Hypoglycemia is one of the most common complications to achieve a good metabolic control, and has been listed by several scientific associations as a common indication to start treatment with continuous subcutaneous insulin infusion (CSII). Use of CSII is still residual in Spain as compared to neighboring countries, and cost of acquisition cost is one of the main reasons.
This study estimates the budget impact of treatment with CSII, as compared to multiple daily insulin injections, of patients with type 1 diabetes mellitus who experience recurrent severe hypoglycemia episodes from the National Healthcare System perspective.
MethodsBudget impact was based on a retrospective, observational study evaluating the efficacy of CSII in patients with type 1 diabetes mellitus conducted at Hospital Clínic i Universitari in Barcelona, where one of the main indications for switching to CSII were recurrent severe hypoglycemia episodes. The mean number of annual episodes was 1.33 in the two years prior to CSII start and 0.08 in the last two years of follow up (p=0.003). Costs of treatment and major hypoglycemic events over a four-year period were considered. Costs were taken from different Spanish data sources and expressed in € of 2016.
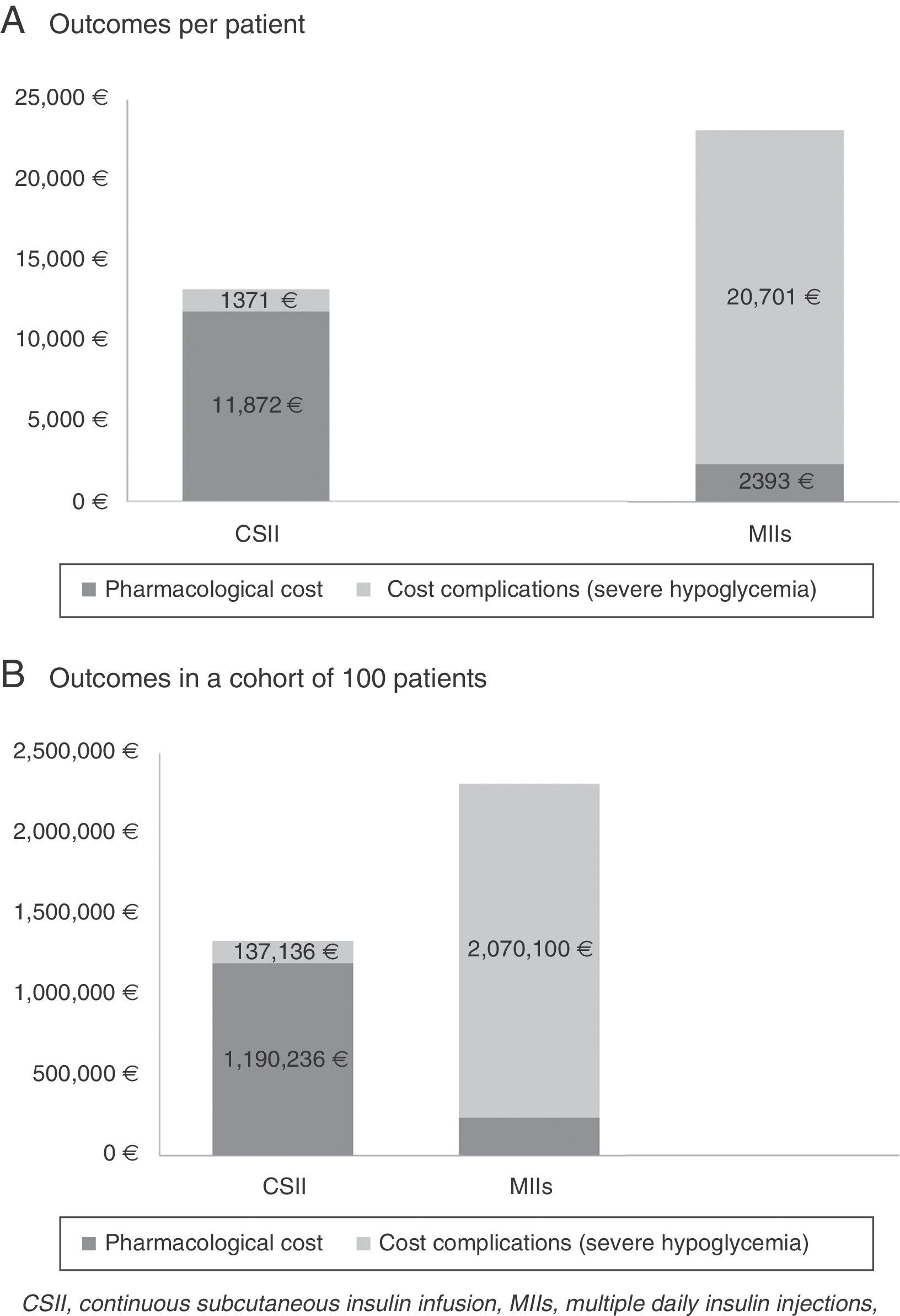
ResultsTreatment with CSII increased costs by €9509 per patient as compared to multiple daily insulin injections (€11,902–€2393). Cost associated to severe hypoglycemic events decreased by €19,330 per patient treated with CSIII (€1371–€20,701). Results suggest mean total savings of €9821 per patient during the four-year study period.
