Anterior pituitary dysfunction (APD) is one of the commonest mass-effect related symptoms in pituitary adenomas (PA).1
Few data exist evaluating macroscopic or histological data and their association with anterior pituitary function. Regarding macroscopic features, tumour size is a classical risk factor of APD.2 It is also known that aggressive3 and invasive PAs4 usually present a higher risk of recurrence and hypopituitarism. Moreover, patients with hard tumours are more likely to present hypopituitarism.5 Regarding the association of histological features such as grade of fibrosis, ki67 index and presence of necrosis with APD, to the best of our knowledge, no previous studies have investigated this aspect.
In this study we investigate if there are any intrinsic PA factor, either macroscopic or histological, that could be associated with a higher risk of preoperative APD in a cohort of 232 surgically treated PA for the first time.
A retrospective, two-centres study was conducted, studying 232 consecutive patients with PAs who underwent pituitary surgery for the first time between January 2009 and December 2019 in the Neurosurgery Department of the Hospital Ramón y Cajal and Hospital HM Puerta del Sur.
APD was defined as the evidence of ACTH, TSH, FSH/LH and/or GH deficits. Preoperative morning serum cortisol <5μg/dL or a peak serum cortisol <18μg/dL after 250μg of cosyntropin was taken as evidence of ACTH deficit. The diagnosis of GH deficit was performed by the presence of an IGF-1 level of – 2 standard deviations for age and sex in the presence of 3 or more hormonal deficits. TSH and FSH/LH deficits were defined as we have previously reported.6
The macroscopic variables analysed were: tumour consistency (tumours difficult to remove with ring curettes and required sharp dissection, bipolar cautery and/or surgical aspirator were termed hard tumours; the easily suckable were classified as soft tumours); PA diameters (cranio-caudal and laterolateral, based on MRI reports); invasiveness based on Knosp radiological classification (invasive PAs were considered those with Knosp >2)7 and macroscopic information (dural, periosteal, or mucosal tissues invasion). The histological features evaluated were, pituitary hormone immunostaining (ACTH, Prolactin and LH (polyclonal, Ventana), GH (clone A0570, Dako), TSH (clone 0042, Dako) and FSH (clone C10, Dako)), the proliferation index based on Ki67 immunoexpression (clone MIB-1, Dako), the presence of ischaemic necrosis or haemorrhagic necrosis and the grade of fibrosis based on the percentage of collagen (1: <5%, 2: 5–15% and 3: >15%) in relation to the whole area of tissue stained with haematoxylin-eosin.8
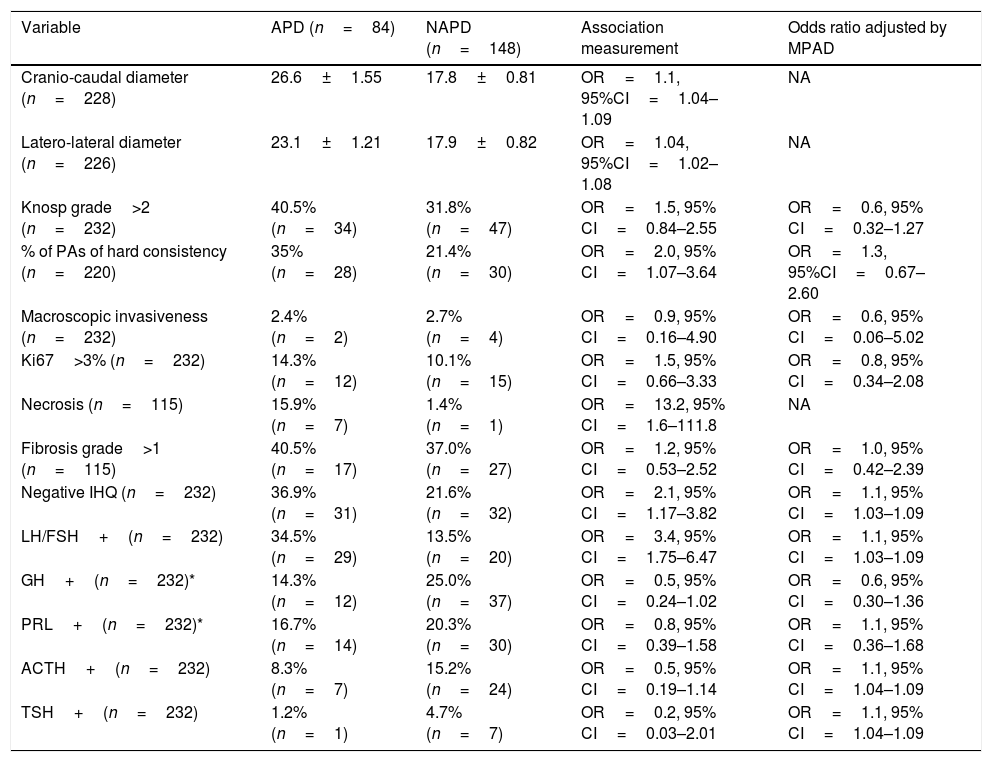
The mean age was 53.3±15.4 years and 53.0% were women. One hundred and forty-six patients presented non-functioning pituitary macroadenomas, 52 acromegaly, 21 Cushing disease, 12 prolactinomas and one patient a TSH-secreting PA. Hypopituitarism was present in 36.2% (n=84) of the patients, of which 61.9% presented ≥2 hormonal deficits and 21.4% panhypopituitarism. The most common APD was of LH/FSH in 31.9%, followed by ACTH in 18.1%; TSH in 16.4% and GH in 13.8%.
