This consensus aims to clarify the role of Dipeptidyl Peptidase-4 inhibitors (iDPP-4) in managing patients with diabetes during the COVID-19 pandemic.
Materials and methodsA PubMed bibliographic search was carried out (December 2019–February 2021). Oxford methodology was used for the evaluation of evidence and possible recommendations were established by consensus.
ResultsDiabetes appears to be an independent factor in COVID-19 disease (evidence 2b). No increased risk of contagion with iDPP-4 is demonstrated (evidence 2b), and its use has been shown to be safe (evidence 2b). The use of this drug may present a specific benefit in reducing mortality, particularly in in-hospital use (evidence 2a), reducing admission to intensive care units (evidence 2b) and the need for mechanical ventilation (evidence 2b).
ConclusionsThe use of iDPP-4 appears to be safe in patients with COVID-19, and quality studies are needed to clarify their possible advantages further.
El objetivo de este consenso es esclarecer el papel de los iDPP-4 en el manejo de los pacientes con diabetes durante la pandemia por COVID-19.
Material y métodosSe llevó a cabo una búsqueda bibliográfica en PubMed (diciembre 2019–febrero de 2021). Se empleó la metodología Oxford y se establecieron de forma consensuada posibles recomendaciones.
ResultadosLa diabetes parece ser un factor independiente en la enfermedad de COVID-19 (evidencia 2b). No se demuestra mayor riesgo de contagio con iDPP-4 (evidencia 2b) y su uso ha demostrado ser seguro (evidencia 2b). Los iDPP-4 pueden presentar un cierto beneficio en la reducción de la mortalidad, particularmente su uso intrahospitalario (evidencia 2a), reduciendo la admisión a unidades de cuidados intensivos (evidencia 2b) y la necesidad de ventilación mecánica (evidencia 2b).
ConclusionesLos iDPP-4 parecen ser fármacos seguros en pacientes con COVID-19 y se necesitan estudios de calidad que aclaren sus posibles ventajas.
The management of patients with diabetes during the COVID-19 pandemic is an aspect that has taken on particular relevance, as diabetic patients with SARS-CoV-2 infection have been found to have a higher risk of a worse prognosis and mortality.1 The reason for poorer outcomes in patients with diabetes is not yet entirely clear, but it may be due to their reduced immune response to SARS-CoV-2 as a result of chronic hyperglycaemia2 and/or to the chronic low-grade inflammation associated with diabetes.3
Some antidiabetic agents (ADA), such as dipeptidyl peptidase-4 (DPP-4) inhibitors, have provoked considerable interest as it has been suggested that they may play a role in reducing the chance of infection with the virus and mitigating the severity of complications associated with COVID-19.4
The objective of this review and consensus document is to summarise all of the available data on the potential impact of DPP-4 inhibitors on the clinical outcomes of patients with diabetes during COVID-19, both in diabetic patients treated outside of the hospital setting and in those who required intrahospital management, and to establish recommendations based on the evidence available to date. The full document can be accessed via the following link: Appendix B see additional material.
MethodologySeveral scientific associations and one foundation, all connected with clinical diabetes management (Sociedad Española de Medicina Interna [Spanish Society of Internal Medicine], Sociedad Española de Diabetes [Spanish Diabetes Society], Sociedad Española de Endocrinología y Nutrición [Spanish Society of Endocrinology and Nutrition] and Fundación redGDPS [Diabetes in Primary Care Study Groups Network Foundation]) were brought together to prepare this review and consensus document.
After reviewing and selecting the reference materials, conducting an appropriate analysis of the available evidence and agreeing the clinical questions to address, potential recommendations were established by consensus.
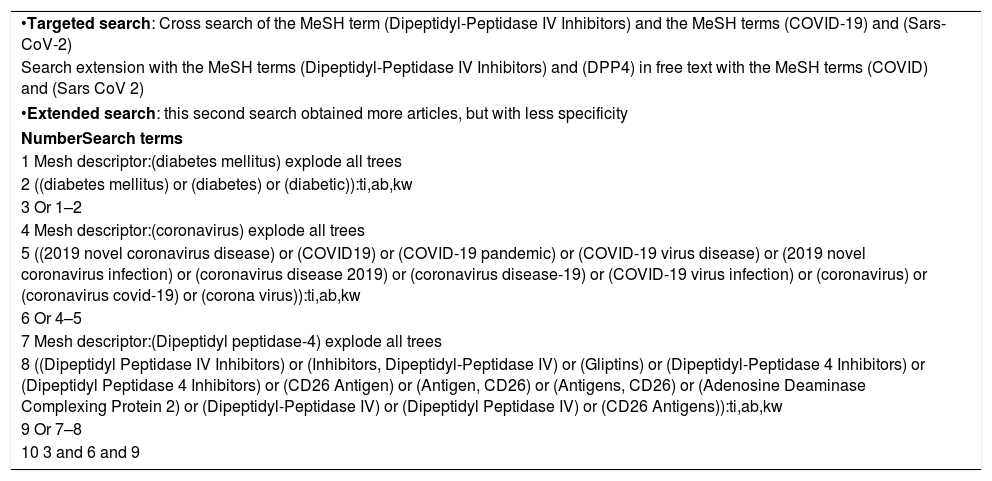
Literature searchA literature search was performed in the PubMed database from December 2019 to February 2021 based on the search strategy outlined in Table 1.
