Metabolic control in type 1 diabetes (T1D) depends on many factors such as eating habits, exercise and lifestyle. The objective of this study was to investigate how these factors were affected during the coronavirus disease 2019 (COVID-19) lockdown and impacted metabolic control in children with T1D.
Materials and methodOne hundred children with T1D were enrolled in the study. Anthropometric measurements, snack and meal frequency, carbohydrate consumption, HbA1c levels, and exercise patterns were recorded and compared before and after the lockdown. Subjects were divided into two subgroups—patients with decreased and patients with increased HbA1c levels after the lockdown—and comparisons of the same parameters were also made between these two subgroups.
ResultsIn the overall group, the mean HbA1c level was significantly higher after the lockdown compared to before (p=0.035). Meal schedules changed due to delayed sleep and waking times, and total daily carbohydrate consumption increased in the subgroup with increased HbA1c while it decreased in the subgroup with decreased HbA1c (p<0.001 for both).
ConclusionOur study supports the notion that blood sugar management in children with T1D worsened during the COVID-19 pandemic. Although it is not possible to explain this with any one factor, some behavioral changes observed in our study, such as inactivity, irregular meal frequency and timing, and irregular sleep and waking patterns appeared to be associated with blood sugar management.
El control metabólico en la diabetes tipo 1 (DT1) depende de muchos factores, como los hábitos alimentarios, el ejercicio físico y el estilo de vida. En este estudio, el objetivo fue investigar cómo estos factores se vieron afectados durante la cuarentena de la enfermedad por coronavirus 2019 (COVID-19) y cómo afectaron al control metabólico en niños con DT1.
Materiales y métodoSe incluyó a 100 niños con DT1 en el estudio. Las mediciones antropométricas, la frecuencia de comidas principales y refrigerios, el consumo de carbohidratos, los niveles de HbA1c y los patrones de ejercicio se registraron y compararon antes y después de la cuarentena. Los sujetos se dividieron en 2 subgrupos: pacientes con niveles de HbA1c disminuidos o aumentados después de la cuarentena y también se realizaron comparaciones con los mismos parámetros entre estos 2 subgrupos.
ResultadosEn el grupo general, el nivel medio de HbA1c fue significativamente más alto después de la cuarentena en comparación con antes (p=0,035). El horario de las comidas se cambió por el retraso de la hora de dormir y despertar, y el consumo diario total de carbohidratos aumentó en el subgrupo con HbA1c aumentada, mientras que disminuyó en el subgrupo con HbA1c disminuida (p <0,001 para ambos).
ConclusiónNuestro estudio apoya que el control glucémico de los niños con DT1 empeoró en el período de la pandemia de la COVID-19. Aunque no es posible explicar esto por un solo factor, algunos cambios de comportamiento observados en nuestro estudio, como la inactividad, la frecuencia y el horario irregulares de las comidas, los patrones irregulares de sueño y vigilia, parecen asociarse con el control glucémico.
Coronavirus disease 2019 (COVID-19) pandemic began in China's Wuhan city in December 2019 spreading rapidly across the world. The first case was announced on March 11 in Turkey, and schools were suspended nationwide on March 16. The Turkish government introduced quarantine to limit viral transmission, and a lockdown was declared for people under 20 as of April 3.
It is expected that during vacations, children and adolescents are physically less active, have more unhealthy diets, longer screen time and irregular sleep-wake patterns.1 These factors could be even worse in children staying at home without outdoor activities and contact with friends.2 Thus, an expected decrease in exercise and an increase in sedentary behavior could have a detrimental effect on glycemic control.3 Instead, a steady daily routine, physical activity and healthy diet contribute to a more effective management of diabetes in children and adolescents.4,5
Since scheduled visits in-person were canceled during COVID-19 pandemics, telemedicine (not requiring physical proximity) seems to be the only way to provide healthcare service for individuals with type 1 diabetes (T1D) and to check glycemic control during this unexpected event.6,7
The aim of this study is to determine how metabolic control is affected by daily routine changes, sleep patterns and eating habits in children and adolescents with T1D during COVID-19 pandemic.