Mean craniocaudal and latero-lateral diameters were 21.0±12.4 and 19.8±10.6mm, respectively. It was found that patients with APD presented a greater craniocaudal and latero-lateral diameters than patients with normal pituitary function (26.6±14.3 vs. 17.8±9.8, p<0.001 and 23.1±11.1 vs. 17.9±16.2, p<0.001, respectively). More than one third (n=81) presented Knosp grade >2 PAs, showing no differences in its prevalence between patients with and without APD (p=0.181).
One quarter of patients harboured hard tumours (n=58). The risk of APD was twice as high in hard compared with soft tumours, but the differences disappeared after adjusted by cranio-caudal diameter (Table 1). Only 2.6% (n=6) presented histological data of invasion. No differences in the prevalence of APD were observed depending on macroscopic features of invasiveness (p=0.882).
Prevalence of APD depending on macroscopic and histological phenotype of pituitary adenomas features.
| Variable | APD (n=84) | NAPD (n=148) | Association measurement | Odds ratio adjusted by MPAD |
|---|---|---|---|---|
| Cranio-caudal diameter (n=228) | 26.6±1.55 | 17.8±0.81 | OR=1.1, 95%CI=1.04–1.09 | NA |
| Latero-lateral diameter (n=226) | 23.1±1.21 | 17.9±0.82 | OR=1.04, 95%CI=1.02–1.08 | NA |
| Knosp grade>2 (n=232) | 40.5% (n=34) | 31.8% (n=47) | OR=1.5, 95% CI=0.84–2.55 | OR=0.6, 95% CI=0.32–1.27 |
| % of PAs of hard consistency (n=220) | 35% (n=28) | 21.4% (n=30) | OR=2.0, 95% CI=1.07–3.64 | OR=1.3, 95%CI=0.67–2.60 |
| Macroscopic invasiveness (n=232) | 2.4% (n=2) | 2.7% (n=4) | OR=0.9, 95% CI=0.16–4.90 | OR=0.6, 95% CI=0.06–5.02 |
| Ki67>3% (n=232) | 14.3% (n=12) | 10.1% (n=15) | OR=1.5, 95% CI=0.66–3.33 | OR=0.8, 95% CI=0.34–2.08 |
| Necrosis (n=115) | 15.9% (n=7) | 1.4% (n=1) | OR=13.2, 95% CI=1.6–111.8 | NA |
| Fibrosis grade>1 (n=115) | 40.5% (n=17) | 37.0% (n=27) | OR=1.2, 95% CI=0.53–2.52 | OR=1.0, 95% CI=0.42–2.39 |
| Negative IHQ (n=232) | 36.9% (n=31) | 21.6% (n=32) | OR=2.1, 95% CI=1.17–3.82 | OR=1.1, 95% CI=1.03–1.09 |
| LH/FSH+(n=232) | 34.5% (n=29) | 13.5% (n=20) | OR=3.4, 95% CI=1.75–6.47 | OR=1.1, 95% CI=1.03–1.09 |
| GH+(n=232)* | 14.3% (n=12) | 25.0% (n=37) | OR=0.5, 95% CI=0.24–1.02 | OR=0.6, 95% CI=0.30–1.36 |
| PRL+(n=232)* | 16.7% (n=14) | 20.3% (n=30) | OR=0.8, 95% CI=0.39–1.58 | OR=1.1, 95% CI=0.36–1.68 |
| ACTH+(n=232) | 8.3% (n=7) | 15.2% (n=24) | OR=0.5, 95% CI=0.19–1.14 | OR=1.1, 95% CI=1.04–1.09 |
| TSH+(n=232) | 1.2% (n=1) | 4.7% (n=7) | OR=0.2, 95% CI=0.03–2.01 | OR=1.1, 95% CI=1.04–1.09 |
APD=anterior pituitary disfunction; IHQ=inmunostaining; MPAD=maximum pituitary adenoma diameter; NAPD=no anterior pituitary dysfunction; NA=not applicable; Positive immunostaining refers to both, diffuse and focal immunostaining; Aggressive invasiveness refers to invasion of the surrounding dural, periosteal, or mucosal tissues.
