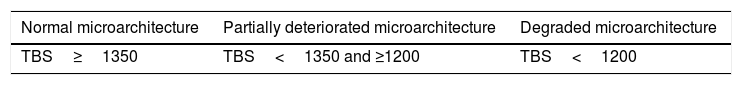Bone mineral density using dual-energy X-ray absorptiometry is the gold standard for the assessment of bone and an important predictor of fracture risk. However, most fragility fractures occur in people without densitometric osteoporosis, especially in endocrinological diseases. Fracture risk estimation tools such as FRAX have improved diagnostic sensitivity but do not include additional skeletal features. Bone microarchitecture research represents an improvement in the treatment of these patients. In this document members of the Mineral and Bone Metabolism Working Group of the Spanish Society of Endocrinology and Nutrition review new advances in dual-energy X-ray absorptiometry and other complex techniques for the study of bone microarchitecture as well as the available data on type 2 diabetes and parathyroid pathology.
La medición de la densidad mineral ósea mediante la absorciometría radiológica de doble energía es la técnica de elección para la valoración ósea y un predictor importante del riesgo de fractura. Sin embargo, la mayoría de las fracturas por fragilidad ocurren en personas sin osteoporosis densitométrica, especialmente en enfermedades endocrinológicas. Las herramientas para la estimación del riesgo de fracturas como FRAX han mejorado la sensibilidad diagnóstica aunque no consideran otras características óseas adicionales. La investigación de la microarquitectura ósea supone una mejoría en el abordaje de estos pacientes. En este documento elaborado por miembros del grupo de trabajo de Metabolismo Mineral y Óseo de la Sociedad Española de Endocrinología y Nutrición se revisan los nuevos avances en absorciometría radiológica de doble energía y otras técnicas más complejas para el estudio de la microarquitectura ósea así como los datos disponibles en diabetes tipo 2 y patología paratiroidea.
Bone densitometry is a noninvasive technique that allows for the measurement of bone mineral density (BMD) in certain locations (typically the lumbar spine and femur, and also the distal third of the radius). It is based on dual-energy X-ray absorptiometry (DXA) and represents the gold standard for the assessment of BMD, and thus for the diagnosis and monitoring of osteoporosis. The advantages of this technique are its high precision and very low radiation exposure, and it can be performed quickly and easily.1–3 In addition, it is relatively inexpensive compared to other techniques.
Bone mineral density is considered to be the key variable reflecting bone resistance, and may account for 70–85% of overall bone strength. In this respect, BMD is one of the main factors conditioning fracture risk. However, it does not take into account other factors that contribute to bone biomechanical behavior and condition bone quality.4 The concept of “bone quality” was introduced to differentiate the fundamental components determining bone resistance: bone mass (the amount of tissue), bone geometry (spatial distribution/size) and bone quality (the microarchitecture and composition of bone tissue).5
The introduction of “altered bone strength” as the defining concept of increased fragility fracture risk involves recognition of the limitations of bone mass assessment based on DXA as the most relevant clinical predictor of fracture risk.5,6 The greatest limitation of BMD measurements by DXA is the existence of a significant overlap between individuals who experience fractures and those who do not, particularly in the context of endocrine disorders such as type 2 diabetes mellitus (DM2) and primary hyperparathyroidism. Accordingly, although BMD measured by DXA is highly specific in predicting fracture risk, its sensitivity is low, and most fragility fractures occur in people who do not have densitometric osteoporosis (T score≥−2.5).
In addition to BMD, consideration is required of those factors that contribute to an increased probability of fracture.7 In this regard it seems more appropriate to assess absolute fracture risk, taking into consideration the combination of risk factors in order to quantify individual fracture risk, than to only consider BMD, thereby increasing fracture detection sensitivity while maintaining specificity.1,2 With this aim in mind, a number of fracture risk assessment tools have been developed. The most widely used tool of this kind is the Fracture Risk Assessment Tool (FRAX), probably because it is endorsed by the World Health Organization (WHO) and has been widely and effectively disseminated, with adaptation to a large number of countries. The FRAX assesses fracture risk by integrating risk factors predisposing to an increased fracture risk, with or without BMD data. Its use has spread rapidly, and the FRAX has been included in clinical practice guides. However, this tool has not been free from criticism and limitations, particularly population biases, which make it difficult to generalize the established thresholds for the indication of therapeutic intervention (20% for major fracture and 3% for hip fracture). In this respect, subsequent studies have shown that the FRAX underestimates the risk of osteoporotic fractures in the Spanish population, specifically when estimating the risk of major fracture and in application to certain patient groups.7
The measurement of BMD and the estimation of fracture risk remain insufficient for considering all the aspects that can influence bone resistance and fracture risk.6 The investigation of bone microarchitecture through new advances in DXA and other more complex techniques has improved the management of bone fragility.8 The present document was prepared by members of the Mineral and Bone Metabolism working group of the Spanish Society of Endocrinology and Nutrition (Sociedad Española de Endocrinología y Nutrición), and reviews the technologies for assessing bone fragility and the data available in patients with DM2 and parathyroid disease.