Subjects, materials and methodsThis is a cross-sectional study conducted with a total of 100 patients with a diagnosis of T1D for at least one year. All patients had visited our outpatient clinic in the last 30 days before the start of the quarantine (April 3, 2020) and were also called for control within 15 days after the end of the quarantine (June 1, 2020). The patients who did not use carbohydrate counting or did not attend regular visits were excluded from the study.
Data regarding date of birth, date of T1D diagnosis, anthropometric parameters and pubertal status according to Tanner stages8 were extracted from patients’ medical files. Body mass index (BMI) was calculated as the ratio of the weight in kilograms to height in meters squared (kg/m2, Quetelet index), and Z-scores of each anthropometric parameter were calculated according to the standards for Turkish Children.9 ISPAD and ADA recommend an HbA1c level of <%7, as the optimal goal, %7–9 as suboptimal control and >%9 as poor control.10,11
Snack and meal frequency, carbohydrate consumption, laboratory results, number of hypoglycemic events, physical findings and exercise patterns were recorded. Carbohydrate requirements were calculated according to height, age and gender based on Turkish dietary guideline.12 Seven-day food consumption records were kept by the patients and were analyzed using the BE-BIS program.13 Physical activity was assessed using Physical Activity Questionnaire-A (PAQ-A) or Physical Activity Questionnaire-C (PAQ-C),14 which has been validated for use in Turkish children,15 and patients were scored between 8 and 40 points based on this questionnaire. The average sleep and waking hours of the patients before and during the quarantine period were recorded based on their statements.
Informed consent of the cases was obtained according to the Helsinki Declaration, and the study was approved by the Istanbul University-Cerrahpaşa ethics committee (number: 83045809-604.01.02).
For statistical analysis and graphical demonstration, Microsoft Excel® and IBM corp. SPSS Statistics® version 21.0 were used. Results were presented as mean±standard deviation (SD) or mean±standard error (SE). Subgroups were created in terms of the change in HbA1c (the patients with increased and decreased HbA1c during the lockdown period), and the glycemic control before the start of the quarantine (optimal and suboptimal-poor). Comparison of the means in different groups and subgroups was performed with Student's T-test for paired samples if the data were normally distributed. Wilcoxon Signed Ranks Test was used for non-normally distributed data. A p value of 0.05 or less was considered statistically significant.
