, urinary calcium/creatinine and bone remodelling markes of formation and resorption than TT+TC subjects. However, the magnitude of these differences is not enough to suggest a more severe expression of asymptomatic PHPT in CC homozygous subjects and the clinical expression of symptomatic PHPT (nephrolithiasis and bone fractures) is similar in both groups. Serum calcium levels are similar in the two groups although the PTH levels are higher in CC genotype group, this could suggest that C allele confers certain resistance to the calcium dependent PTH regulation.
Previous research studies showed that TT genotype was related to increased Gsα mRNA expression in different tissues9 and such increase was associated with enhanced apoptosis in in vitro experiments.10–12 Since cellular proliferative rate and survival is enhanced in parathyroid adenomas our aim was to study whether it was a C allele related severity of PHPT or not. The slight differences found between genotype groups are not enough to agree with most of previous studies on malignant tumours such as bladder cancer,9 small cell lung cancer,25 squamous cell carcinoma of the larynx,15 severe tumour progression in malignant melanoma,26 disease progression or tumour related death in clear cell renal cell carcinoma14 in which CC genotype has been associated with adverse clinical course. On the contrary, TT homozygosity has been associated with a decreased overall survival in invasive breast cancer,27 worse outcomes (recurrence and survival) in esophageal cancer,28 higher proliferation rate and lower apoptotic rate in intrahepatic cholangiocarcinoma.29
The main limitations of our study are the sample size and the lack of complementary functional study. We accept that limited results could be obtained from the study of only one SNP of a gene in the GWAS era.
We can conclude that there is not a genetic susceptibility to PHPT related with GNAS T393C single nucleotide polymorphism or a major influence in its development and clinical expression. Further and larger functional studies should be carried out to confirm this results.
Ethical disclosureThe authors declare that all procedures followed were in accordance with the ethical standards of the ethics committee on human experimentation and with the Declaration of Helsinki and the World Medical Association. The authors declare that written informed consent was obtained from all patients included in the study after proper information was given to them. The authors declare that no identifying information from patients is included in this article.
Conflict of interestThe authors declare that there is no conflict of interest related to the contents of this article and that no funding from pharmaceutical industry has been received.