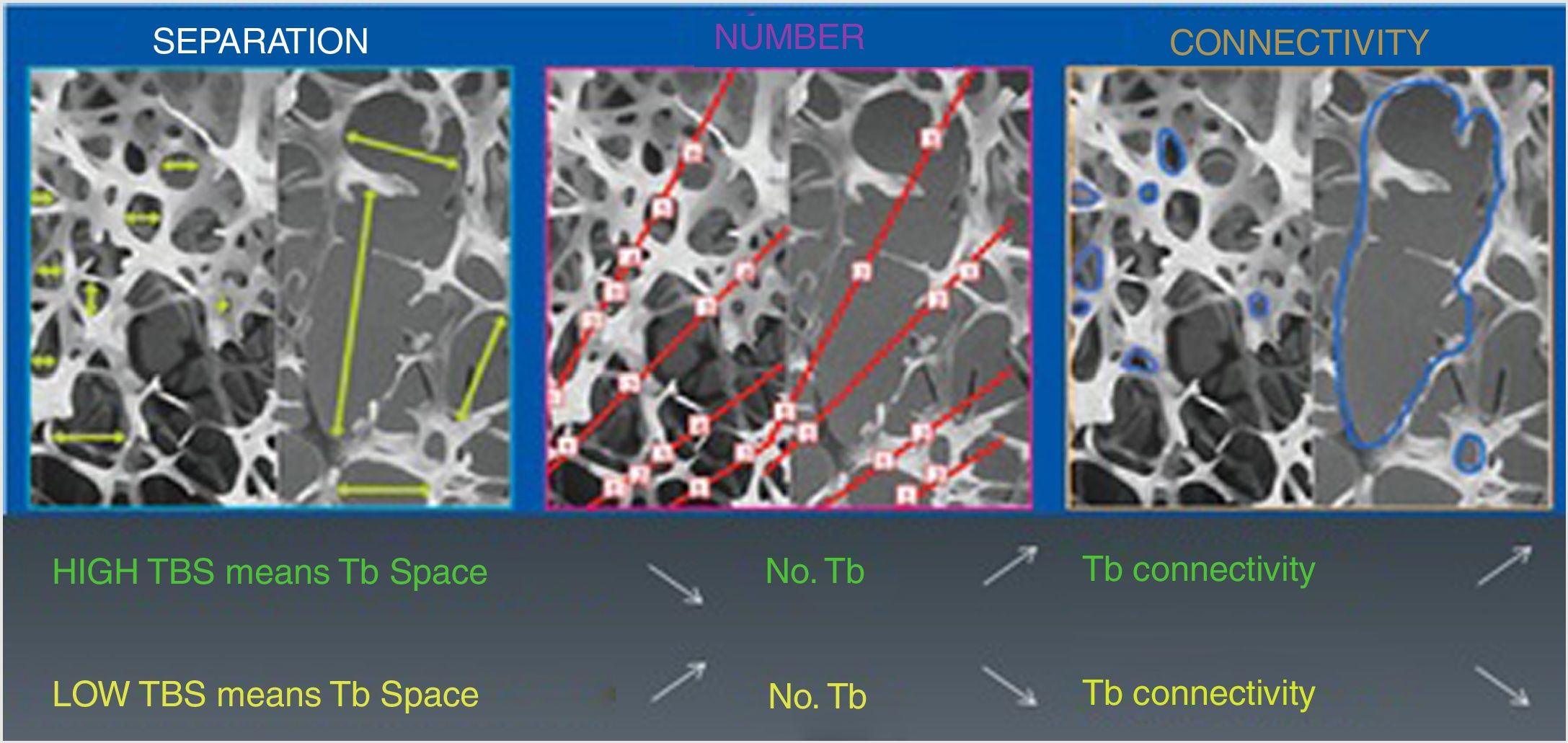
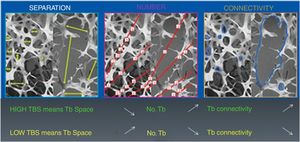
New advances in bone densitometryTrabecular Bone ScoreThe Trabecular Bone Score (TBS) is a novel numerical score that allows for the estimation of trabecular bone microarchitecture based on image texture analysis of DXA of the lumbar spine using the application TBS iNsight® developed by Medimaps.9 Since the TBS is based on DXA, it can be made widely available because no new equipment is required. The application analyzes the variations in intensity of each pixel, bone microarchitecture being estimated independently of BMD, based on a mathematical algorithm.10 There is a significant correlation between TBS and different bone histomorphometric parameters: the score is directly correlated to trabecular number and connectivity, and negatively correlated to intertrabecular space. In other words, a high TBS score means that the bone microarchitecture is dense and well connected, with small spaces between the trabeculae. By contrast, a low TBS score means that bone tissue microarchitecture is incomplete and poorly connected, with large spaces between the trabeculae (Figs. 1 and 2).10–12
Correlation of the TBS to bone microarchitecture parameters. Reproduced from Del Río et al.11
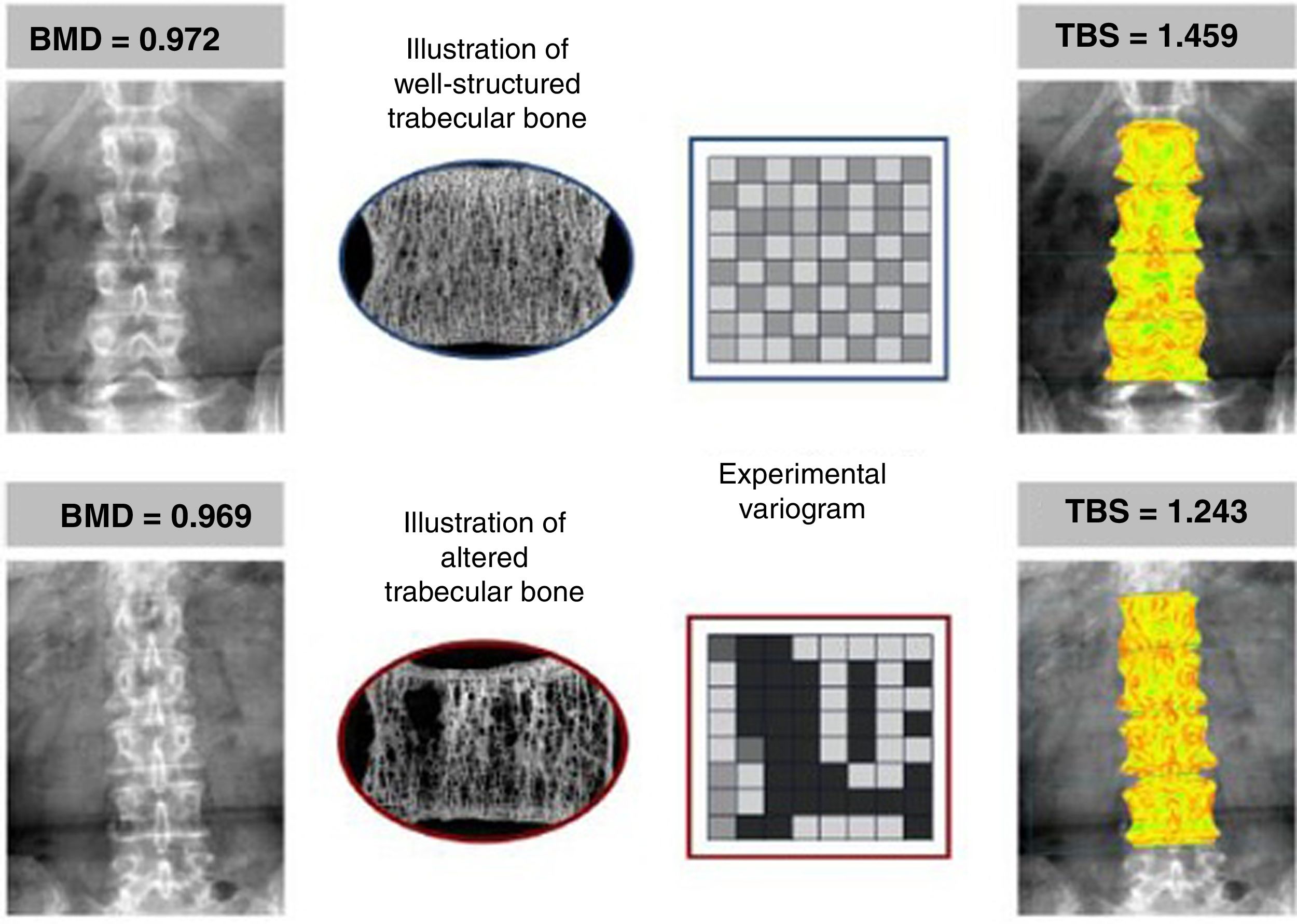
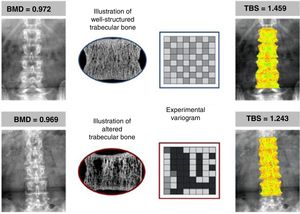
An example of two patients with similar BMD values (in g/cm2) but with different TBS values, according to the characteristics of their trabecular bone. Upper panel showing well-structured bone (more numerous and well-connected trabeculae), yielding a high TBS. Lower panel showing few and poorly connected trabeculae, where the TBS is low, indicating a deterioration of the microarchitecture and a greater probability of fracture. Reproduced from Silva et al.12
