We present a case of severe hyponatraemia in a 67-year-old male patient, admitted to a tertiary hospital with a diagnosis of SARS-CoV-2 coronavirus respiratory infection confirmed by PCR. The patient had a medical-surgical history of ischaemic heart disease with revascularised acute myocardial infarction (AMI) in 2009, congenital right hemiparesis, mild intellectual disability, hydrocephalus and pharmacologically well-controlled epilepsy.
At 20 days following admission, he developed symptoms compatible with facial myoclonus, temporary disorientation and dysarthria lasting four minutes, with complete recovery. On examination he was conscious and oriented, with a blood pressure of 169/89 mmHg, afebrile, with capillary blood glucose of 180 mg/dl and arrhythmic tachycardia at 130 beats per minute, with known atrial fibrillation confirmed by ECG at admission.
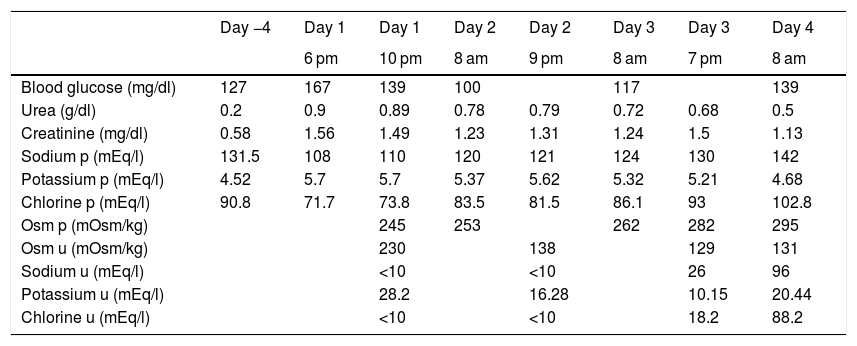
Previously, the patient did not have fluid therapy. Treatment with 300 mg of amiodarone was then started and urgent blood tests were requested, highlighting hyponatraemia of 108 mEq/l (results in Table 1, 2nd column, with previous tests in 1 st column). The patient was re-evaluated and still did not present any neurological abnormalities, so admission to the ICU was ruled out and an infusion was started of 250 ml of 3% hypertonic saline in four hours. At that time, thyroid dysfunction (TSH: 1.62 mU/l) and adrenal insufficiency (ACTH: 24.2 pg/ml and basal cortisol: 15 mcg/dl) were also ruled out. The lab test results after four hours are reflected in Table 1 (3rd column).
Lab test evolution of the patient in relation to the hours of evolution.
| Day −4 | Day 1 | Day 1 | Day 2 | Day 2 | Day 3 | Day 3 | Day 4 | |
|---|---|---|---|---|---|---|---|---|
| 6 pm | 10 pm | 8 am | 9 pm | 8 am | 7 pm | 8 am | ||
| Blood glucose (mg/dl) | 127 | 167 | 139 | 100 | 117 | 139 | ||
| Urea (g/dl) | 0.2 | 0.9 | 0.89 | 0.78 | 0.79 | 0.72 | 0.68 | 0.5 |
| Creatinine (mg/dl) | 0.58 | 1.56 | 1.49 | 1.23 | 1.31 | 1.24 | 1.5 | 1.13 |
| Sodium p (mEq/l) | 131.5 | 108 | 110 | 120 | 121 | 124 | 130 | 142 |
| Potassium p (mEq/l) | 4.52 | 5.7 | 5.7 | 5.37 | 5.62 | 5.32 | 5.21 | 4.68 |
| Chlorine p (mEq/l) | 90.8 | 71.7 | 73.8 | 83.5 | 81.5 | 86.1 | 93 | 102.8 |
| Osm p (mOsm/kg) | 245 | 253 | 262 | 282 | 295 | |||
| Osm u (mOsm/kg) | 230 | 138 | 129 | 131 | ||||
| Sodium u (mEq/l) | <10 | <10 | 26 | 96 | ||||
| Potassium u (mEq/l) | 28.2 | 16.28 | 10.15 | 20.44 | ||||
| Chlorine u (mEq/l) | <10 | <10 | 18.2 | 88.2 |
p: plasma value; u: urine value.