Search strategy approach (MeSH descriptors).
| •Targeted search: Cross search of the MeSH term (Dipeptidyl-Peptidase IV Inhibitors) and the MeSH terms (COVID-19) and (Sars-CoV-2) |
| Search extension with the MeSH terms (Dipeptidyl-Peptidase IV Inhibitors) and (DPP4) in free text with the MeSH terms (COVID) and (Sars CoV 2) |
| •Extended search: this second search obtained more articles, but with less specificity |
| NumberSearch terms |
| 1 Mesh descriptor:(diabetes mellitus) explode all trees |
| 2 ((diabetes mellitus) or (diabetes) or (diabetic)):ti,ab,kw |
| 3 Or 1–2 |
| 4 Mesh descriptor:(coronavirus) explode all trees |
| 5 ((2019 novel coronavirus disease) or (COVID19) or (COVID-19 pandemic) or (COVID-19 virus disease) or (2019 novel coronavirus infection) or (coronavirus disease 2019) or (coronavirus disease-19) or (COVID-19 virus infection) or (coronavirus) or (coronavirus covid-19) or (corona virus)):ti,ab,kw |
| 6 Or 4–5 |
| 7 Mesh descriptor:(Dipeptidyl peptidase-4) explode all trees |
| 8 ((Dipeptidyl Peptidase IV Inhibitors) or (Inhibitors, Dipeptidyl-Peptidase IV) or (Gliptins) or (Dipeptidyl-Peptidase 4 Inhibitors) or (Dipeptidyl Peptidase 4 Inhibitors) or (CD26 Antigen) or (Antigen, CD26) or (Antigens, CD26) or (Adenosine Deaminase Complexing Protein 2) or (Dipeptidyl-Peptidase IV) or (Dipeptidyl Peptidase IV) or (CD26 Antigens)):ti,ab,kw |