Table 1 shows the TBS reference scores.11
TBS reference scores.
| Normal microarchitecture | Partially deteriorated microarchitecture | Degraded microarchitecture |
|---|---|---|
| TBS≥1350 | TBS<1350 and ≥1200 | TBS<1200 |
Reproduced from Del Río et al.11
Different studies have shown TBS to be a predictor of osteoporotic fracture risk independently of BMD, and to be moreover an independent contributor that improves the predictive capacity of fracture risk assessment tools such as the FRAX.13–15
The Spanish Society of Bone and Mineral Metabolism Research (Sociedad Española de Investigación Ósea y del Metabolismo Mineral [SEIOMM]) has recently published a position paper on the clinical usefulness of TBS.9Table 2 summarizes the official positions of the SEIOMM according to the review of the scientific evidence made by the expert committee.
Review of the scientific evidence on the clinical use of the TBS: Official positions of the SEIOMM (summarized).
| 1. Can the TBS be used to assess fracture risk in clinical practice? |
| • It may be used to assess the risk of vertebral and femoral fracture, and global fragility, in men and women aged 50 years or older [Level of evidence, 2++. Grade of recommendation, B] |
| • It may be used together with BMD to assess vertebral, femoral and global fragility in men and women aged 50 years or older [Level of evidence, 2++. Grade of recommendation, B] |
| 2. Can the TBS be used to monitor patients with osteoporosis? |
| • It may be used in the monitoring of patients with osteoporosis to assess changes over time [Level of evidence, 2+. Grade of recommendation, C] |