It was decided to leave an infusion of 500 ml 3% hypertonic saline to be administered in eight hours. After that, lab tests were performed again, including the N-terminal fraction of brain natriuretic peptide (NT-proBNP): 5350 pg/ml (previously 6830 pg/ml) (rest of the parameters in Table 1, 4th column). On examination, he presented with arterial hypertension (172/100 mmHg) and controlled heart rate, was afebrile and maintained a baseline oxygen saturation of 98%. There were no data on decompensated heart failure. When questioned in a targeted manner, he reported a daily intake of more than four litres of water, with preserved diuresis (he wore adult absorbent briefs at that time) and an increase of up to five bowel movements in 24 h. He had a good level of consciousness and in the rest of the examination only palpation of a non-painful infraumbilical mass stood out which was first assessed as possibly being a bladder overdistension, but that was ruled out due to good daily diuresis. At that time, abdominal CT was requested to rule out underlying neoplasia. The patient had undergone a pulmonary CT five days beforehand, which ruled out the presence of pulmonary thromboembolism, as well as neoplastic pathology, reporting the presence of lesions consistent with the underlying infectious process.
The treatment with omeprazole was suspended, as well as the daily furosemide tablet (the diuretic had been previously decreased, with decreasing NT-proBNP figures) and a fluid restriction of one litre of water was indicated, initiating an infusion of one litre of isotonic saline with two 10 ml ampoules of 20% hypertonic saline after calculating the sodium deficit, with a maximum correction objective of 8 mEq/l in 24 h.
In the control lab tests after eight hours, renal function remained stable compared to previously, and sodium was 121 mEq/l. At 24 h, the patient was still asymptomatic, without neurological deficit, with unchanged physical examination and the same sensation of palpation of the infraumbilical mass. The lab test results for that day are shown in Table 1 (6th column). Fluid restriction was maintained at one litre per day and perfusion of 1,000 ml of isotonic saline in 24 h with three 10 ml ampoules of 20 % hypertonic saline. Abdominal CT only determined the presence of an overdistended bladder, for which reason the patient was catheterised, obtaining a total diuresis of 1,800 ml.
Six hours after the new perfusion and catheterisation, the lab test values were those shown in Table 1 (7th column), for which hypertonic saline was suspended, maintaining only isotonic saline.
The following day, the patient was normotensive, with a good heart rate, with no neurological abnormalities and, on examination, the abdominal mass had disappeared. The lab test results for that day are shown in Table 1 (8th column). For the rest of his hospital stay, his blood sodium remained within normal levels and he did not develop complications secondary to the rapid correction of sodium after urinary catheterisation.
This was a case of severe hyponatraemia secondary to acute asymptomatic urine retention, with urinary incontinence due to overflow. It was ruled out that hyponatraemia could be secondary to fluid overload (absence of oedema, normal lung auscultation, no hepatic congestion or jugular engorgement). It was unlikely that it was secondary to treatment with antiepileptic drugs (phenobarbital and phenytoin), as it was a chronic treatment without dose modification. Hypothyroidism and adrenal insufficiency were ruled out. Neither was it attributed to the COVID-19 infection itself, since the most critical time period had passed and a clear relationship between COVID-19 infection and hyponatraemia has not been described for the time being.1,2 Dehydration due to increased stool rhythm was not likely because the patient had presented only five not very large stools in the previous 24 h, so syndrome of inappropriate secretion of antidiuretic hormone (SIADH) was initially considered, despite the fact that the ion values in urine were not conclusive with this diagnosis. With fluid restriction and hypertonic saline, plasma sodium levels rose very slowly, and following bladder catheterisation they quickly normalised.
There are not many cases described in the literature.3–7 Bladder distention is believed to cause release of vasopressin at the pituitary level.8 Another theory supports the idea of saline loss due to resistance to the action of aldosterone at the renal tubular level due to an excess of sodium secretion in patients with obstructive uropathy,9 although in cases in which the obstruction dilates the urinary tract and renal calyces, renal vasoconstriction occurs, with decreased renal blood flow and decreased sodium arriving at the nephron. This also causes renal failure with acute tubular necrosis, in which urinary biochemical parameters are consistent with those found in the current case.10
Therefore, acute urinary retention should be considered in the differential diagnosis of hyponatraemia with low blood sodium levels that are not compatible with SIADH.
Data confidentialityThe protocols established by the hospital have been followed in order to access data from the clinical history described in this case.
FundingNo sponsorship of any kind was received for producing this article.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Agudo Tabuenca A, Morales Campoverde KG, García García MB, Peteiro Miranda CM, Sánchez Marteles M. No toda la culpa fue de la COVID. Endocrinol Diabetes Nutr. 2021;68:593–595.