| 9 Or 7–8 |
| 10 3 and 6 and 9 |
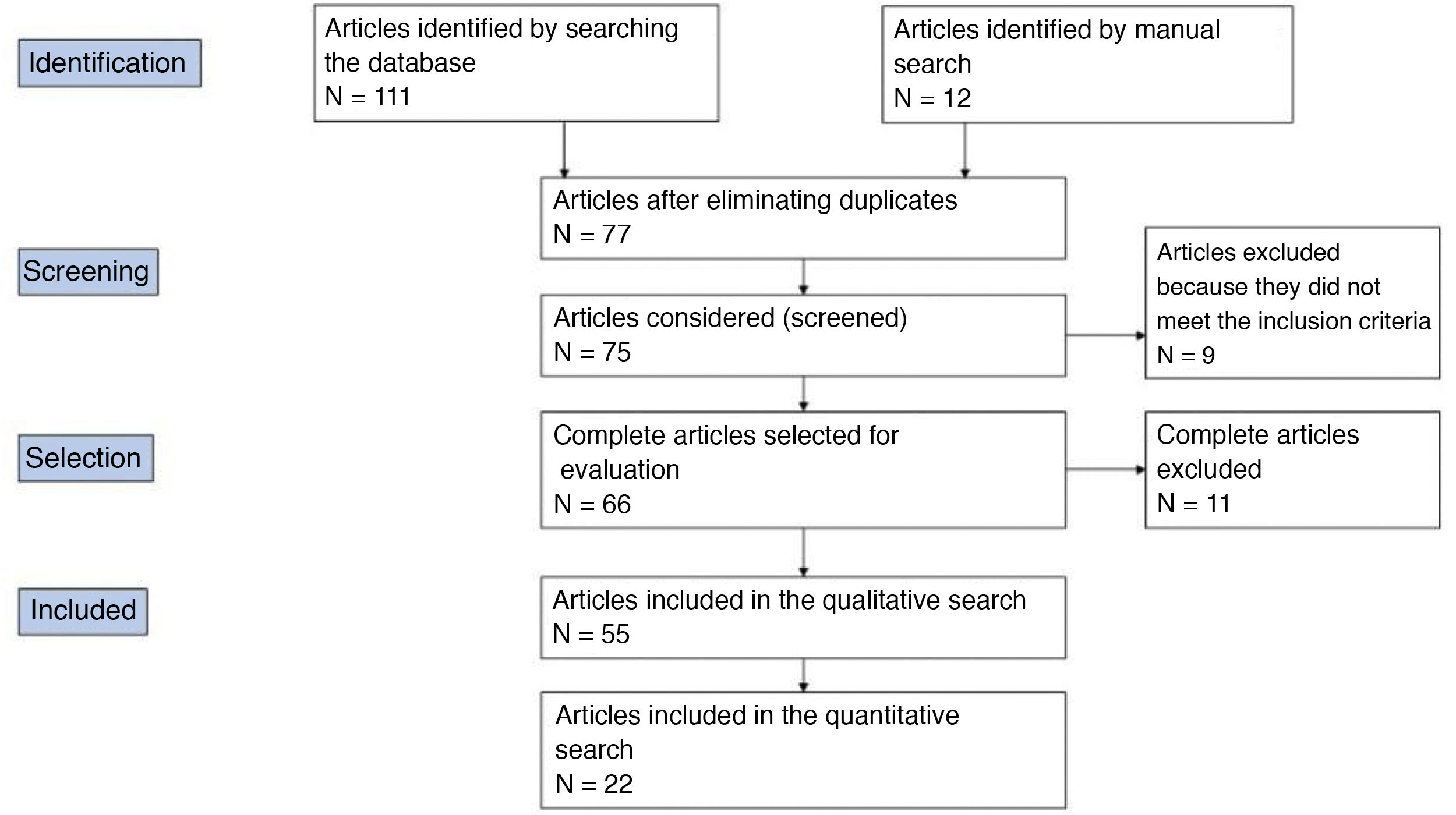
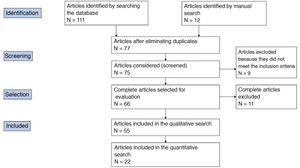
The results were supplemented with a second manual search. The reviewers carried out the literature selection, data analysis, assessment of the most relevant terms and the quality analysis independently. Fig. 1 shows the PRISMA flow diagram of how the article selection process was carried out. The clinical questions of interest were established based on this review.
Oxford methodologyThe quality of the articles selected was assessed using the Oxford Centre for Evidence-Based Medicine (CEBM) Levels of Evidence (Oxford CEBM, 2009).
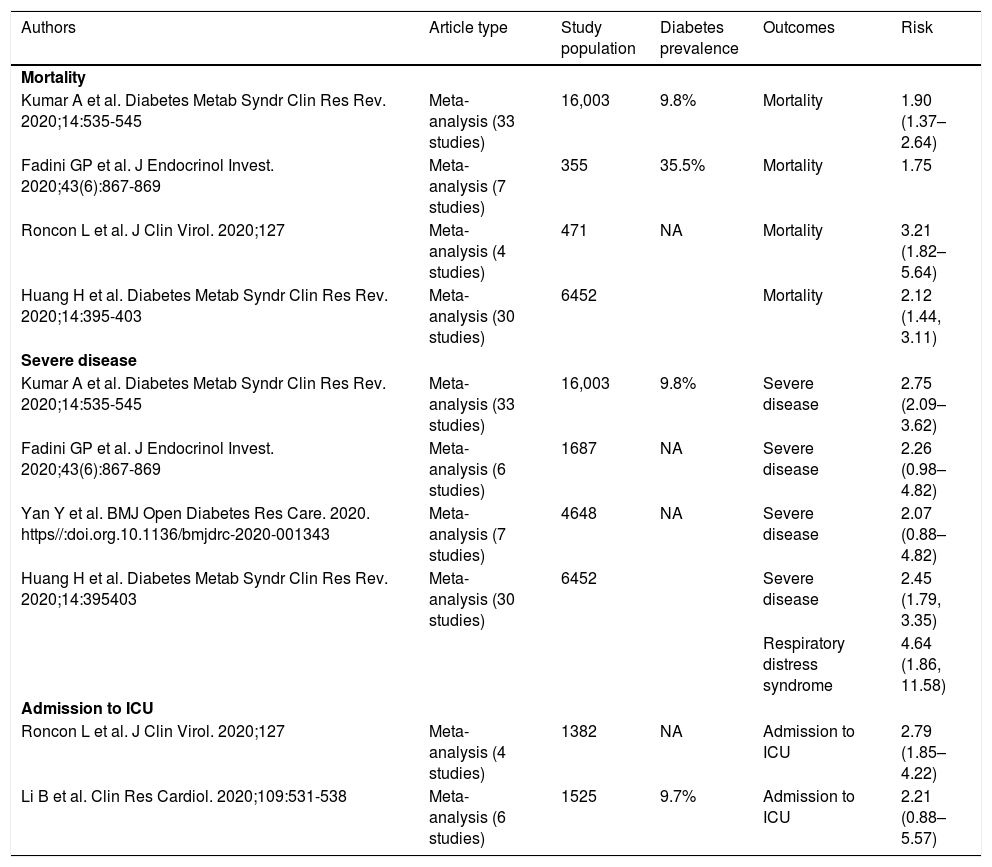
ResultsCan diabetes be considered an independent risk factor in the clinical course of patients with SARS-CoV-2?The data published to date suggest that diabetes does not appear to increase the risk of COVID-19 infection, but the presence of diabetes in patients with COVID-19 is a risk factor with regard to mortality, disease severity and need for admission to an intensive care unit (ICU)1(level of evidence 2a) (Table 2).
