Treatment with recombinant human growth hormone (rhGH) has been shown to improve adult height in pediatric patients with GH deficiency (GHD). However, reassessment of patients after they reach their final height shows some of them have permanent GH deficiency (PGHD), while others had a transient deficiency (TGHD). The study objective was to assess, in a cohort of pediatric patients with GHD, potential differences in response to treatment with rhGH depending on whether deficiency is permanent or transient.
Materials and methodsA retrospective study of 89 patients with GHD, who were monitored from diagnosis to adult height. Clinical, auxological, radiographic and laboratory variables were collected at diagnosis, after the first year of treatment, and when they had reached their adult height.
ResultsPGHD was found in 28% of patients. Initial height was −2.46±0.86 SD and −2.24±0.68 SD in subjects with PGHD and TGHD respectively. Peak GH level at diagnosis was 4.26±2.78 and 6.20±2.01ng/ml (p<0.01) in the PGHD and TGHD groups respectively. After the first year of treatment, increase in growth velocity was greater in the PGHD group: 4.33±3.53 SD vs. 2.95±2.54 SD in the PGHD group (p=0.043). Final height was −0.81±0.87 SD in the PGHD and −0.95±0.83 SD in the TGHD group (p=0.47).
ConclusionsPatients with PGHD had a better short- and long-term response to rhGH. They also showed lower GH levels in stimulation tests at diagnosis, as previously reported.
El tratamiento con hormona de crecimiento recombinante (rhGH) ha demostrado mejorar la talla adulta de los pacientes pediátricos con déficit de GH (DGH). Sin embargo, cuando los pacientes son reevaluados al llegar a la talla final, se evidencia que existen pacientes en los que el déficit de GH es permanente (DPGH) y otros en los que el déficit ha sido transitorio (DTGH). El objetivo es evaluar, en una cohorte de pacientes pediátricos con DGH, si existen diferencias en la respuesta al tratamiento con GH en función de que dicho déficit sea permanente o transitorio.
Materiales y métodosEstudio retrospectivo de 89 pacientes con DGH, que fueron seguidos desde el diagnóstico y durante todo el seguimiento hasta la talla adulta. Se obtuvieron parámetros clínicos, auxológicos, radiológicos y analíticos al diagnóstico, así como tras el primer año de tratamiento y en la revisión de la talla final.
ResultadosEl 28% de los pacientes presentaron un DPGH. Talla inicial de −2,46 ± 0,86 DE en el DPGH y −2,24 ± 0,68 DE en el DTGH. El valor pico de GH al diagnóstico fue de 4,26 ± 2,78 y 6,20 ± 2,01ng/mL, respectivamente (p < 0,01). Tras el primer año de tratamiento el incremento de la velocidad de crecimiento fue mayor en el grupo de DPGH: 4,33 ± 3,53 DE vs. 2,95 ± 2,54 DE (p = 0,043). Talla final de −0,81 ± 0,87 DE los DPGH y de −0,95 ± 0,83 DE los DTGH (p = 0,47).
ConclusionesLos pacientes con DPGH obtienen un mayor beneficio del tratamiento con rhGH tanto a corto como a largo plazo. Además, muestran niveles más bajos de GH en las pruebas de estímulo al diagnóstico, como ha sido descrito previamente.
Short stature and growth retardation in children are common problems in clinical practice. Since the approval of recombinant growth hormone (rhGH) for the treatment of growth hormone (GH) deficiency in 1985 by the United States Food and Drug Administration (FDA), many studies have demonstrated its effectiveness in improving adult height or final height.1–4 In Spain, the use of rhGH has been approved not only for classical GH deficiency, but also for Turner syndrome, small for gestational age cases, chronic renal failure, SHOX gene alterations and Prader Willi syndrome.