ResultsA total of 100 children (M/F: 45/55) with T1D were finally enrolled in the statistical analysis (Fig. 1). Mean decimal age was 14.7±3.4, ranging from 3.5 to 17.9 years, and mean age at the time of the diagnosis of T1D was 7.2±3.6 years.
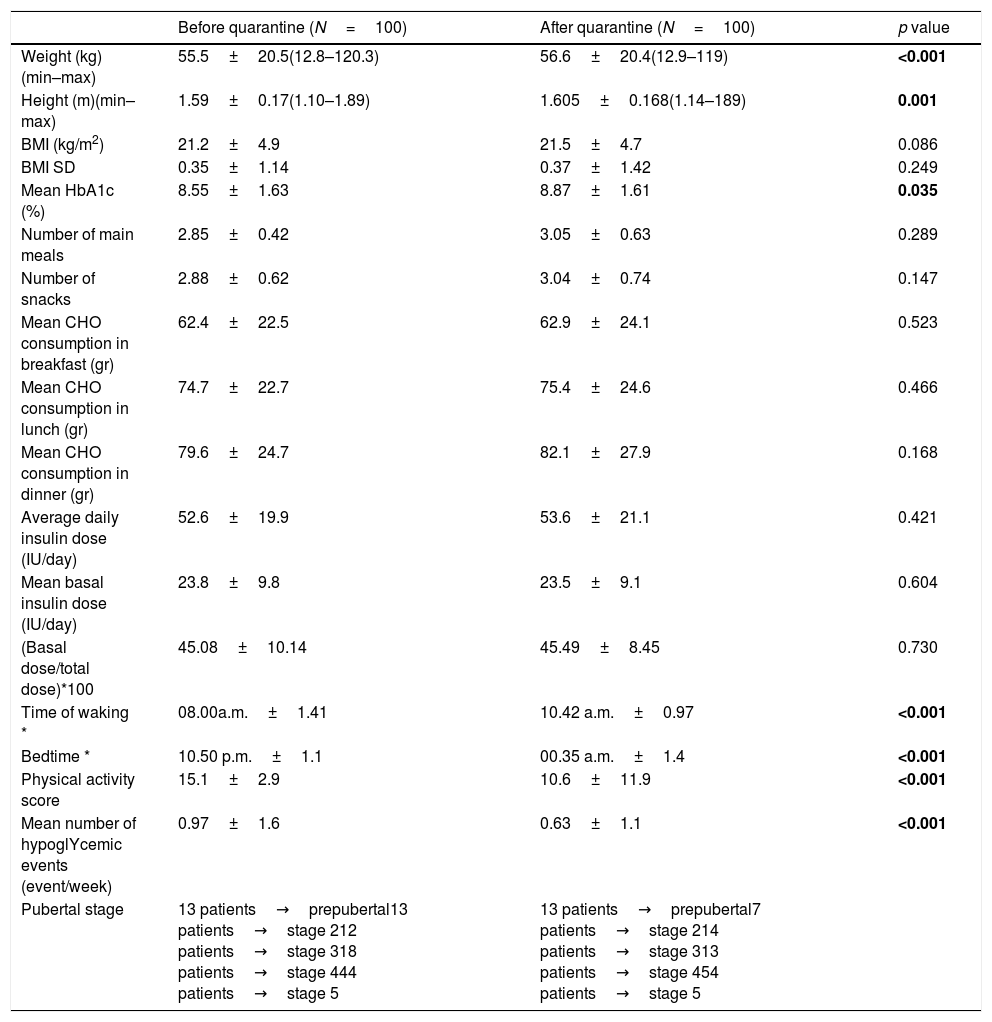
The anthropometric measurements and pubertal status of the patients and parameters of glycemic control, meal frequency, the amount of carbohydrate consumption, insulin requirements, sleep and waking times are compared between the last visit before quarantine and the first visit after quarantine in Table 1.
Comparison of the anthropometric measurements and parameters of glycemic control and insulin requirements between before and after the lockdown.
| Before quarantine (N=100) | After quarantine (N=100) | p value | |
|---|---|---|---|
| Weight (kg)(min–max) | 55.5±20.5(12.8–120.3) | 56.6±20.4(12.9–119) | <0.001 |
| Height (m)(min–max) | 1.59±0.17(1.10–1.89) | 1.605±0.168(1.14–189) | 0.001 |
| BMI (kg/m2) | 21.2±4.9 | 21.5±4.7 | 0.086 |
| BMI SD | 0.35±1.14 | 0.37±1.42 | 0.249 |
| Mean HbA1c (%) | 8.55±1.63 | 8.87±1.61 | 0.035 |
| Number of main meals | 2.85±0.42 | 3.05±0.63 | 0.289 |
| Number of snacks | 2.88±0.62 | 3.04±0.74 | 0.147 |
| Mean CHO consumption in breakfast (gr) | 62.4±22.5 | 62.9±24.1 | 0.523 |
| Mean CHO consumption in lunch (gr) | 74.7±22.7 | 75.4±24.6 | 0.466 |
| Mean CHO consumption in dinner (gr) | 79.6±24.7 | 82.1±27.9 | 0.168 |
| Average daily insulin dose (IU/day) | 52.6±19.9 | 53.6±21.1 | 0.421 |
| Mean basal insulin dose (IU/day) | 23.8±9.8 | 23.5±9.1 | 0.604 |
| (Basal dose/total dose)*100 | 45.08±10.14 | 45.49±8.45 | 0.730 |
| Time of waking * | 08.00a.m.±1.41 | 10.42 a.m.±0.97 | <0.001 |
| Bedtime * | 10.50 p.m.±1.1 | 00.35 a.m.±1.4 | <0.001 |
| Physical activity score | 15.1±2.9 | 10.6±11.9 | <0.001 |
| Mean number of hypoglYcemic events (event/week) | 0.97±1.6 | 0.63±1.1 | <0.001 |
| Pubertal stage | 13 patients→prepubertal13 patients→stage 212 patients→stage 318 patients→stage 444 patients→stage 5 | 13 patients→prepubertal7 patients→stage 214 patients→stage 313 patients→stage 454 patients→stage 5 |
Significant p-values were highlighted in bold.