| • It does not improve upon BMD in assessing the effect of treatment over time. It should not be used for the assessment of response to bisphosphonates [Level of evidence, 2++. Grade of recommendation, B] |
| 3. In which disorders is the TBS particularly useful? |
| • It may be used to assess fracture risk in subjects with diabetes and in subjects treated with glucocorticoids [Level of evidence, 2+]. |
| • It may be used for clinical guidance in the case of patients with hypo- and hyperparathyroidism [Level of evidence, 2+] |
| • It may be used for the clinical guidance of patients in the presence of osteoarthritis [Level of evidence, 2+] |
Adapted from Blanch Rubio et al.9
Levels of evidence and grades of recommendation of the Scottish Intercollegiate Guidelines Network (SIGN).
Bone geometry, microarchitecture or the three-dimensional (3D) distribution of mineral content also play an important role in bone strength together with BMD. Structural parameters have been proposed in order to better quantify the mechanical strength of bone and to estimate the risk of hip fracture, obtained by quantitative computed tomography (QCT). Obtaining a 3D bone reconstruction model from QCT images requires high radiation doses and the use of expensive techniques. Recently, alternatives have emerged with the introduction of 3D models from flat 2D densitometry reconstructions obtained by DXA.16,17 The DXA-3D technique can be used to precisely reconstruct the internal density of the femur in 3D format from DXA-2D images. This may help to obtain new information from clinical DXA imaging in order to simulate the structure and mechanical strength of bone, affording additional data on the true risk of fracture.