COVID-19 disease outcomes by presence of diabetes.
| Authors | Article type | Study population | Diabetes prevalence | Outcomes | Risk |
|---|---|---|---|---|---|
| Mortality | |||||
| Kumar A et al. Diabetes Metab Syndr Clin Res Rev. 2020;14:535-545 | Meta-analysis (33 studies) | 16,003 | 9.8% | Mortality | 1.90 (1.37–2.64) |
| Fadini GP et al. J Endocrinol Invest. 2020;43(6):867-869 | Meta-analysis (7 studies) | 355 | 35.5% | Mortality | 1.75 |
| Roncon L et al. J Clin Virol. 2020;127 | Meta-analysis (4 studies) | 471 | NA | Mortality | 3.21 (1.82–5.64) |
| Huang H et al. Diabetes Metab Syndr Clin Res Rev. 2020;14:395-403 | Meta-analysis (30 studies) | 6452 | Mortality | 2.12 (1.44, 3.11) | |
| Severe disease | |||||
| Kumar A et al. Diabetes Metab Syndr Clin Res Rev. 2020;14:535-545 | Meta-analysis (33 studies) | 16,003 | 9.8% | Severe disease | 2.75 (2.09–3.62) |
| Fadini GP et al. J Endocrinol Invest. 2020;43(6):867-869 | Meta-analysis (6 studies) | 1687 | NA | Severe disease | 2.26 (0.98–4.82) |
| Yan Y et al. BMJ Open Diabetes Res Care. 2020. https//:doi.org.10.1136/bmjdrc-2020-001343 | Meta-analysis (7 studies) | 4648 | NA | Severe disease | 2.07 (0.88–4.82) |
| Huang H et al. Diabetes Metab Syndr Clin Res Rev. 2020;14:395403 | Meta-analysis (30 studies) | 6452 | Severe disease | 2.45 (1.79, 3.35) | |
| Respiratory distress syndrome | 4.64 (1.86, 11.58) | ||||
| Admission to ICU | |||||
| Roncon L et al. J Clin Virol. 2020;127 | Meta-analysis (4 studies) | 1382 | NA | Admission to ICU | 2.79 (1.85–4.22) |
| Li B et al. Clin Res Cardiol. 2020;109:531-538 | Meta-analysis (6 studies) | 1525 | 9.7% | Admission to ICU | 2.21 (0.88–5.57) |
ICU, Intensive care unit; NA, not available.
Adapted from Apicella et al.1
It is not fully clear whether this increase in risk is because of the diabetes per se or because patients with diabetes often have a greater prevalence of other risk factors conducive to a worse prognosis for COVID-19. It has been demonstrated that circulating levels of some cytokines such as interleukin-6 (IL-6) are higher in patients with COVID-19 and diabetes, presenting a worse prognosis than in patients without diabetes. This suggests that in the presence of an underlying proinflammatory environment, diabetes would be an independent risk factor for worse outcomes (level of evidence 5).5 Expert consensus: Based on this, in diabetes, irrespective of adequate control of comorbidities such as obesity or cardiovascular disease, we would recommend raising awareness of the importance of optimal glycaemic control, caution in premature discontinuation of established therapy and optimisation of antidiabetic therapy, weighing up the possible effects of the drugs on inflammation. In patients with diabetes and COVID-19, factors to consider may include selecting drugs that guarantee little fluctuation in glycaemic control and have been found to be safe in different scenarios (pre-hospitalisation, hospitalisation, ICU).
Using a modelling-based focus, a recent publication demonstrated that the S1 domain of the SARS-CoV-2 spike glycoprotein might interact with the human DPP-4 membrane, enabling the entry of the virus.6 However, a registry conducted in Italy explored the potential association between DPP-4 inhibitors for diabetes and the risk of COVID-19 infection and concluded that DPP-4 inhibitors do not appear to have any protective or harmful effect on COVID-19 incidence7(level of evidence 2b), backing up recent data on their neutral role on the incidence of respiratory infections.8 Expert consensus: Although there is a possible interaction between SARS-CoV-2 and DPP-4, the use of DPP-4 inhibitors does not appear to either increase or reduce the risk of SARS-CoV-2 infection.
A case-control study found that the rate of exposure to DPP-4 inhibitors among patients with type 2 diabetes mellitus (T2DM) with SARS-CoV-2 infection hospitalised for COVID-19 was comparable to that of matched patients in the same region or those from the local outpatient clinic.9 These data suggest that DPP-4 inhibitors do not increase the risk of hospitalisation in patients with COVID-19, and they are considered a valid therapeutic option for the management of patients with diabetes and symptomatic COVID-19 (level of evidence 4). Expert consensus: According to the data that currently exist, DPP-4 inhibitors do not increase the risk of hospitalisation in patients with COVID-19. We therefore suggest maintaining treatment with DPP-4 inhibitors in high- or very high-risk epidemiological situations and in mild or moderate COVID-19 disease that does not require hospitalisation.