Over the years, the diagnostic criteria for GH deficiency (GHD) have been modified, and there is controversy regarding its definition, as well as the parameters of good response and treatment benefit after the end of growth. Classically, a diagnosis of GHD was established when the GH peak was 10ng/ml or less after two stimulus tests (clonidine, insulin-induced hypoglycemia, propranolol-exercise, etc.). Deficiency was considered to be severe when the level was 5ng/ml or lower, depending on the analytical technique employed.5,6 At present, a response of <7.7ng/ml after stimulus testing is considered pathological, depending on the measurement technique used.7
Likewise, differences have been described in the short- and long-term response between patients with permanent growth hormone deficiency (PGHD) and those with transient deficiency (TGHD). Patients with TGHD are diagnosed on reaching adult height, when upon re-evaluation (in the transition period) they are seen to reach a normal peak GH value (different cut-off points have been reported: GH ≥5ng/ml, ≥5.62ng/ml, ≥6.1ng/ml) in the insulin-induced hypoglycemia test or exhibit normal IGF-1 levels.8–10 In Spain, adult GHD is diagnosed when a peak GH level of 3ng/ml or less is obtained after a stimulus test (with the insulin-induced hypoglycemia test as the first option), according to the criteria of the Growth Hormone Advisory Committee updated in 2004.11
The study of the auxological data from both groups may help us to better understand the course and response to treatment of these patients, since no firm consensus has been reached on the evaluation of GHD in the transition period to adult age and on the continuity of treatment from that period onwards.
The primary objective of the present study was to assess the response to rhGH after the first year of treatment and at final height (transition to adult age) in two groups of patients diagnosed with PGHD and TGHD.
MethodsA retrospective, cross-sectional descriptive study was made of 89 patients diagnosed with GHD, treated with rhGH and followed-up on to adult height at the Unit of Endocrinology and Pediatric Diabetes of Hospital Universitario Ramón y Cajal (Madrid, Spain) (Fig. 1). “Final height” was defined as the height reached with a growth rate of less than 1cm/year. The inclusion criteria were: patients of short stature (defined by the growth curves and tables of the Faustino Orbegozo Eizaguirre Foundation)12 and a diagnosis of isolated GH deficiency with a pathological GH stimulus test (a clonidine and insulin-induced hypoglycemia test), serum IGF-1 levels below two standard deviations (SD) for age and gender, and who were treated with rhGH for at least one year and reached final or adult height. The exclusion criteria included patients with an incomplete medical history or who discontinued follow-up before reaching final height, and patients with multiple hormone deficiency.
The patients were classified according to peak GH level in the stimulus test. At the start of treatment, a diagnosis of GHD was defined by a peak ≤10ng/ml. After re-evaluation at adult height, TGHD was diagnosed when the GH peak was ≥6.1ng/ml; all other patients were classified as PGHD, because this cut-off point has a high sensitivity (96%) and specificity (100%).10 The techniques used to determine IGF-1 were immunoassay and radioimmunoassay, while GH was also measured using immunoassay methods: several commercial kits were used, since this was a retrospective study covering a prolonged period of time.
Data were collected from the clinical records. The data obtained at the start of treatment included gender, chronological age, baseline height, genetic height, the body mass index (BMI), the pubertal stage, bone age with a prediction of adult height (PAH) calculated using the Bayley-Pinneau method,13 peak GH level in the stimulus test, and the rhGH dose. At evaluation after the first year of treatment and in the final height assessment, we analyzed the following parameters, in addition to those cited above: the rhGH dose, the PAH, the growth rate and the final height. A satisfactory response to the first year of treatment was considered when patient height increased by more than 0.3 SD from its baseline height, taking as reference the study published by Cohen et al.14 Lastly, comparisons were made of the change between final height and baseline height, and between final height and baseline PAH. Data were expressed as absolute values, percentages and mean±SD. The SPSS version 19.0 statistical package was used throughout. The Student's t-test was used for quantitative variables, while the Mann–Whitney U test was used for the comparison of data with a non-normal distribution. Statistical significance was considered for p<0.05.