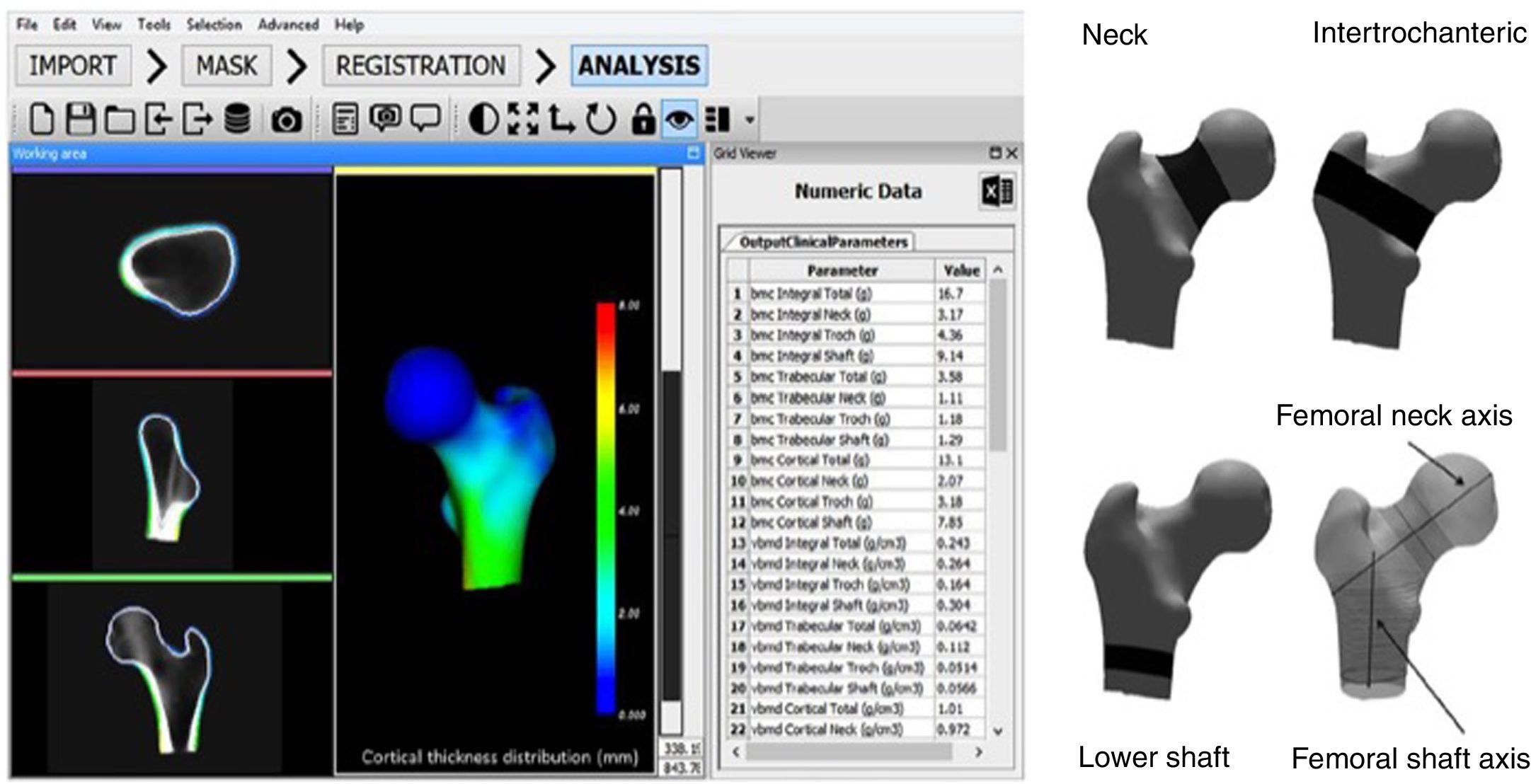
The DXA-3D software algorithm estimates the volumetric densities of the cortical and trabecular compartments of bone and the anatomical distribution of cortical thickness using standard DXA hip scans.