Based on consistency, tumours were classified in two groups: soft tumours if they were easily suckable and hard tumours when they need to be fragmented for removing using ring curettes.
A ki67>3% was present in 11.6% of the patients, and it was not related with APD (p=0.343). The presence of necrosis was evident only in patients with APD, in 15.9% (n=7) of them, except for one patient who debuted with pituitary apoplexy that presented ischaemic and haemorrhagic necrosis on histological examination, but normal function. The grade of fibrosis was not related with APD risk (p=0.711).
Patients with negative immunostaining PAs had a higher prevalence of APD than patients with any positive immunostaining (p=0.012) and, also patients with FSH/LH positive immunostaining than those with other immunostaining patterns (p<0.001). Patients harbouring negative immunostaining adenomas and gonadotrophinomas presented PA of higher tumour size compared with PAs with other immunostaining patterns (28.3±11.5 vs. 18.4±11.6mm, p<0.001 and 27.4±10.4 vs. 19.4±12.3mm, p<0.001, respectively) (Table 1).
In our series of 232 PAs, we found that patients with large tumours presented a higher prevalence of APD. These tumours more commonly presented negative immunostaining or only positive for FSH/LH. The presence of necrosis in histological exams was specific of APD (7 of 8 cases with necrosis presented APD).
In the same way, patients with APD presented PAs with craniocaudal and latero-lateral diameters, 9.0 and 5.3mm higher, respectively, than patients with normal pituitary function. This is in accordance with previous studies supporting that the risk for pituitary dysfunction increases with the increasing size of the PA.9,10 Nomikos2 described that APD was evident in 71% of PAs between 10–20mm, compared with 89.2% between 20–30mm; and Mukai11 concludes that physicians should proactively examine pituitary function in patients with NFPA measuring ≥20mm due to higher risk of APD.
In our study, hard tumours presented a two-fold times higher risk of APD than those of soft consistency, but it was probably size-related (hard PA were larger than softs). Although few data exist about this association, the findings of a previous study, seems to support our results.5 Thotakura et al.5 also found a significant higher proportion of hypopituitarism in hard tumours (66.7% vs. 9.6%, p<0.001), but they reported a low proportion of patients with hard tumours, of 6%. Moreover, the size of the PAs in hard and soft tumours were similar.
The only independent histological data associated with APD was the presence of necrosis, as it was evident only in patients with APD with the exception of one patient. There have been recognised some intrinsic features of PAs that render them vulnerable to necrosis/ischaemia, such as their high metabolic demand, paucity of angiogenesis, and sparse vascularity. These findings are typical or pituitary apoplexy, which present a high prevalence of APD, in up to 80%.12
The conclusion of our study is that preoperative APD is relatively frequent in PAs (up to 35.8% of the patients) and the only independent intrinsic PA features associated with APD were the size and the presence of necrosis. A hard consistence and a negative or FSH/LH positive immunostaining were also associated with a higher risk but probably influenced by the higher tumour size in these patients. So, given the clear relationship between tumour size and pituitary function status, preoperative hormonal study should be performed in all patients with PAs, but especially in those of greater tumour size.
Ethical approvalAll procedures performed in the participants of the study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consentInformed consent was obtained from all individual participants included in the study.