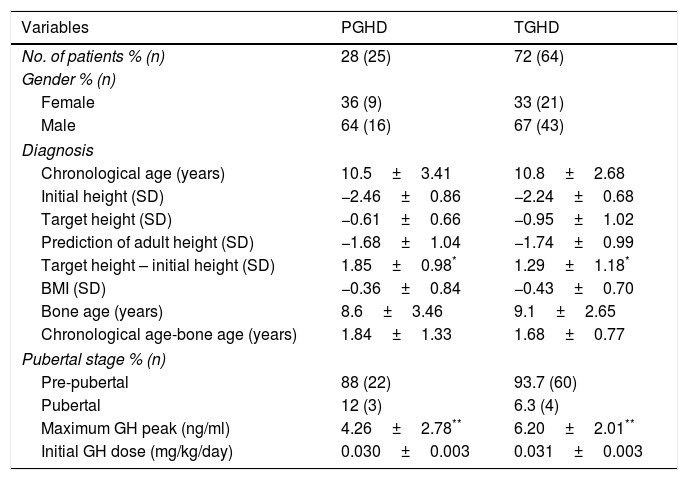
ResultsA total of 153 medical records of rhGH-treated patients, and 89 patients (67% male) met the inclusion criteria for the diagnosis of isolated GHD (Fig. 1). The data before starting treatment and during follow-up are analyzed according to the type of GHD (Tables 1 and 2). Of these patients, 25 had PGHD at final reassessment (28%), while 64 had TGHD (72%). The chronological age at the start of treatment was 10.5±3.41 years, and the initial height was −2.46±0.86 SD (boys: –2.47±0.83 SD and girls: –2.44±0.96 SD; 68% of the total with initial height <–2 SD and 52% with PAH-TH (target height) >1 SD) for the PGHD patients. In turn, the chronological age was 10.8±2.68 years, and the initial height was –2.24±0.68 SD (boys: –2.12±0.64 SD and girls: –2.49±0.72 SD; 59% of the total with initial height <–2 SD and 42% with PAH-TH>1 SD) for the TGHD patients. In the patients with PGHD, we observed greater genetic height and shorter initial height, with a statistically significant difference between them as compared to the TGHD group (p=0.026). With regard to gender differences, the target height in the first group was –0.41±0.67 SD (172.2±4.5cm) for boys and –0.96±0.51 SD (156.5±3.3cm) for girls. The respective values in the second group were –1.05±0.87 SD (168.7±5.5cm), and –0.76±1.27 SD (156.8±6.8cm). With regard to the initial PAH according to gender, the figure in the first group was –1.33±0.86 SD (166.4±5.7cm) in boys and –2.31±1.08 SD (148.5±6.5cm) in girls, while in the second group the PAH values were –1.71±1.06 SD (164.5±6.5cm) and –1.81±0.86 SD (151.5±5.0cm), respectively.
Comparison of data between the patients with permanent (PGHD) and transient GH deficiency (TGHD) at the start of treatment.
| Variables | PGHD | TGHD |
|---|---|---|
| No. of patients % (n) | 28 (25) | 72 (64) |
| Gender % (n) | ||
| Female | 36 (9) | 33 (21) |
| Male | 64 (16) | 67 (43) |
| Diagnosis | ||
| Chronological age (years) | 10.5±3.41 | 10.8±2.68 |
| Initial height (SD) | −2.46±0.86 | −2.24±0.68 |
| Target height (SD) | −0.61±0.66 | −0.95±1.02 |
| Prediction of adult height (SD) | −1.68±1.04 | −1.74±0.99 |
| Target height – initial height (SD) | 1.85±0.98* | 1.29±1.18* |
| BMI (SD) | −0.36±0.84 | −0.43±0.70 |
| Bone age (years) | 8.6±3.46 | 9.1±2.65 |
| Chronological age-bone age (years) | 1.84±1.33 | 1.68±0.77 |
| Pubertal stage % (n) | ||
| Pre-pubertal | 88 (22) | 93.7 (60) |
| Pubertal | 12 (3) | 6.3 (4) |
| Maximum GH peak (ng/ml) | 4.26±2.78** | 6.20±2.01** |
| Initial GH dose (mg/kg/day) | 0.030±0.003 | 0.031±0.003 |
BMI: body mass index.
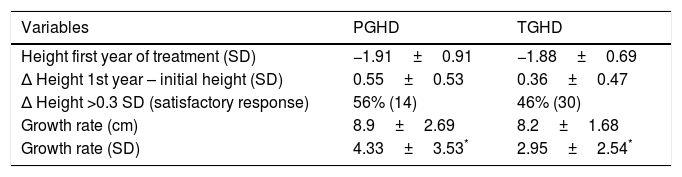
Comparison of data between patients with permanent (PGHD) and transient GH deficiency (TGHD) after the first year of rhGH treatment.
| Variables | PGHD | TGHD |
|---|---|---|
| Height first year of treatment (SD) | −1.91±0.91 | −1.88±0.69 |
| Δ Height 1st year – initial height (SD) | 0.55±0.53 | 0.36±0.47 |
| Δ Height >0.3 SD (satisfactory response) | 56% (14) | 46% (30) |
| Growth rate (cm) | 8.9±2.69 | 8.2±1.68 |
| Growth rate (SD) | 4.33±3.53* | 2.95±2.54* |
Δ: Difference between 2 values/increase in value.