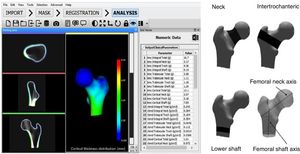
The DXA-3D software developed by Galgo Medical (Barcelona, Spain) is based on a statistical model of the shape and density of the proximal femur generated from a QCT database of Caucasian women and men.17 The algorithm is based on a statistical model of density and form that is recorded on the DXA image to obtain a personalized 3D model of the proximal femur. Cortical thickness and density are calculated by fitting a mathematical function to the calculated density profile along the normal vector at each node of the proximal femoral surface grid. The main output measures of DXA-3D are cortical bone surface density (in mg/cm2) and the volumetric BMD of trabecular and integral bone (in mg/cm3) (Fig. 3).
DXA-3D software interface (left) and regions of interest used in calculation of the DXA-3D structural parameters (right). BMC: bone mineral content; DXA: dual-energy X-ray absorptiometry; vBMD: volumetric bone mineral density; 3D: three-dimensional.
Reproduced from Clotet et al.19
Recent studies have shown DXA-3D techniques to afford precise estimates of trabecular and cortical volumetric BMD values and cortical thickness, with a close correlation to the structural parameters measured with QCT.16–19 The advantages of this technique are superimposable and complementary to those of TBS, since the fact that it is based on DXA means that it is more accessible, less expensive and involves less radiation exposure than QCT. Analyses on previous DXA scans may also be made.
On a practical level, the technique has been shown to be precise in short-term repeated measurements among postmenopausal women20; the DXA-3D measurements are seen to be associated with hip fracture21 and vertebral fracture22; and the technique is useful for assessing the effects of osteoporotic drugs.23
Densitometric vertebral fracture assessmentDensitometric vertebral fracture assessment (VFA) was developed to assess vertebral integrity in a lateral image of the spine using DXA. This technique can be used at the time of BMD assessment, and offers the advantage of lesser radiation exposure for the patient compared with conventional X-rays (<1%), as well as a lower cost. Several studies have compared the ability of VFA to detect vertebral fractures versus conventional X-rays. While there is a high level of agreement between the two techniques, it is lower for mild fractures (grade 1) than for moderate or severe fractures (grades 2 or 3).24,25 It should be taken into account that with VFA alone it is not possible to determine whether the nature of the fracture is related to non-osteoporotic disease. Therefore, in addition to the clinical signs of the patient, it is advisable to assess other imaging techniques when there are two or more mild deformities and no moderate or severe deformities; in the case of vertebral lesions not attributable to benign causes; or if the patient has a known history of tumor disease. Vertebral fracture assessment is designed to detect vertebral fractures but not other anomalies.26 The semiquantitative method of Genant based on conventional radiology is generally the best approach for initial image evaluation.27
Other technologiesHigh-resolution peripheral quantitative computed tomographyHigh-resolution peripheral quantitative computed tomography (HR-pQCT) allows for three-dimensional assessment of bone microarchitecture in vivo. It is able to distinguish the cortical and trabecular compartments of both the distal radius and the tibia. The technique offers significant advantages over two-dimensional measurements, since it assesses not only BMD, but also structure and strength in both of the mentioned compartments. High-resolution peripheral quantitative computed tomography acquires images based on the same principles as traditional QCT, but can achieve a much higher resolution, though in a smaller field of view. It only allows for the exploration of peripheral locations such as the radius and tibia. Radiation exposure during an HR-pQCT scan is lower than that of a full body CT scan.28,29
- -
Density analysis: volumetric BMD (total, trabecular bone and cortical bone) (mgHA/cm3) measurements can be made. The images can be used to simulate two-dimensional BMD with a close correlation to DXA of the extreme distal radius.
- -
Analysis of trabecular bone structure: trabecular measurements are generally calculated rather than measured directly from imaging, as the resolution of HR-pQCT is very close to the size of the individual trabeculae. Bone volume fraction, average trabecular number and average trabecular thickness and separation are obtained with HR-pQCT. Other metric parameters are more affected by image resolution.
- -
Analysis of cortical structure: cortical thickness and cortical porosity.
- -
Estimation of bone strength: this can be estimated using finite element analysis. Such analysis evaluates the mechanical behavior of bone under certain conditions through simulation.