Mean HbA1c level in the overall group was significantly higher after quarantine period as compared to before (p=0.035). No differences were observed in total carbohydrate consumption during the lockdown period in the overall group. However, the meal schedule was changed due to delayed sleep and waking times. Thus, during quarantine period, patients’ breakfast consumption before 10.00a.m. decreased from 78% to 20% (p<0.001). Likewise, dinner consumption after 07.00p.m. increased during the quarantine period. The number of snacks increased in 27% and decreased in 10% of the patients while the number of main meals increased in 14%, decreased in 18%, and remained unchanged in 68% of the patients.
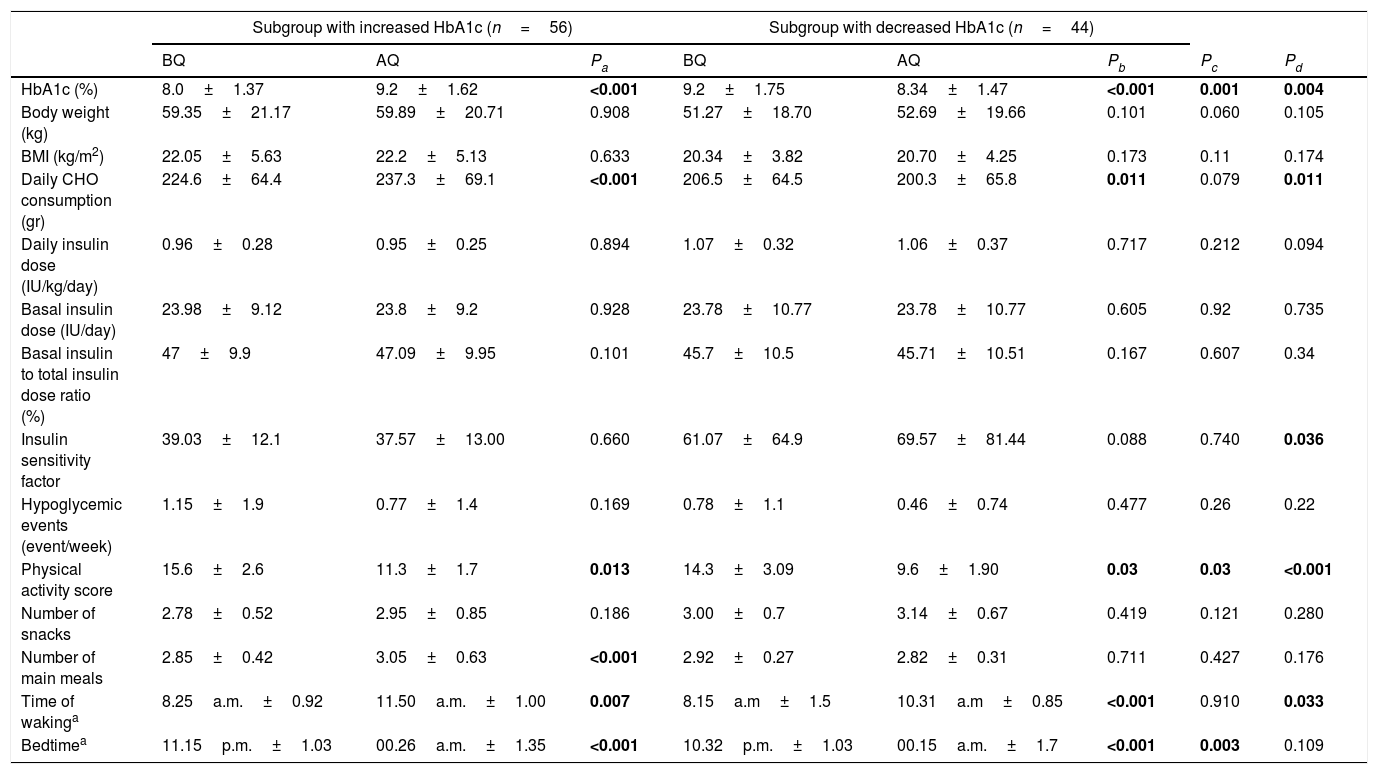
The group was further divided into two subgroups: patients with decreased (n=56, 56%) or increased HbA1c levels (n=44, 44%) after the lockdown period. The mean age of the subgroups with increased and decreased HbA1c was 14.97±4.23. and 14.41±4.65 respectively (p=0.995), and the mean durations of diabetes were 7.16±3.92 and 7.16±3.38 respectively (p=0.54 Anthropometric measurements, parameters of glycemic control, insulin requirements, sleep and waking times and carbohydrate consumption were compared between the two subgroups before and after quarantine in Table 2. In both subgroups, the change in the mean HbA1c was statistically significant (p<0.001 for both) (Table 2). Change in HbA1c (%) during the lockdown of the subgroups with increased and decreased HbA1c was 12.88±10.81 and −17.59±9.63, respectively (p=0.04). When the insulin doses per weight was calculated, no significant change was found in neither of the groups before and after quarantine (0.96±0.28IU/kg/d and 0.95±0.25IU7kg/d, p=0.894 in the subgroup