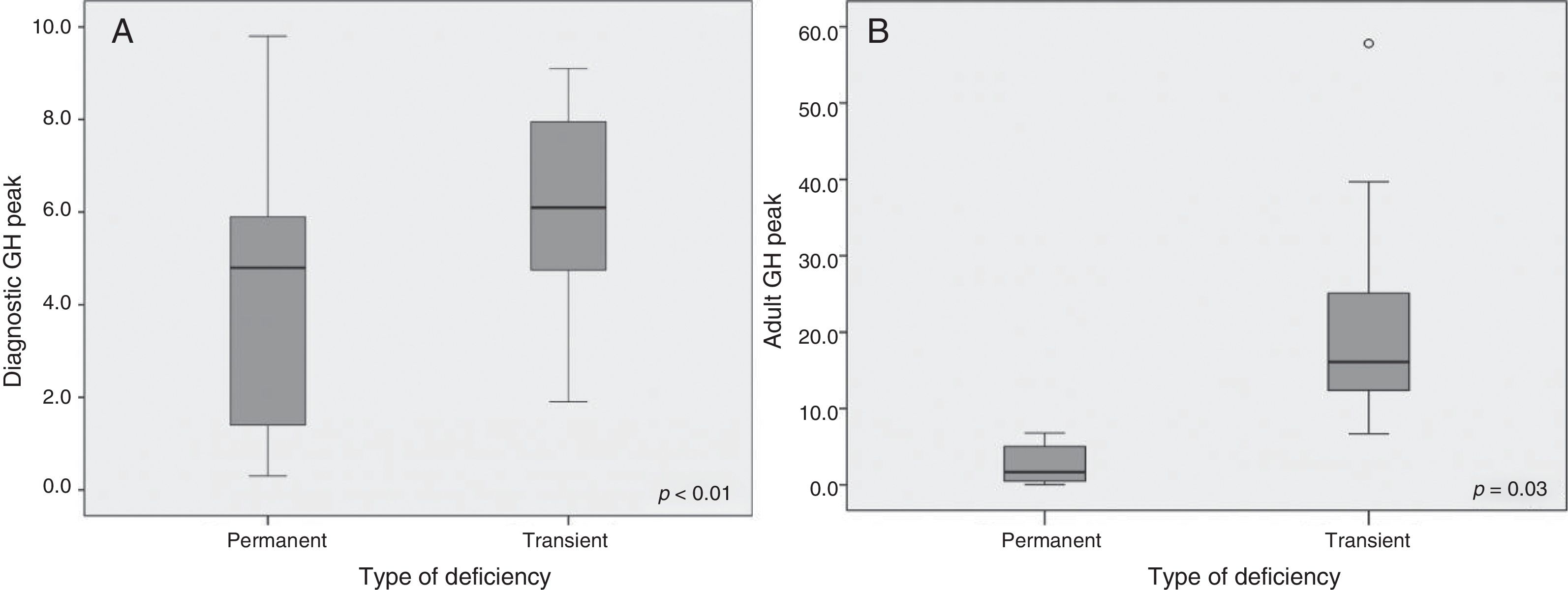
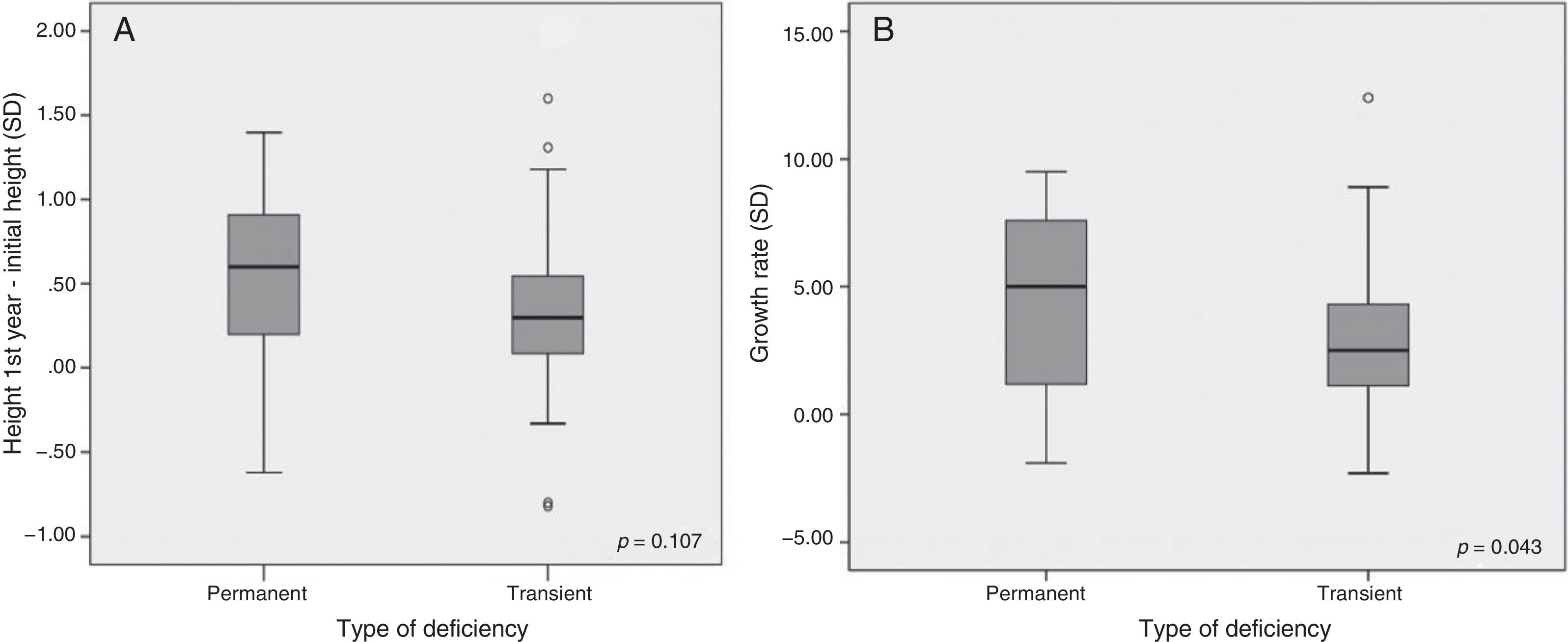
Significant results were found regarding GH determination after the stimulus tests: the peak GH value at diagnosis was 4.26±2.78ng/ml in PGHD and 6.20±2.01ng/ml in TGHD (p<0.01) (Fig. 2A). Significant results were likewise recorded at re-evaluation with the insulin-induced hypoglycemia test on reaching final height (GH peak 2.60±2.54 vs. 19.59±10.75ng/ml, respectively; p=0.003) (Fig. 2B). No significant differences were found in terms of the rhGH starting dose, the BMI, bone age or the pubertal stage between the two groups. After the first year of treatment (Table 2), the good responder rate was 56% in the patients with PGHD and 46% in those with TGHD on assessing a change in height of over 0.3SD (an increase in height of 0.55±0.53 SD vs. 0.36±0.47 SD, respectively). In addition, the change in growth rate was significantly greater in the PGHD group (4.33±3.53 SD vs. 2.95±2.54 SD in the TGHD group; p=0.043) (Fig. 3A and B).