The HR-pQCT technique has been shown to be useful for assessing microarchitectural differences between different ages and genders, as well as for assessing fracture risk, with better results than with DXA alone.30 Many clinical studies with HR-pQCT have examined the association of fracture in postmenopausal women with osteopenia and osteoporosis, and found both fracture risk and severity to be related to bone microarchitecture.31,32 Other studies with HR-pQCT have been conducted in secondary osteoporosis and related bone diseases, particularly since these disorders may affect the cortical and trabecular compartments differently.28 Finally, its usefulness has also been demonstrated in therapeutic monitoring, for assessing the effects of different treatments upon bone microarchitecture. One of the first randomized, placebo-controlled clinical trials compared the effects of denosumab and alendronate after 12 months of treatment in 247 postmenopausal women. Prevention of the decline in bone microarchitecture parameters was observed, with better outcomes in patients treated with denosumab.33 Studies have been made to define the effects of alendronate,34 teriparatide and zoledronic acid, among other drugs.35,36
Bone microindentationMicroindentation is a technique that can directly assess the mechanical characteristics of cortical bone in vivo, and represents a new dimension in the assessment of bone resistance to fracture. On inserting a probe on the surface of the anterior tibia and inducing microscopic fractures, microindentation measures the mechanical resistance of bone at tissue level.37 The technique is minimally invasive and is performed under local anesthesia. A cyclic indentation technique was initially introduced, and impact microindentation was subsequently developed. Cyclic microindentation is expressed in terms of indentation distances, which measure the distances to which the microindenting device is able to penetrate. Impact microindentation results are expressed as the Bone Material Strength Index (BMSi), representing the relationship between probe penetration of the bone and probe penetration of a methyl methacrylate reference spectrum.
Microindentation has been shown to discriminate between individuals with and without fragility fracture, independently of BMD.38 Lower BMSi scores have also been observed in patients with fragility fractures, independently of the presence of osteopenia or osteoporosis as assessed by BMD.39
Application in endocrinologyType 2 diabetes mellitusType 2 diabetes mellitus (DM2) is a highly prevalent disease associated with increased bone fragility, in which BMD measured by DXA and the FRAX underestimates the fracture risk.40 The mechanisms underlying increased bone fragility in DM2 are complex. The different factors involved include low bone turnover, the accumulation of advanced glycation end products, anomalies of bone microarchitecture and macroarchitecture, and tissue alterations.41 Studies made with new bone quality assessment technologies are therefore of considerableinterest.
Large epidemiological studies have shown the TBS to be an independent predictor of fracture in both diabetic and non-diabetic individuals. Leslie et al.42 analyzed 29,407 Canadian women aged 50 years or older with available DXA scans, of whom 2356 had been diagnosed with diabetes. After adjusting for clinical risk factors, the women with diabetes were found to be more likely to fall within the lower tertile of the lumbar TBS, but less likely to fall within the lower tertiles of lumbar spine or femoral neck BMD, or the total femur area. The TBS scores were predictive of incident fractures independently of BMD. Other studies have found the TBS to be negatively related to glycosylated hemoglobin, fasting plasma glucose and insulin levels.43
The application of HR-pQCT to the study of bone structure in patients with DM2 has shown cortical porosity to be greater in diabetics, and this deficit of the cortical component has been found to be more manifest in diabetic patients with microvascular complications.44 The effects of DM2 upon volumetric BMD, bone geometry and bone resistance in the proximal femur have been investigated, with contradictory results. In the study published by Melton et al.,45 trabecular volumetric BMD was higher in patients with DM2 when compared with non-diabetic subjects, while cortical volumetric BMD, bone cross-sectional area, and cortical thickness were similar in diabetics and controls. By contrast, Heilmeier et al.46 compared femoral volumetric BMD and bone geometry in a cross-sectional study of diabetic postmenopausal women with and without fractures. The results revealed significantly lower integral and trabecular volumetric BMD values and also a lesser cortical thickness in patients with fracture. Therefore, deficits have been observed in cortical bone structure, while the results referring to trabecular bone structure are inconclusive.
There are ongoing DXA-3D studies, but it is not yet clear whether these parameters will be useful for better identifying bone fragility in diabetic patients.
The so-called impact microindentation technique has been used in different studies to determine bone quality in patients with DM2. These studies have revealed poorer bone quality in diabetic patients presenting similar BMD values.47 However, due to its invasive nature, impact microindentation does not appear to be open to general use in routine clinical practice.