with increased HbA1c; 1.07±0.32IU/kg/d and 1.06±0.37IU/kg/d, p=0.717 in the subgroup with decreased HbA1c before and after quarantine, respectively). Daily intake of carbohydrate significantly increased in the subgroup with increased HbA1c while decreased in the subgroup with decreased HbA1c (p<0.001 for both). Physical activity scores were found to be significantly decreased in both subgroups during the COVID-19 lockdown.
Comparison of the parameters of glycemic control and insulin requirements between the subgroups with increased and decreased HbA1c before and after quarantine.
| Subgroup with increased HbA1c (n=56) | Subgroup with decreased HbA1c (n=44) | |||||||
|---|---|---|---|---|---|---|---|---|
| BQ | AQ | Pa | BQ | AQ | Pb | Pc | Pd | |
| HbA1c (%) | 8.0±1.37 | 9.2±1.62 | <0.001 | 9.2±1.75 | 8.34±1.47 | <0.001 | 0.001 | 0.004 |
| Body weight (kg) | 59.35±21.17 | 59.89±20.71 | 0.908 | 51.27±18.70 | 52.69±19.66 | 0.101 | 0.060 | 0.105 |
| BMI (kg/m2) | 22.05±5.63 | 22.2±5.13 | 0.633 | 20.34±3.82 | 20.70±4.25 | 0.173 | 0.11 | 0.174 |
| Daily CHO consumption (gr) | 224.6±64.4 | 237.3±69.1 | <0.001 | 206.5±64.5 | 200.3±65.8 | 0.011 | 0.079 | 0.011 |
| Daily insulin dose (IU/kg/day) | 0.96±0.28 | 0.95±0.25 | 0.894 | 1.07±0.32 | 1.06±0.37 | 0.717 | 0.212 | 0.094 |
| Basal insulin dose (IU/day) | 23.98±9.12 | 23.8±9.2 | 0.928 | 23.78±10.77 | 23.78±10.77 | 0.605 | 0.92 | 0.735 |
| Basal insulin to total insulin dose ratio (%) | 47±9.9 | 47.09±9.95 | 0.101 | 45.7±10.5 | 45.71±10.51 | 0.167 | 0.607 | 0.34 |
| Insulin sensitivity factor | 39.03±12.1 | 37.57±13.00 | 0.660 | 61.07±64.9 | 69.57±81.44 | 0.088 | 0.740 | 0.036 |
| Hypoglycemic events (event/week) | 1.15±1.9 | 0.77±1.4 | 0.169 | 0.78±1.1 | 0.46±0.74 | 0.477 | 0.26 | 0.22 |