DPP-4 inhibitors have anti-adipogenic, anti-platelet and anti-inflammatory effects. Studies have found that starting DPP-4 inhibitors leads to a reduction in cytokine production, and a recent meta-analysis also identified a significant reduction in polymerase chain reaction (PCR) levels after treatment with DPP-4 inhibitors compared to placebo.10 Expert consensus: The anti-inflammatory, anti-platelet and anti-adipogenic effects of DPP-4 inhibitors, as well as the possible modulating role of DPP-4 receptors on virus entry, are potential physiological mechanisms that could contribute to a more favourable clinical course of COVID-19 disease.
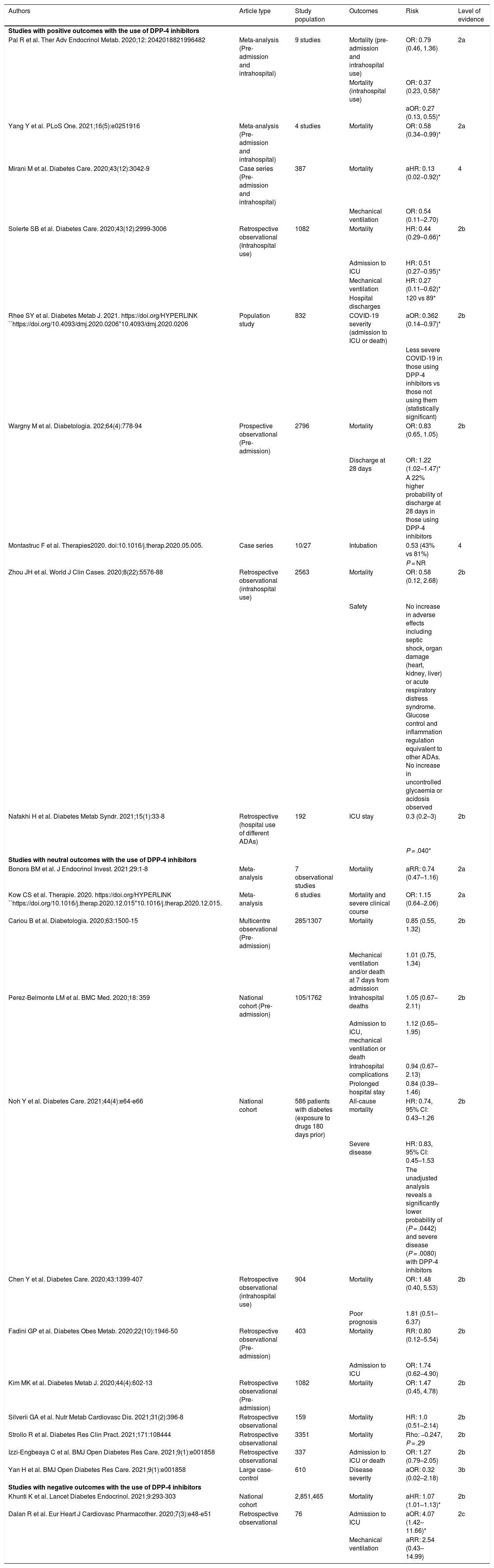
The results of the assessment of the studies suggest a beneficial or neutral effect from this group of ADAs (level of evidence 2a). Four meta-analyses also suggest a beneficial or neutral effect of DPP-4 inhibitors on mortality. A cohort study suggests a small (1.07 [1.01–1.13]) but significant increase in mortality, which can be explained by the large sample size and a probable selection bias in being prescribed preferentially to older, more frail patients (Table 3).
Outcomes of studies with DPP-4 inhibitors in patients with COVID-19.
| Authors | Article type | Study population | Outcomes | Risk | Level of evidence |
|---|---|---|---|---|---|
| Studies with positive outcomes with the use of DPP-4 inhibitors | |||||
| Pal R et al. Ther Adv Endocrinol Metab. 2020;12: 2042018821996482 | Meta-analysis (Pre-admission and intrahospital) | 9 studies | Mortality (pre-admission and intrahospital use) | OR: 0.79 (0.46, 1.36) | 2a |
| Mortality (intrahospital use) | OR: 0.37 (0.23, 0.58)* | ||||
| aOR: 0.27 (0.13, 0.55)* | |||||
| Yang Y et al. PLoS One. 2021;16(5):e0251916 | Meta-analysis (Pre-admission and intrahospital) | 4 studies | Mortality | OR: 0.58 (0.34–0.99)* | 2a |
| Mirani M et al. Diabetes Care. 2020;43(12):3042-9 | Case series (Pre-admission and intrahospital) | 387 | Mortality | aHR: 0.13 (0.02−0.92)* | 4 |
| Mechanical ventilation | OR: 0.54 (0.11–2.70) | ||||
| Solerte SB et al. Diabetes Care. 2020;43(12):2999-3006 | Retrospective observational (Intrahospital use) | 1082 | Mortality | HR: 0.44 (0.29–0.66)* | 2b |
| Admission to ICU | HR: 0.51 (0.27–0.95)* | ||||
| Mechanical ventilation | HR: 0.27 (0.11–0.62)* | ||||
| Hospital discharges | 120 vs 89* | ||||
| Rhee SY et al. Diabetes Metab J. 2021. https://doi.org/HYPERLINK ``https://doi.org/10.4093/dmj.2020.0206''10.4093/dmj.2020.0206 | Population study | 832 | COVID-19 severity (admission to ICU or death) | aOR: 0.362 (0.14–0.97)* | 2b |
| Less severe COVID-19 in those using DPP-4 inhibitors vs those not using them (statistically significant) | |||||
| Wargny M et al. Diabetologia. 202;64(4):778-94 | Prospective observational (Pre-admission) | 2796 | Mortality | OR: 0.83 (0.65, 1.05) | 2b |
| Discharge at 28 days | OR: 1.22 (1.02–1.47)* | ||||
| A 22% higher probability of discharge at 28 days in those using DPP-4 inhibitors | |||||
| Montastruc F et al. Therapies2020. doi:10.1016/j.therap.2020.05.005. | Case series | 10/27 | Intubation | 0.53 (43% vs 81%) | 4 |
| P = NR | |||||
| Zhou JH et al. World J Clin Cases. 2020;8(22):5576-88 | Retrospective observational (intrahospital use) | 2563 | Mortality | OR: 0.58 (0.12, 2.68) | 2b |
| Safety | No increase in adverse effects including septic shock, organ damage (heart, kidney, liver) or acute respiratory distress syndrome. Glucose control and inflammation regulation equivalent to other ADAs. No increase in uncontrolled glycaemia or acidosis observed | ||||