ConclusionThe higher costs associated to CSII therapy may be totally offset by the severe hypoglycemic events prevented.
Las hipoglucemias suponen una de las complicaciones más habituales para alcanzar un buen control metabólico y figuran entre las indicaciones comúnmente aceptadas por diferentes sociedades para iniciar tratamiento con infusión subcutánea continua de insulina (ISCI). La utilización de ISCI en España es aún residual en comparación con países de nuestro entorno, siendo el coste de adquisición una de las principales motivaciones.
Este trabajo estima el impacto presupuestario asociado a ISCI frente a múltiples dosis de insulina en pacientes con diabetes mellitus tipo 1 e hipoglucemias graves recurrentes desde la perspectiva del Sistema Nacional de Salud.
MétodosEl impacto presupuestario está basado en los resultados de un estudio observacional que evaluó la efectividad de ISCI en el Hospital Clínic i Universitari de Barcelona, donde el promedio anual de hipoglucemias graves en los dos años anteriores al inicio con ISCI fue 1,33 y 0,08 en los últimos dos años de seguimiento (p=0,003). Se contemplaron los costes asociados al tratamiento y al manejo de hipoglucemias graves durante cuatro años. Los costes unitarios (€, 2016) fueron obtenidos de bases de datos nacionales.
ResultadosEl coste del tratamiento con ISCI resultó en un incremento de 9.509€/paciente frente a múltiples dosis de insulina (11.902€-2.393€). El coste asociado a las hipoglucemias graves disminuyó 19.330€/paciente en aquellos tratados con ISCI (1.371€-20.701€). Los resultados indican un ahorro medio de 9.821€/paciente para el SNS en los cuatro años de estudio.
ConclusiónEl incremento asociado al coste del tratamiento podría quedar totalmente compensado gracias a los episodios de hipoglucemia grave evitados.
The comorbidities associated with diabetes mellitus have a considerable cost impact on the Spanish National Health System (Sistema Nacional de Salud [SNS]).1 Specifically, hypoglycemia is one of the most common short-term complications and one of the main obstacles to achieving good metabolic control.2
Severe hypoglycemia has a considerable effect upon patient health and often requires hospital admission, drug treatment, medical care and home visits, with resulting productivity losses and/or sick leave.3
The existing scientific evidence warrants intensive treatment in the form of multiple daily insulin injections (MIIs) or continuous subcutaneous insulin infusion (CSII) as the best option for the management of type 1 diabetes mellitus (DM1).4
The benefits of insulin pump therapy in terms of glycemic control and patient quality of life5,6 have been widely demonstrated and described in the literature, and the existence of recurrent severe and non-severe hypoglycemic episodes has been recognized as an indication of CSII by different scientific bodies. Despite this, however, the adoption of CSII in the management of DM1 in Spain is still largely limited compared with other countries in Europe. One of the main reasons for this is the greater initial investment needed compared with MII.7
The present study was carried out to estimate the budget impact (BI) of CSII in patients with DM1 and a history of recurrent severe hypoglycemia from the perspective of the SNS, based on local data obtained in routine clinical practice.