Hyperparathyroidism and hypoparathyroidismPrimary hyperparathyroidism (HPT) is one of the most common endocrine diseases, and one of its classical manifestations is bone disease. Mainly in bones with a predominantly cortical composition, excess parathyroid hormone (PTH) may lead to osteitis fibrosa cystica, manifesting as bone pain, and can cause fractures. The typical radiographic signs include subperiosteal resorption of the middle and distal phalangeal bones, a mottled or “salt and pepper” cranial appearance, bone cysts, and brown tumors in the long bones and pelvis. Lumbar and hip BMD measured by DXA is usually well preserved, but is decreased in the distal third of the radius, where the bone is of a cortical nature.48 This suggests an increase in non-vertebral fractures in this disease. However, many studies indicate that the fracture risk is globally incremented in HPT.49 In other words, the bone phenotype of HPT as determined by DXA is discordant with the data obtained from bone fragility studies. The new technologies in bone fragility therefore may be useful in this disease.8
Trabecular bone involvement in HPT has also been corroborated by the TBS.50 In a case-control study, the TBS was found to be lower in patients with HPT than in the controls, despite no difference in spinal BMD, and the vitamin D levels were similar. Furthermore, in this patient population, the TBS was seen to correlate with many HR-pQCT parameters.51 Likewise, it has been shown that patients with HPT suffer greater bone mass loss in both cortical and trabecular bone. Stein et al. reported a reduction in cortical volume in both the distal third of the radius and the tibia.52 Furthermore, the radius showed a reduction not only in trabecular volume, but also in trabecular number and thickness, together with increased trabecular separation. In the tibia, the abnormalities were less evident, with only trabecular volume and separation demonstrating significant reductions. The different studies with HR-pQCT confirm the involvement of trabecular bone in HPT, and are consistent with the clinical observations of a general increase in fractures in these patients. Analysis of individual trabecular segmentation of the radius and tibia based on HR-pQCT demonstrated a greater alteration of the trabecular microarchitecture in postmenopausal women with HPT.52 These alterations of trabecular bone microarchitecture resulted in impaired bone strength, as shown by microstructural finite element analysis.
Hypoparathyroidism is an uncommon endocrine disorder characterized by a chronic deficiency or the absence of parathyroid hormone, which causes a highly significant decrease in bone remodeling. Individuals with hypoparathyroidism generally exhibit BMD values measured by DXA above the mean for their age in all bones of the skeleton, and particularly in the lumbar spine. Different studies have shown no differences in the TBS between patients with hypoparathyroidism and controls, and that the score in patients with hypoparathyroidism is very close to normal.53 High-resolution peripheral quantitative computed tomography confirms the increase in cortical volume, but reveals a decrease in cortical thickness, associated with lesser porosity of the cortex, in patients with hypoparathyroidism.53 Discrepancies have been observed with regard to the trabecular bone phenotype. Rubin et al.54 reported increased trabecular thickness in patients with hypoparathyroidism as compared to controls, while Cusano et al.55 described a lesser trabecular thickness in these patients. This discrepancy can be explained in part by technical differences in the trabecular compartment measurements of the two studies.53 It is not clear how these structural changes affect bone resistance or fracture risk.56
Summary and conclusionsOnly 40–50% of all patients with fragility fracture have densitometrically manifest osteoporosis, the three-dimensional distribution of the mineral content of cortical and trabecular bone being a determining factor in fracture resistance.
Direct methods for measuring bone quality, such as microindentation, are invasive, and the results differ among cohorts and are inconsistent with the mechanical properties of the bone. Quantitative computed tomography allows for the assessment not only of BMD, but also of the structure and strength of bone microarchitecture in vivo regarding both the cortical and trabecular compartments. The new advances in densitometry with the incorporation of software for assessing trabecular microarchitecture at the lumbar level (TBS) or for the 3D reconstruction of the internal density of the femur with the attainment of structural parameters from planar DXA images (DXA-3D), as well as the evaluation of vertebral fractures (VFA), offer advantages in the forms of lower cost and the lesser radiation exposure of the patient.
The development of new technologies for assessing bone quality and fracture risk in patients with DM2 or primary hyperparathyroidism is of considerable interest, in view of the characteristics inherent to bone involvement in these diseases. At present, the best validated technique is the TBS, which is already available for use in clinical practice and can be combined with the FRAX scale. However, further studies of a prospective nature are needed to determine its importance in the management of these patients.
Conflicts of interestNone of the authors have relevant conflicts of interest in relation to this study. No specific funding has been received for preparing this article.
Please cite this article as: García Martín A, de la Higuera López-Frías M, Cortés Berdonces M, Jodar Gimeno E, Ávila Rubio V, Alhambra MR, et al. Nuevas tecnologías en la evaluación de la fragilidad ósea y su aplicación en Endocrinología. Endocrinol Diabetes Nutr. 2020;67:602–610.