| Nafakhi H et al. Diabetes Metab Syndr. 2021;15(1):33-8 | Retrospective (hospital use of different ADAs) | 192 | ICU stay | 0.3 (0.2–3) | 2b |
| P = .040* | |||||
| Studies with neutral outcomes with the use of DPP-4 inhibitors | |||||
| Bonora BM et al. J Endocrinol Invest. 2021;29:1-8 | Meta-analysis | 7 observational studies | Mortality | aRR: 0.74 (0.47–1.16) | 2a |
| Kow CS et al. Therapie. 2020. https://doi.org/HYPERLINK ``https://doi.org/10.1016/j.therap.2020.12.015''10.1016/j.therap.2020.12.015. | Meta-analysis | 6 studies | Mortality and severe clinical course | OR: 1.15 (0.64−2.06) | 2a |
| Cariou B et al. Diabetologia. 2020;63:1500-15 | Multicentre observational (Pre-admission) | 285/1307 | Mortality | 0.85 (0.55, 1.32) | 2b |
| Mechanical ventilation and/or death at 7 days from admission | 1.01 (0.75, 1.34) | ||||
| Perez-Belmonte LM et al. BMC Med. 2020;18: 359 | National cohort (Pre-admission) | 105/1762 | Intrahospital deaths | 1.05 (0.67–2.11) | 2b |
| Admission to ICU, mechanical ventilation or death | 1.12 (0.65–1.95) | ||||
| Intrahospital complications | 0.94 (0.67–2.13) | ||||
| Prolonged hospital stay | 0.84 (0.39–1.46) | ||||
| Noh Y et al. Diabetes Care. 2021;44(4):e64-e66 | National cohort | 586 patients with diabetes (exposure to drugs 180 days prior) | All-cause mortality | HR: 0.74, 95% CI: 0.43–1.26 | 2b |
| Severe disease | HR: 0.83, 95% CI: 0.45–1.53 | ||||
| The unadjusted analysis reveals a significantly lower probability of (P = .0442) and severe disease (P = .0080) with DPP-4 inhibitors | |||||
| Chen Y et al. Diabetes Care. 2020;43:1399-407 | Retrospective observational (intrahospital use) | 904 | Mortality | OR: 1.48 (0.40, 5.53) | 2b |
| Poor prognosis | 1.81 (0.51–6.37) | ||||
| Fadini GP et al. Diabetes Obes Metab. 2020;22(10):1946-50 | Retrospective observational (Pre-admission) | 403 | Mortality | RR: 0.80 (0.12–5.54) | 2b |
| Admission to ICU | OR: 1.74 (0.62–4.90) | ||||
| Kim MK et al. Diabetes Metab J. 2020;44(4):602-13 | Retrospective observational (Pre-admission) | 1082 | Mortality | OR: 1.47 (0.45, 4.78) | 2b |
| Silverii GA et al. Nutr Metab Cardiovasc Dis. 2021;31(2):396-8 | Retrospective observational | 159 | Mortality | HR: 1.0 (0.51–2.14) | 2b |
| Strollo R et al. Diabetes Res Clin Pract. 2021;171:108444 | Retrospective observational | 3351 | Mortality | Rho: −0.247, P = .29 | 2b |
| Izzi-Engbeaya C et al. BMJ Open Diabetes Res Care. 2021;9(1):e001858 | Retrospective observational | 337 | Admission to ICU or death | OR: 1.27 (0.79–2.05) | 2b |
| Yan H et al. BMJ Open Diabetes Res Care. 2021;9(1):e001858 | Large case-control | 610 | Disease severity | aOR: 0.32 (0.02–2.18) | 3b |
| Studies with negative outcomes with the use of DPP-4 inhibitors | |||||
| Khunti K et al. Lancet Diabetes Endocrinol. 2021;9:293-303 | National cohort | 2,851,465 | Mortality | aHR: 1.07 (1.01–1.13)* | 2b |
| Dalan R et al. Eur Heart J Cardiovasc Pharmacother. 2020;7(3):e48-e51 | Retrospective observational | 76 | Admission to ICU | aOR: 4.07 (1.42–11.66)* | 2c |
| Mechanical ventilation | aRR: 2.54 (0.43–14.99) | ||||
The possible effect of these drugs might be more apparent in hospitalised patients, as is reflected in the meta-analysis by Pal et al.,11 which identified nine high-quality observational studies on 7008 patients with diabetes suffering from COVID-19. In the subgroup analysis, the authors observed that intrahospital use of DPP-4 inhibitors was associated with a reduction in mortality (adjusted OR: 0.27; 95% CI: 0.13–0.55; P = .0003).