Material and methodsThe model used for this analysis was developed using MS Excel® 2010 and following the international recommendations for evaluations of this kind.8
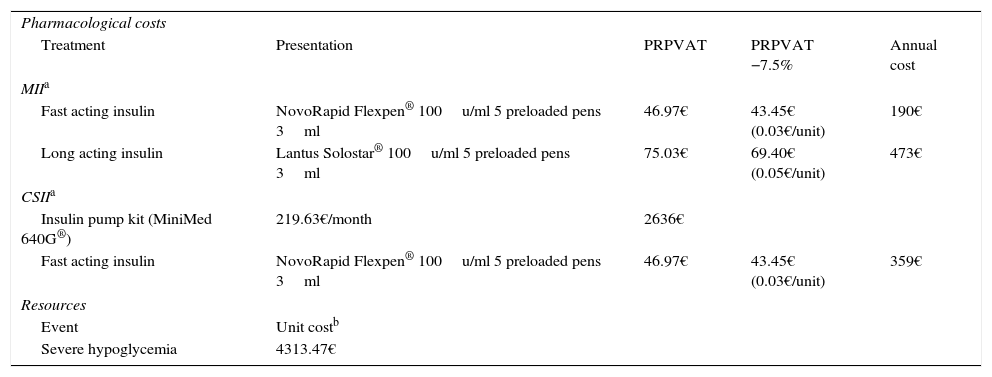
Target populationThe target population consisted of patients that switched from MII to CSII, whose main reason for the change in treatment was the appearance of recurrent severe hypoglycemic episodes. The baseline characteristics of the patients are shown in Table 1
Baseline characteristics of the patients included in the study.
| Group of patients with recurrent severe hypoglycemia | |
|---|---|
| No. | 43 |
| Females, n (%) | 24 (55.8) |
| Duration of diabetes, years | 19.9±9.5 |
| Age at start of CSII, years | 39.1±9.0 |
| BMI (kg/m2) | 23.6±4.7 |
| HbA1c, mmol/mol (%) | 56±11 (7.3±1.0) |
BMI: body mass index; CSII: continuous subcutaneous insulin infusion.
Source: Quirós et al.9
The effectiveness data on which the analysis was based were obtained from a retrospective observational study carried out during 2003–2008 at the Diabetes Unit of the Department of Endocrinology and Nutrition of Hospital Clínic i Universitari de Barcelona (Barcelona, Spain), with the aim of determining the long-term outcomes of CSII, and in which the mean number of annual severe hypoglycemic episodes per patient in the two years before the start of CSII was 1.33±2.55 versus 0.08±0.30 in the last two years of follow-up (p=0.003). In all, 93% of the patients benefited from the change to treatment with CSII. HbA1c levels at the start and end of the study were 56±11mmol/mol (7.3±1.0%) vs 57±9mmol/mol (7.4±0.8%) (p=0.280), respectively.9
This study showed treatment with the insulin pump to be an alternative capable of reducing the number of severe hypoglycemic episodes to a greater extent than MII over the long term, and without any worsening of patient HbA1c levels.
Time horizon, perspective and discount rateThe time horizon evaluated in the analysis was four years, long enough to identify and quantify patient evolution.
The direct healthcare costs generated by the management of recurrent severe hypoglycemia (cost of therapy and cost associated with management of the event) were identified, estimated and quantified from the perspective of the SNS.
No discount rate was considered, in compliance with the good clinical practice recommendations for conducting BI analyses of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR).8
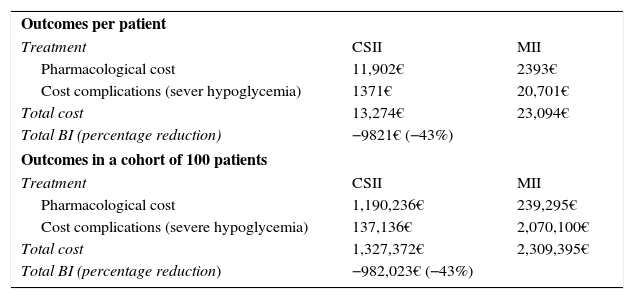
Resources and costsThe analysis took into account differences in posology according to the therapy selected, i.e., MII or CSII. These were established after consultation with experts (Table 2).
Resource utilization and costs.
| Pharmacological costs | ||||
| Treatment | Presentation | PRPVAT | PRPVAT −7.5% | Annual cost |
| MIIa | ||||
| Fast acting insulin | NovoRapid Flexpen® 100u/ml 5 preloaded pens 3ml | 46.97€ | 43.45€ (0.03€/unit) | 190€ |
| Long acting insulin | Lantus Solostar® 100u/ml 5 preloaded pens 3ml | 75.03€ | 69.40€ (0.05€/unit) | 473€ |
| CSIIa | ||||
| Insulin pump kit (MiniMed 640G®) | 219.63€/month | 2636€ | ||
| Fast acting insulin | NovoRapid Flexpen® 100u/ml 5 preloaded pens 3ml | 46.97€ | 43.45€ (0.03€/unit) | 359€ |
| Resources | ||||
| Event | Unit costb | |||
| Severe hypoglycemia | 4313.47€ | |||
CSII: continuous subcutaneous insulin infusion; MII: multiple daily insulin injection; PRP: public retail price.