| Physical activity score | 15.6±2.6 | 11.3±1.7 | 0.013 | 14.3±3.09 | 9.6±1.90 | 0.03 | 0.03 | <0.001 |
| Number of snacks | 2.78±0.52 | 2.95±0.85 | 0.186 | 3.00±0.7 | 3.14±0.67 | 0.419 | 0.121 | 0.280 |
| Number of main meals | 2.85±0.42 | 3.05±0.63 | <0.001 | 2.92±0.27 | 2.82±0.31 | 0.711 | 0.427 | 0.176 |
| Time of wakinga | 8.25a.m.±0.92 | 11.50a.m.±1.00 | 0.007 | 8.15a.m±1.5 | 10.31a.m±0.85 | <0.001 | 0.910 | 0.033 |
| Bedtimea | 11.15p.m.±1.03 | 00.26a.m.±1.35 | <0.001 | 10.32p.m.±1.03 | 00.15a.m.±1.7 | <0.001 | 0.003 | 0.109 |
CHO: Carbohydrate; BMI: Body mass index; BQ: Before quarantine; AQ: After quarantine.
Pa: Comparison of the subgroup with increased HbA1c before and after quarantine.
Pb: Comparison of the subgroup with decreased HbA1c before and after quarantine.
Pc: Comparison between the subgroups with increased and decreased HbA1c before quarantine.
Pd: Comparison between the subgroups with increased and decreased HbA1c after quarantine.
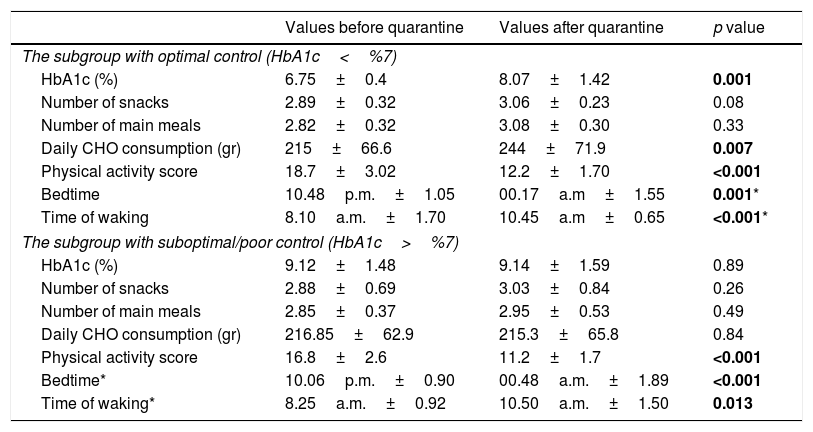
The patients were also divided into 2 subgroups according to their HbA1c levels before quarantine: the patients with optimal control (HbA1c below 7%) and suboptimal/poor control (HbA1c above 7%) according to International Society for Pediatric and Adolescent Diabetes (ISPAD) criteria.10 The mean HbA1c levels of those with optimal control were 6.74%±0.4 and 8.07%±1.14 before and after quarantine, respectively (p=0.001). The remaining 74 children had mean HbA1c levels of 9.12%±1.4 and 9.14±1.54 before and after quarantine, respectively, which was statistically insignificant (p=0.89). Of these, 39 children had a decrease in HbA1c levels, while 35 children had an increase. The comparative data of the children with good and poor glycemic control are shown in Table 3.