Given the potential positive impact of DPP-4 inhibitor use, particularly in hospitalised patients, various randomised studies have been launched to clarify this aspect (NCT04365517, NCT04341935 and NCT04371978). Expert consensus: Out-of-hospital use of DPP-4 inhibitors has been found not to increase the risk of mortality in patients with diabetes and COVID-19. Intrahospital use of DPP-4 inhibitors may be associated with a significant reduction in mortality in patients with diabetes and COVID-19. It would therefore be prudent to initiate or continue DPP-4 inhibitors in patients with COVID-19 if not contraindicated.
The meta-analysis by Kow et al. found that there is no increase in the risk of greater severity (level of evidence 2a).12 Other evidence that DPP-4 inhibitors do not increase patients' severity is found in the study Coronavirus Disease and Diabetes Outcome (CORONADO). In this study, no association was observed between severe clinical course of COVID-19 and treatment with DPP-4 inhibitors prior to admission (OR: 1.01; 95% CI: 0.75–1.34).
However, use of these drugs at hospitalisation appears to significantly reduce the need for mechanical ventilation (HR: 0.27 [0.11–0.62]) and admission to the ICU (HR: 0.51 [0.27–0.95]), and increase the number of patients discharged at 30 days according to a multicentre retrospective study conducted in 338 patients with T2DM and COVID-19 (level of evidence 2b).13 Expert consensus: Treatment with DPP-4 inhibitors in stable hospitalised patients appears to be a safe treatment option that does not affect the severity of the clinical course of the disease.
Some reviews and recent guidelines13 indicate that DPP-4 inhibitors are very safe, and may even be potentially beneficial for most patients hospitalised with diabetes and COVID-19.
DPP-4 inhibitors have been found to be safe drugs, and also do not require adjustment on days when patients with diabetes and COVID-19 feel ill, in comparison with other ADAs (Table 4).14 Although only a randomised trial can definitively answer this question, based on molecular, pathophysiological and retrospective studies, the use of DPP-4 inhibitors is safe and certainly beneficial for metabolic control. Expert consensus: These data support the safety of DPP-4 inhibitors for diabetes control during the COVID-19 pandemic. DPP-4 inhibitors are a group of drugs that offer many advantages, including in severe cases of COVID-19, because they are well-tolerated, entail a low risk of hypoglycaemia and can be used, with dose adjustment if appropriate, in patients with decreased kidney function. In this sense, the use of DPP-4 inhibitors can be considered, including in patients hospitalised with diabetes and COVID-19.
Recommendations with different antidiabetic drugs in patients with suspected COVID-19.
| Panel: Consideration of possible metabolic interference effects of antidiabetic drugs in patients with type 2 diabetes and suspected or confirmed COVID-19 |
| Metformin |
| • In an acute process, like others it increases the risk of dehydration and lactic acidosis, so it is recommended to suspend the drug and follow recommendations for days of illness |
| Sodium-glucose cotransporter 2 inhibitors |
| • Include canagliflozin, dapagliflozin, empagliflozin and ertugliflozin |
| • In the acute phase there is a risk of dehydration and diabetic ketoacidosis during illness, so patients must suspend the drugs and follow recommendations for days of illness, with the exception of dapagliflozin, which, according to the DARE-19 data, appears to be a safe drug in monitored hospitalised patients |
| • Patients must not start treatment during respiratory illness |
| Glucagon-like peptide-1 receptor agonists |
| • Include dulaglutide, exenatide, liraglutide, lixisenatide and semaglutide |
| • Digestive complaints can cause dehydration and lead to severe disease, so patients must be closely monitored |
| • Adequate fluid intake and regular meals should be encouraged |
| Dipeptidyl peptidase-4 inhibitors |
| • Include alogliptin, linagliptin, saxagliptin, sitagliptin and vildagliptin |
| • These drugs are generally well-tolerated and can be continued |
| Insulin |
| • Insulin treatment should not be interrupted |
| • Regular self-monitoring of blood glucose every 2–4 h or continuous glucose monitoring should be encouraged |
| • Carefully adjust regular therapy if appropriate to achieve therapeutic objectives based on type of diabetes, comorbidities and state of health |
| • Online and telemedicine healthcare models should be used to continue regular check-ups and educational programmes on self-management virtually and ensure that patients adhere to therapy |
Source: adapted from Bornstein et al.14
In patients with COVID-19, the hypothesis has been suggested that due to their anti-inflammatory effect, they might help to prevent complications associated with COVID-19.15 Studies such as the SIDIACO Sitagliptin study (NCT04365517) are currently ongoing that will enable the possible advantages of these drugs in patients with these underlying diseases to be elucidated. Expert consensus: Patients with diabetes and COVID-19 might hypothetically benefit from using DPP-4 inhibitors if they have additional cardiovascular disease or chronic kidney disease.