The total cost estimate for each treatment alternative consisted of the following cost chapters: the pharmacological cost and that derived from the management of a severe hypoglycemic event. The cost associated with insulin therapy (NovoRapid Flexpen®, Lantus Solostar®) was calculated from the public retail price, including VAT,10 but taking into consideration the deduction established by Spanish Royal Decree 8/2010.11 The costs associated with the device (MiniMed® 640G) and consumables were provided by Medtronic Ibérica, S.A. The unit costs of the healthcare resources (severe hypoglycemia) were recorded from the hospital discharge registries of the Minimum Basic Data Set of the Spanish Ministry of Health, Social Services and Equality corresponding to the year 2013, taking into account the records documenting diagnostic category 251 of the International Classification of Diseases (ICD-9-CM).12
All costs were expressed in euros corresponding to 2016 and updated according to variations in the consumer price index, where necessary.13
Sensitivity analysisDifferent univariate deterministic sensitivity analyses (SAs) were made in order to evaluate the robustness of the model and to identify the variables with the strongest impact upon the outcomes. The total cost impact of modifying the value of the parameters associated with greater uncertainty (which can vary according to the different hospitals in Spain) was evaluated by varying the monthly cost of the insulin pump kit (−10%) and the cost associated with the management of severe hypoglycemic episodes (3500€,3 −50% and the cost implied by a neutral BI). In addition, we evaluated different time horizons and values referring to the severe hypoglycemia rate with both treatment alternatives (1, 2 and 3 years and ±10%, respectively).
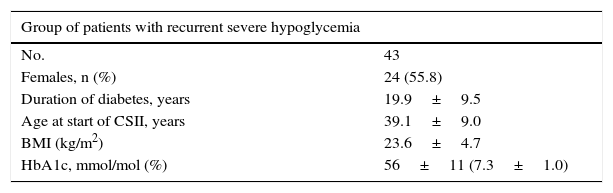
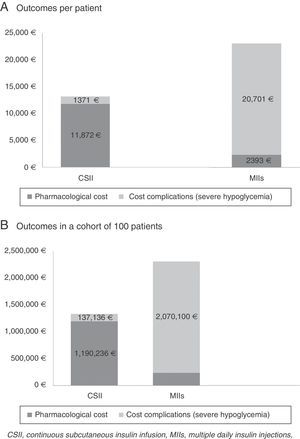
ResultsThe cost of CSII resulted in an increase of 9509€ per patient compared with MII (11,902€ versus 2393€). On the other hand, the cost associated with the management of severe hypoglycemic episodes decreased 19,330€ per patient in those administered CSII compared with MII (1371€ versus 20,701€). Thus, compared with MII, the BI of CSII for the treatment of DM1 patients with severe hypoglycemic episodes corresponded to an average saving of 9821€ per patient for the SNS in the course of the studied time horizon. In a hypothetical cohort of 100 patients, the saving would be in the order of 982,023€.
Table 3 and Fig. 1 describe the results of the BI analysis, stratified according to the different cost concepts (therapy and severe hypoglycemia).
Results referring to the base case.
| Outcomes per patient | ||
| Treatment | CSII | MII |
| Pharmacological cost | 11,902€ | 2393€ |
| Cost complications (sever hypoglycemia) | 1371€ | 20,701€ |
| Total cost | 13,274€ | 23,094€ |
| Total BI (percentage reduction) | −9821€ (−43%) | |
| Outcomes in a cohort of 100 patients | ||
| Treatment | CSII | MII |
| Pharmacological cost | 1,190,236€ | 239,295€ |
| Cost complications (severe hypoglycemia) | 137,136€ | 2,070,100€ |
| Total cost | 1,327,372€ | 2,309,395€ |
| Total BI (percentage reduction) | −982,023€ (−43%) | |
MII: multiple daily insulin injection; BI: budget impact; CSII: continuous subcutaneous insulin infusion.