Comparison of the factors affecting glycemic control between the subgroups with optimal and suboptimal/poor glycemic control according to HbA1c level.
| Values before quarantine | Values after quarantine | p value | |
|---|---|---|---|
| The subgroup with optimal control (HbA1c<%7) | |||
| HbA1c (%) | 6.75±0.4 | 8.07±1.42 | 0.001 |
| Number of snacks | 2.89±0.32 | 3.06±0.23 | 0.08 |
| Number of main meals | 2.82±0.32 | 3.08±0.30 | 0.33 |
| Daily CHO consumption (gr) | 215±66.6 | 244±71.9 | 0.007 |
| Physical activity score | 18.7±3.02 | 12.2±1.70 | <0.001 |
| Bedtime | 10.48p.m.±1.05 | 00.17a.m±1.55 | 0.001* |
| Time of waking | 8.10a.m.±1.70 | 10.45a.m±0.65 | <0.001* |
| The subgroup with suboptimal/poor control (HbA1c>%7) | |||
| HbA1c (%) | 9.12±1.48 | 9.14±1.59 | 0.89 |
| Number of snacks | 2.88±0.69 | 3.03±0.84 | 0.26 |
| Number of main meals | 2.85±0.37 | 2.95±0.53 | 0.49 |
| Daily CHO consumption (gr) | 216.85±62.9 | 215.3±65.8 | 0.84 |
| Physical activity score | 16.8±2.6 | 11.2±1.7 | <0.001 |
| Bedtime* | 10.06p.m.±0.90 | 00.48a.m.±1.89 | <0.001 |
| Time of waking* | 8.25a.m.±0.92 | 10.50a.m.±1.50 | 0.013 |
CHO: Carbohydrate.
We did not have any patients using continuous glucose monitoring system (CGMS), and only 7 patients with a mean age of 14.1±5.1 years and mean duration since diagnosis of 4.8±1.79 years were using insulin pump. The mean HbA1c levels were %7.54±0.56 and %7.75±0.75 before and after quarantine, respectively, with an increase in 5 and a decrease in 2 of the 7 patients. However, these changes were not statistically significant (p=0.896).
DiscussionOur results showed that glycemic control was impaired, and HbA1c values were increased in children with T1D during the lockdown period.
HbA1c level is known to be associated with age, treatment modality and duration of diabetes.16 In a large prospective multicenter database, 3,38,330 HbA1c measurements from 27,035 patients with T1D were recorded between 1995 and 2005, and the median HbA1c level was 7.9%.17 In a cross-sectional study involving a total of 1032 patients from 12 centers in Turkey, mean HbA1c level was found to be 8.5%±1.6.18 Similarly, in a study from Jordan, a developing country, the mean HbA1c level of 259 patients was reported as 8.77%±1.48.19 The mean HbA1c levels of our patients were 8.55%±1.63 and 8.87%±1.61 before and after quarantine, respectively, and we think that our results are in line with these studies. Also, 85% of our cases were pubertal, and it is known that HbA1c levels tend to be higher during puberty.20
The mean HbA1c level of the patients with optimal metabolic control before quarantine showed a significant increase after the lockdown period. In one study evaluating the effects of COVID-19 quarantine on blood glucose control and determinants of glucose variability in adults with T1D, blood glucose control significantly worsened,21 while three studies reported that there is no difference in glycemic control during the COVID-19 lockdown period in both adults22 and children23,24 with T1D on hybrid closed loop pump. Interestingly, Bonora et al.25 have shown better metabolic control in adult patients with T1D who had stopped working. However, no difference in glycemic control was reported in those who continued to work during the lockdown period. In a study conducted in Italy, continuous glucose monitoring system (CGMS) records of 22 pre-school and school children (mean age 8.7±1.9 years) before COVID-19 and in the first 2 weeks of the stay-at-home period were evaluated, and the forced return to parental care due to the “stay at home” rule was found to be related with a better metabolic control with higher rates of time in range and lower mean values of time above range.26 The authors interpreted this result by suggesting that metabolic control in this age group benefits from parental care rather than physical activity. As in our study, it is an expected result that metabolic control worsened during the lockdown period in older children. During the pandemic period, adolescents continued to manage their diabetes by themselves, but the irregular sleep and waking times affected the frequency and timing of meals causing higher glycemic variability, and thus resulting in higher HbA1c levels.