DPP-4 inhibitors have been found to be safe drugs and do not require adjustment on days of illness in patients with diabetes and COVID-19, in comparison with other oral ADAs16; their use in comparison with other oral ADAs has been associated with shorter ICU stay (level of evidence 2b) and possible benefits in hospitalised patients. Expert consensus: Based on the studies published to date, DPP-4 inhibitors could be considered to have a suitable profile for the treatment of diabetes in hospitalised patients.
DPP-4 inhibitors are a group of drugs associated with many advantages in the context of the COVID-19 pandemic, including in severe cases, because they are well-tolerated, have a low risk of hypoglycaemia, do not require a dose adjustment on days of illness and can be used in patients with decreased kidney function.
After evaluating all the available literature, there is no evidence of sufficient quality to conclusively recommend treatment with DPP-4 inhibitors in patients with diabetes and COVID-19. However, there is also insufficient evidence to advise against their use.
The literature reviewed suggests that there might potentially be substantial benefits to treatment with DPP-4 inhibitors, and we are hopeful that this question will shortly be answered by the high-quality prospective, randomised clinical trials that are currently ongoing.
AuthorshipAll the authors contributed equally to the preparation of the article.
FundingMedical writing assistance was funded by the Menarini Group. However, none of its members attended the meetings held to review the literature and agree on decisions, nor did they influence the decisions made by the experts.
Conflicts of interestFrancisco Javier Carrasco-Sánchez: consultant and/or adviser for Boehringer-Lilly, Novo Nordisk, Sanofi, AstraZeneca, MSD and Mundipharma. Remuneration received from AstraZeneca, Boehringer-Lilly, Novartis, Novo-Nordisk and Sanofi.
Enrique Carretero-Anibarro: research, speaker and/or consultant for Almirall, AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Esteve, Janssen, Menarini, MSD, Mundipharma, Novartis, Novo Nordisk and Sanofi Diabetes.
Manuel Ángel Gargallo: speaker and/or consultant for Sanofi Diabetes, Mundipharma, Novo Nordisk, Lilly and AstraZeneca.
Ricardo Gómez-Huelgas: consultant and/or adviser for Boehringer-Lilly, Novo Nordisk, Sanofi, AstraZeneca, MSD and Janssen. Remuneration received from AstraZeneca, Boehringer-Lilly, Novartis, Novo-Nordisk and Sanofi. Research studies for Boehringer-Lilly, Novo-Nordisk, Sanofi and Janssen.
Juan Francisco Merino-Torres: researcher, speaker and/or consultant for Abbott, Amgen, AstraZeneca, Ascensia Diabetes Care, Bristol-Myers Squibb, Esteve, GlaxoSmithKline, Janssen, Kabi-Fresenius, Lilly, Menarini, Merck-Sharp-Dohme, Novartis, Novo-Nordisk, Nutricia, Pfizer, Rovi and Sanofi-Aventis.
Domingo Orozco-Beltrán: participation in training activities funded unconditionally by MSD, Lilly, Novo Nordisk, Boehringer and Fundación Bamberg, as well as others sponsored by scientific associations.
Pedro José Pines Corrales: speaker and/or consultant for AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Menarini, MSD, Novo Nordisk and Sanofi Diabetes.
Manuel Antonio Ruiz Quintero: speaker for Sanofi/Aventis, GSK, Novartis, Novo Nordisk, Lilly, Boehringer Ingelheim, Almirall, Janssen, Mundifarma, AstraZeneca and Servier, and consultant/speaker for MSD and Esteve.
The authors would like to thank Ana Isabel Ortega on behalf of Springer Healthcare for the literature search and its classification, as well as assistance with the preparation of the manuscript. They would also like to thank M. Ángeles Caldeiro, of Springer Healthcare, for coordinating all phases of the project. This assistance was funded by the Menarini Group.
Please cite this article as: Carrasco-Sánchez FJ, Carretero-Anibarro E, Gargallo MÁ, Gómez-Huelgas R, Merino-Torres JF, Orozco-Beltrán D, et al. Resumen Ejecutivo del Consenso de expertos sobre la eficacia y seguridad de los iDPP-4 en el tratamiento de pacientes con diabetes y COVID-19. Endocrinol Diabetes Nutr. 2022;69:209–218.