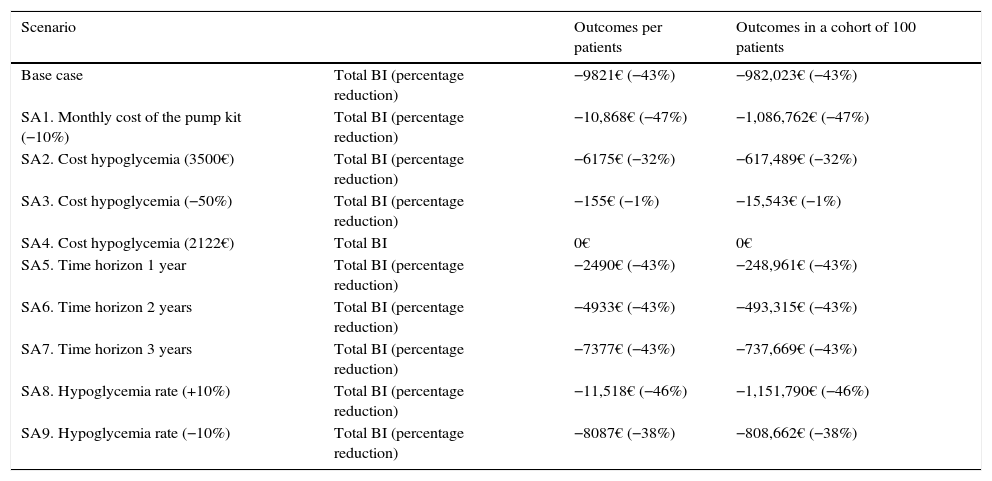
The results corresponding to the 8 evaluated scenarios expressed in total BI and percentage reduction per patient, and in a cohort of 100 patients, are shown in Table 4. The deterministic SAs confirmed the robustness of the model in all the scenarios, attesting to the total cost savings associated with CSII versus MII.
Results of the deterministic sensitivity analysis.
| Scenario | Outcomes per patients | Outcomes in a cohort of 100 patients | |
|---|---|---|---|
| Base case | Total BI (percentage reduction) | −9821€ (−43%) | −982,023€ (−43%) |
| SA1. Monthly cost of the pump kit (−10%) | Total BI (percentage reduction) | −10,868€ (−47%) | −1,086,762€ (−47%) |
| SA2. Cost hypoglycemia (3500€) | Total BI (percentage reduction) | −6175€ (−32%) | −617,489€ (−32%) |
| SA3. Cost hypoglycemia (−50%) | Total BI (percentage reduction) | −155€ (−1%) | −15,543€ (−1%) |
| SA4. Cost hypoglycemia (2122€) | Total BI | 0€ | 0€ |
| SA5. Time horizon 1 year | Total BI (percentage reduction) | −2490€ (−43%) | −248,961€ (−43%) |
| SA6. Time horizon 2 years | Total BI (percentage reduction) | −4933€ (−43%) | −493,315€ (−43%) |
| SA7. Time horizon 3 years | Total BI (percentage reduction) | −7377€ (−43%) | −737,669€ (−43%) |
| SA8. Hypoglycemia rate (+10%) | Total BI (percentage reduction) | −11,518€ (−46%) | −1,151,790€ (−46%) |
| SA9. Hypoglycemia rate (−10%) | Total BI (percentage reduction) | −8087€ (−38%) | −808,662€ (−38%) |
SA: sensitivity analysis; BI: budget impact.
The 50% decrease in the cost of severe hypoglycemia was the parameter with the strongest impact upon the results. On the other hand, the cost implied in the case of a neutral BI was 2122€. The evaluated scenarios also showed a directly proportional relationship between the time horizon of the analysis and the savings generated, the latter increasing with the length of the time horizon examined (from 1 to 4 years).
DiscussionThe clinical and social consequences of severe hypoglycemia have been extensively studied. However, its economic impact on routine clinical practice has not been so thoroughly quantified to date.
The results obtained in the present analysis show that CSII for the treatment of DM1 patients with severe hypoglycemic episodes results in an average saving for the SNS of up to 9821€ four years after its introduction. Furthermore, even after taking into consideration a 50% reduction in the unit cost of a severe hypoglycemic episode, CSII continues to afford a mean saving of 155€ per patient.
When resources are limited, it is necessary to assign them efficiently in order to maximize the healthcare outcomes, in accordance with the investment made. In this context, economic analyses of healthcare technologies in general, with methodologically sound studies assessing both the costs and the outcomes of the different alternatives, and BI analyses in particular, constitute useful tools capable of complementing informed decision making based on purely clinical data.