In our study, adolescents comprised 87% of the subjects, and deterioration of the metabolic control can be attributed to irregular sleep and waking times leading to irregular frequency and timing of meals and snacks in this age group. Although mean daily carbohydrate consumption did not change in the overall group, it increased in the subgroup with increased HbA1c and decreased in the subgroup with decreased HbA1c, supporting the direct relation between carbohydrate consumption and HbA1c levels. In addition, the patients with optimal metabolic control before quarantine also had significantly higher carbohydrate consumption during the lockdown period. When the insulin doses per weight was calculated, no significant change was found in neither of the groups before and after quarantine Even though total insulin dose per weight did not increase in the subgroup with decreased HbA1c during the lockdown period, total carbohydrate consumption significantly decreased. Thus, the improvement seems to be achieved by the increase in total insulin dose per gram carbohydrate. Increased mean weight SD may also result from irregular eating habits during the lockdown period. Ahola et al.27 reported that in adults with T1D, skipping breakfast was associated with lower odds of reaching good glycemic control and higher blood glucose values. Among adolescents with T1D, irregular or infrequent food consumption was associated with poorer metabolic control.5 In a study of 105 adolescents with diabetes in Finland, low body mass index, high daily meal numbers, high daily variability in energy intake, and a long interval between insulin injection and eating were associated with good metabolic control.28 Overby et al.29 showed skipping meal or having more than two snacking events during the day was associated with higher HbA1c, higher intake of added sugar and spending more time in front of the screen.29 Recently, skipping breakfast was associated with an increased postprandial glycemic response after lunch in an experimental study with healthy young individuals.30
Mean physical activity score was significantly lower during the lockdown period in the overall group compared to before (p<0.001), and the subgroup with increased HbA1c had a significantly higher mean physical activity score both before and during the lockdown period compared to the subgroup with decreased HbA1c. Similarly, the physical activity score significantly decreased in the patients with optimal metabolic control whose mean HbA1c level showed an increase after the lockdown period. In patients with T1D, regular physical activity is associated with several positive health effects which was supported by Beraki et al.31 in a cross-sectional analysis of data from 4655 children and adolescents with T1D, concluding that physical activity seemed to influence HbA1c levels in children and adolescents with T1D. During the COVID-19 pandemic, glycemic control of 13 patients with a median age of 14.2 years was retrospectively analyzed and compared between patients who exercised regularly (n=8) and those who did not (n=5).23 Glycemic control improved in the patients who exercised at home as expected but did not worsen in those who did not exercise, which was attributed to the continual presence of parents, who could monitor their children more closely than usual, and a more regular timetable during the day. The COVID-19 pandemic restriction does not necessarily mean that all types of physical activity must be eliminated. Physical activity at home using a variety of safe, simple and easily applicable exercises (e.g., bodyweight exercise, skipping rope, and online classes) can help maintain fitness levels while maintaining social distance.32
There are some limitations in our study. Firstly, we did not have any patients using CGMS and therefore could not make any comparisons based on time in range values. Another limitation is that since the number of patients using insulin pump in the study was inadequate, patients using insulin pump or pen could not be compared. Also, our study only reflects a 3-month interval analysis, and we think that a clinically significant result may have been obtained if the study had been continued for a longer period of time. ISPAD and ADA recommend an HbA1c level of <%7, as the optimal goal, %7–9 as suboptimal control and >%9 as poor control.10,33 As the majority of our patients had HbA1c levels between %7 and %9, even though the change during the lockdown period was found to be statistically significant, it does not seem to reach a clinical significance.
ConclusionOur study supports that glycemic control of the children with T1D has worsened in COVID-19 pandemic period. Even though it is not possible to explain this with a certain factor, some behavior changes observed in our study, such as inactivity, irregular meal frequency and timing, irregular sleep and waking patterns seem to associate with the glycemic control.
AuthorshipAll named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Ethical statementWritten consent of the cases and volunteers was obtained according to the Helsinki. Declaration and the study were approved by the Istanbul University-Cerrahpasa ethics committee (number: 83045809-604.01.02).
Data availabilityThe datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
FundingNo funding or sponsorship was received for this study or publication of this article.
Conflict of interestHande Turan, Didem Güneş Kaya, Gürkan Tarçın, Olcay Evliyaoğlu declare that they have no conflict of interest