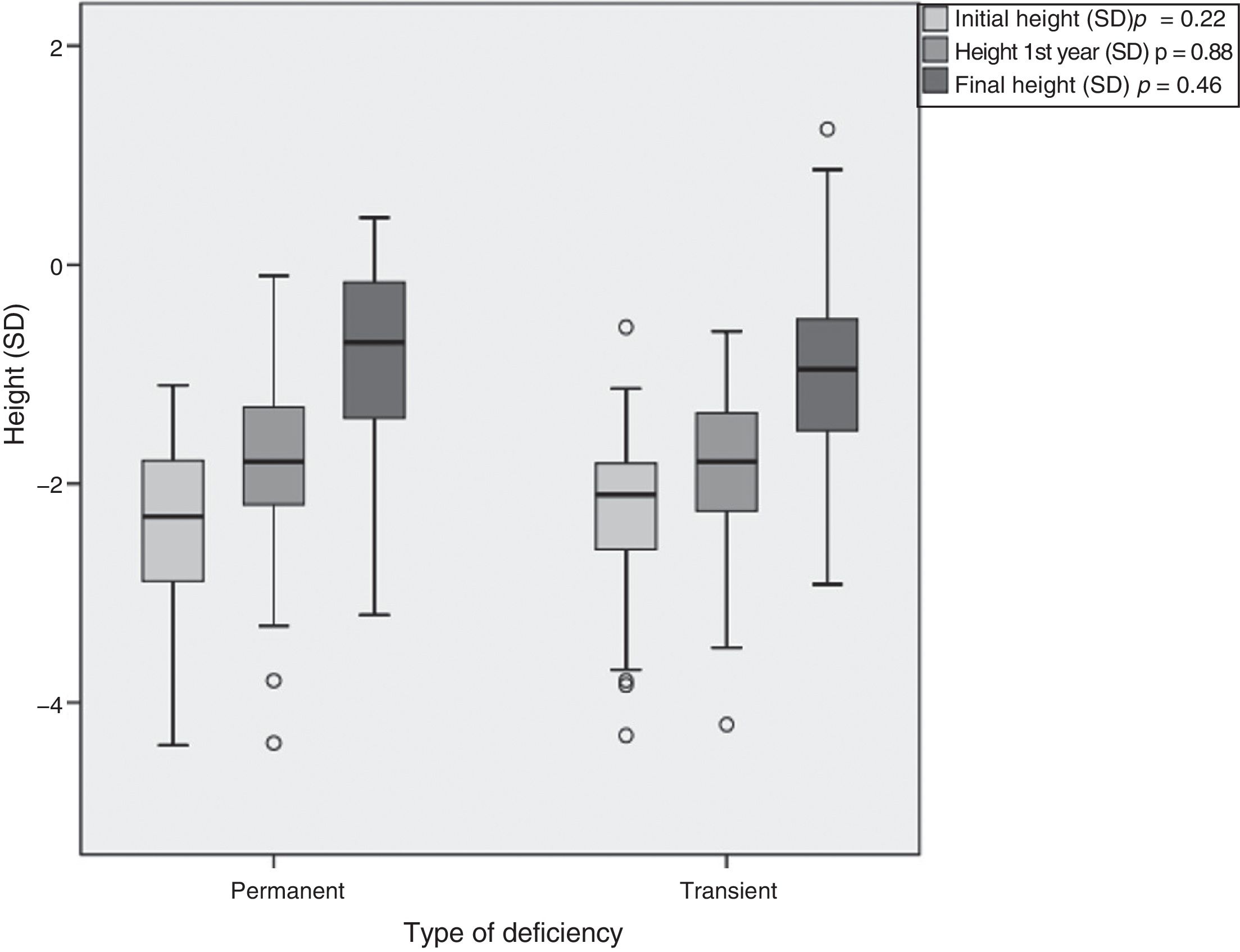
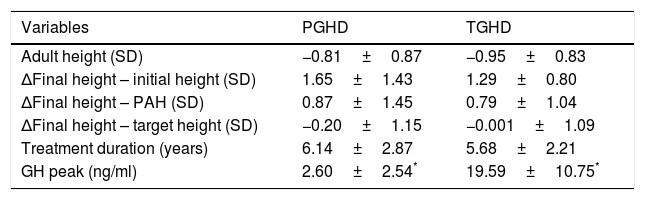
Treatment duration was 6.14±2.87 years for the patients with PGHD and 5.68 years±2.21 years in the TGHD group (Table 3). The patients with PGHD reached a final height of −0.8±0.87 SD (boys −0.68±0.86 SD [170.6±6.0cm] and girls −1.03±0.84 SD [156.4±4.1cm]), while the patients with TGHD reached a final height of −0.95±0.83 SD (boys −0.86±0.80 SD [168.3±5.4cm] and girls −1.15±0.89 SD [155.2±5.5cm]). Although a greater recovery with respect to the initial height was seen in the patients with PGHD (1.65±1.43 vs. 1.29±0.80 SD), the differences were not statistically significant (p=0.46) (Fig. 4).
Comparison of data between the patients with permanent (PGHD) and transient GH deficiency (TGHD) at the end of treatment and on reaching final height.
| Variables | PGHD | TGHD |
|---|---|---|
| Adult height (SD) | −0.81±0.87 | −0.95±0.83 |
| ΔFinal height – initial height (SD) | 1.65±1.43 | 1.29±0.80 |
| ΔFinal height – PAH (SD) | 0.87±1.45 | 0.79±1.04 |
| ΔFinal height – target height (SD) | −0.20±1.15 | −0.001±1.09 |
| Treatment duration (years) | 6.14±2.87 | 5.68±2.21 |
| GH peak (ng/ml) | 2.60±2.54* | 19.59±10.75* |
Δ: Difference between 2 values/increase in value; PAH: prediction of adult height.