In this regard, a recent systematic review has evaluated and synthesized the contents of 11 cost-effectiveness studies carried out in 8 different countries, in which CSII and MII were the compared treatments. The results of this review, which also includes a study carried out in the Spanish setting, suggest that CSII is a cost-effective alternative to MII, taking into account the willingness to pay thresholds in the different countries analyzed.14,15 In the concrete case of the study carried out in the Spanish setting, an incremental cost-effectiveness ratio of 29,947€ per quality-adjusted life year gained was recorded for CSII versus MII. Given that a willingness to pay a threshold of 30,000€ per quality-adjusted life year gained is commonly accepted in Spain,16 CSII constitutes an efficient treatment option compared with MII.
To the best of our knowledge, this is the first BI analysis to examine the economic consequences of CSII in DM1 patients with recurrent severe hypoglycemia in Spain based on data obtained from local clinical practice and unit costs, thereby affording a general view of the costs and benefits associated with both treatment interventions over the short/middle term. In this regard, and in agreement with our own analysis, a recent study published in Germany analyzed the clinical and economic benefits associated with the introduction of CSII in DM1 patients with poor glycemic control in the course of a four-year period. The study found CSII to be associated with a reduction in severe hypoglycemic episodes and in the micro- and macrovascular complications of the disease compared with MII. Accordingly, the costs associated with the introduction of CSII are largely compensated for by the savings associated with the management of the avoided complications over the short/middle term.17
The results of the present study, as with all model-based analyses, are not without limitations. The difficulties associated with the projection of a future time horizon define cost estimates as one of the common limitations in studies of this kind. On the other hand, since the percentage of severe hypoglycemic episodes requiring hospital care was not known in the study on which this analysis is based, we assumed that such care was required in 100% of the cases, leading to a possible overestimation of the savings associated with CSII. However, it is important to emphasize that recurrent non-severe hypoglycemic episodes and severe hypoglycemic episodes that do not require hospital admission also imply healthcare resource utilization (health professionals or ambulance to the home of the patient, emergency visits, increased reactive strip use and a number of visits to the specialist and/or nursing staff on the days following the event, sick leave, etc.), not to mention the consequent associated costs. Assuming that the percentage of severe hypoglycemic episodes requiring hospital admission is 35–25% of the total18 and that the remaining percentage implies no cost, the BI of DM1 patient treatment with CSII compared with MII would entail an incremental cost of between 2744€ and 4677€, respectively per patient for the SNS in the course of four years.
Another possible limitation is the sample size of the retrospective observational study in which differences were seen in terms of the number of hypoglycemic episodes.9 In the study, the number of patients that started CSII on the basis of the therapeutic indication of the analysis was 43. Another limitation of the study is that it is not possible to fully guarantee the external validity of the results, since the study was based on the findings of a single reference center for CSII in Spain. In turn, quality of life parameters or indirect costs associated with work productivity losses were not taken into account. As a result, the present study does not fully take into consideration the value of the different alternatives.
As a strength of the analysis, mention should be made of the fact that it is based on data referring to the clinical practice setting in Hospital Clinic i Universitari de Barcelona, a reference center in CSII for the treatment of DM1 patients in Spain.
Lastly, we must emphasize the importance of registries that compile clinical and economic outcomes prospectively or even in real time, thus affording improved knowledge of the natural history of the disease, evaluation or supervision of the quality and safety of medical care, and making it possible to conduct pharmacoeconomic analyses that contribute to improving therapeutic selection, along with efficient resource allotment.
In conclusion, the results of our analysis show that the increase in cost associated with CSII versus MII in patients with DM1 and recurrent severe hypoglycemia in Spain could be entirely compensated for and could even result in savings for the SNS, due to the reduction in severe hypoglycemic episodes.
However, further studies are needed to assess the economic impact of these treatments, taking into account both the direct and indirect costs of the severe/non-severe hypoglycemic episodes and the cost of the out-hospital management of such episodes.
Financial supportThis study has been financed by Medtronic Ibérica, S.A.
Conflicts of interestIE and MA work for Medtronic Ibérica, S.A. MG, CQ and IC declare that they have no conflicts of interest in relation to this study.
Please cite this article as: Giménez M, Elías I, Álvarez M, Quirós C, Conget I. Impacto presupuestario de la infusión subcutánea continua de insulina en el tratamiento de pacientes con diabetes tipo 1 que presentan episodios de hipoglucemia grave recurrente en España. Endocrinol Diabetes Nutr. 2017;64:377–383.