Since 1998, when the use of rhGH in adults with GHD was first approved in Spain, the treatment indications have been modified in both pediatric and adult patients: the indications accepted in 2008 are the ones currently applicable.15 In the pediatric population, the treatment criteria refer to a height <−2 SD, height or a PAH below 1 SD with respect to genetic height, a growth rate
Different ways of assessing the patient response to GH treatment have been described, with a broad range of effectiveness in terms of adult height.16–18 Most publications confirm a better response to treatment in patients with severe GH deficiency, these being the individuals with lower peak GH values after stimulation testing and who continue to present GHD in adulthood.19–21
This study presents data on the short- and long-term response of patients diagnosed with GHD in childhood, classifying them into two groups (PGHD and TGHD) upon re-evaluation during the transition to adult life. The two groups showed an increase in height (measured in terms of SD) when compared with both height at the start of treatment and the corresponding PAH. However, greater height recovery was noted in the group of patients with permanent GH deficiency or a final diagnosis of PGHD.
In the reported sample, the patients with PGHD had lower initial height and a greater difference with respect to genetic height at the time of diagnosis. This agrees with the observations of Westphal et al.22 In addition, this patient group represented 28% of the total sample, a somewhat lower proportion as compared to the 40% in the study published by Tauber et al.23 and the 44% reported by Deillon et al.2 Most patients were male (66%), with a 2:1 male/female ratio, which was consistent with the findings of other studies.24–26 Likewise, a significantly lower peak GH value was recorded in PGHD versus TGHD, as well as a better height response, as commented above.
With regard to the effectiveness of rhGH treatment after the first year, an increase of >0.3–0.5 SD versus baseline is defined as representing a good response to therapy, though this response is considered to be dose-dependent and varies greatly.14 In our study, height was seen to increase by 0.55±0.53 SD and 0.36±0.47 SD in the PGHD and TGHD groups, respectively. With regard to the growth rate, the initial recovery or catch-up during the first year of treatment was 4.33±3.53 SD (8.9±2.69cm) and 2.95±2.54 SD (8.2±1.68cm) in the patients with PGHD and TGHD, respectively, these results being statistically significant. Salah et al.20 reported an improvement in height of 0.8 and 0.6 SD in patients with severe and partial GH deficiency, after the first year of treatment. Likewise, they reported an increase in the growth rate of 4.7 SD (9.3cm) and 3.4 SD (8.3cm), respectively, which agrees with our own findings. By contrast, Cardoso et al.19 recorded a greater increase in both parameters in the first year of treatment among patients with PGHD (gain in height 0.41 SD versus 0.52 SD and growth rate 7.43cm vs. 8.42cm, respectively).
The safety of rhGH treatment was not assessed in this study, since a monitoring period after discontinuation is required in order to do so. Nevertheless, no serious side effects were observed during administration. Moreover, several authors have shown that rhGH treatment may be regarded as safe, provided that the indications are established on an individual basis, with close patient follow-up.27,28
With regard to the limitations of our study, mention should be made of the small sample size, attributable to the strict inclusion criteria applied, and the fact that we only included patients with data referring to final height and re-evaluation through stimulus testing after the end of therapy. Another limitation could be related to the analytical techniques used in the course of the time period covered by the study, specifically for the determination of IGF-1 and GH, as previously commented upon. In addition, we did not have a control group (untreated idiopathic short stature) with which to establish comparisons.
In conclusion, we have described the auxological characteristics of a group of pediatric patients diagnosed with GHD, in which greater treatment effectiveness was seen in terms of growth over both the short and long term, in the group with a final diagnosis of PGHD. However, studies involving a larger number of patients are needed to assess the overall benefit in terms of growth, the occurrence of adverse effects, and the improvement of patient quality of life.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Villafuerte B, Barrio R, Martín-Frías M, Alonso M, Roldán B. Características auxológicas en pacientes pediátricos con déficit aislado permanente o transitorio de hormona del crecimiento. Respuesta al tratamiento y talla final. Endocrinol Diabetes Nutr. 2019;66:368–375.