Sarcopenia is a syndrome characterized by the loss of muscle mass and strength. The study objective was to determine the association between muscle density and overall survival (OS) in patients with metastatic onset prostate cancer (MPCa).
Patients and methodsThis was a retrospective study of patients diagnosed with MPCa between 2009 and 2015 who received androgen deprivation therapy alone as initial treatment. Muscle density was calculated using the Hounsfield Unit Average Calculation (HUAC) in both psoas muscles in the computed tomography (CT) scan performed for diagnosis.
ResultsA total of 59 patients diagnosed with MPCa, with a mean age of 57.5±72.47 years, were found. Median PSA level at diagnosis was 68.25ng/dl (IQR 37.26-290). Gleason scores ≥8 were recorded in 90.75% of the patients, bone metastases in 88.13%, and visceral metastases in 10.16%. Median HUAC was 20.32 HU (IQR 15.46–22.83).
In a univariate analysis, the number of bone metastases, the presence of visceral metastases, and testosterone levels ≥50ng/dl at follow-up were associated with poorer OS, while high HUAC levels were associated with better OS. In a multivariate analysis, the number of bone metastases [hazard ratio (HR)=1.573, 95% confidence interval (CI)=1.103–2.243, p=0.012], the presence of visceral metastases (HR=7.404, CI=2.233–24.549, p=0.001), and the Gleason score (HR=2.001, CI=1.02–3.923, p=0.044) were associated with greater overall mortality, and HUAC (HR=0.902, CI=0.835–0.973, p=0.008) was associated with better OS.
ConclusionsIn our series, increased HUAC values in the psoas muscles, as a reflection of muscle density, when MPCa was diagnosed had a protective effect on OS in these patients.
La sarcopenia es un síndrome caracterizado por pérdida de masa y fuerza muscular. El objetivo del estudio es determinar la asociación entre la densidad muscular y la supervivencia global (SG) de los pacientes con cáncer de próstata de debut metastásico (CaPM).
Materiales y métodosEstudio retrospectivo de pacientes diagnosticados de CaPM entre 2009 y 2015 que recibieron únicamente terapia de privación androgénica como tratamiento inicial. La densidad muscular se calculó usando el Hounsfield Unit Average Calculation (HUAC) de ambos psoas en la tomografía computarizada (TC) del diagnóstico.
ResultadosIdentificamos 59 pacientes diagnosticados de CaPM. La media de edad fue 72.47 años. La mediana de PSA al diagnóstico fue de 68.25ng/dl (RIC 37.26-290). El 90.75% presentaban un Gleason ≥8, 88.13% metástasis óseas y 10.16% viscerales. La mediana de HUAC fue de 20.32 UH (RIC 15.46–22.83).
En el análisis univariante, el número de metástasis óseas, la presencia de metástasis viscerales y la presencia de niveles de testosterona ≥50ng/dl en el seguimiento se asociaron a una peor SG, mientras que niveles elevados de HUAC se asociaron a mejor SG. En el análisis multivariante, el número de metástasis óseas[Hazard ratio (HR)=1.573, intervalo confianza (IC) 95%=1.103–2.243, p=0.012], la presencia de metástasis viscerales (HR=7.404, IC=2.233–24.549, p=0.001) y el Gleason (HR=2.001, IC=1.02–3.923, p=0.044) se asociaron a un aumento de la mortalidad global y el HUAC(HR=0.902,IC=0.835–0.973, p=0.008) se asoció a una mejor SG.
ConclusionesEn nuestra serie, el aumento de los valores de HUAC de los músculos del psoas, como reflejo de la densidad muscular, en el diagnóstico del CaPM, tuvo un efecto protector sobre la SG en estos pacientes.
Different overall survival (OS) prognostic factors have been described in patients with metastatic prostate cancer (MPCa). The classical factors have been the Gleason score and the levels of prostate-specific antigen (PSA) and testosterone 6 months after the start of androgen deprivation therapy (ADT).1 Other factors associated with OS in these patients have been the presence of pain, visceral metastases, elevated LDH and alkaline phosphatase levels, and the presence of anemia.2
In 2010, the European Working Group on Sarcopenia in Older People (EWGSOP) defined sarcopenia as a syndrome characterized by a loss of muscle mass and strength that may be associated with poor physical performance.3 In addition, they defined pre-sarcopenia as a decrease in muscle mass not affecting muscle strength or physical performance.
In 2019, the EWGSOP2 updated the definition of sarcopenia. The latter was defined as a generalized and progressive skeletal muscle disorder associated with an increased probability of adverse outcomes including falls, fractures, physical disability and mortality.4 The EWGSOP2 also updated the diagnostic criteria for sarcopenia (Table 1).
Definition of sarcopenia according to the EWGSOP2.4
| Probable sarcopenia is identified by criterion 1. The diagnosis of sarcopenia is confirmed by the additional documentation of criterion 2. If all criteria 1, 2 and 3 are met, sarcopenia is considered to be severe | |
| EWGSOP2 criteria | 1. Low muscle strength |
| 2. Low muscle quantity or quality | |
| 3. Low physical performance | |
EWGSOP=European Working Group on Sarcopenia in Older People.
Reference: Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age and Ageing 2019;48:16–31. doi:10.1093/ageing/afy169.
Various imaging techniques may be used to calculate muscle mass: DXA (dual energy X-ray absorptiometry), BIA (bioelectric impedance analysis), magnetic resonance imaging (MRI) and computed tomography (CT).5
A number of studies have shown the presence of sarcopenia in the diagnosis of a solid tumour to have a negative impact upon OS.6
The present study was carried out to assess the prognostic impact of muscle density in patients diagnosed with metastatic onset prostate cancer treated with ADT alone.
Material and methodsA retrospective study was made of patients diagnosed with metastatic onset prostate cancer between 2009 and 2015. Patients without a baseline CT scan were excluded from the analysis. All patients received ADT alone as first treatment, comprising the initial use of antiandrogens (bicalutamide 50mg/day) to avoid flare-up phenomena, and subsequently 6-monthly LHRH analogues, with the suspension of bicalutamide 30 days after the start of the latter treatment. All patients were advised to maintain an adequate level of physical activity, with resistance and strength exercises.
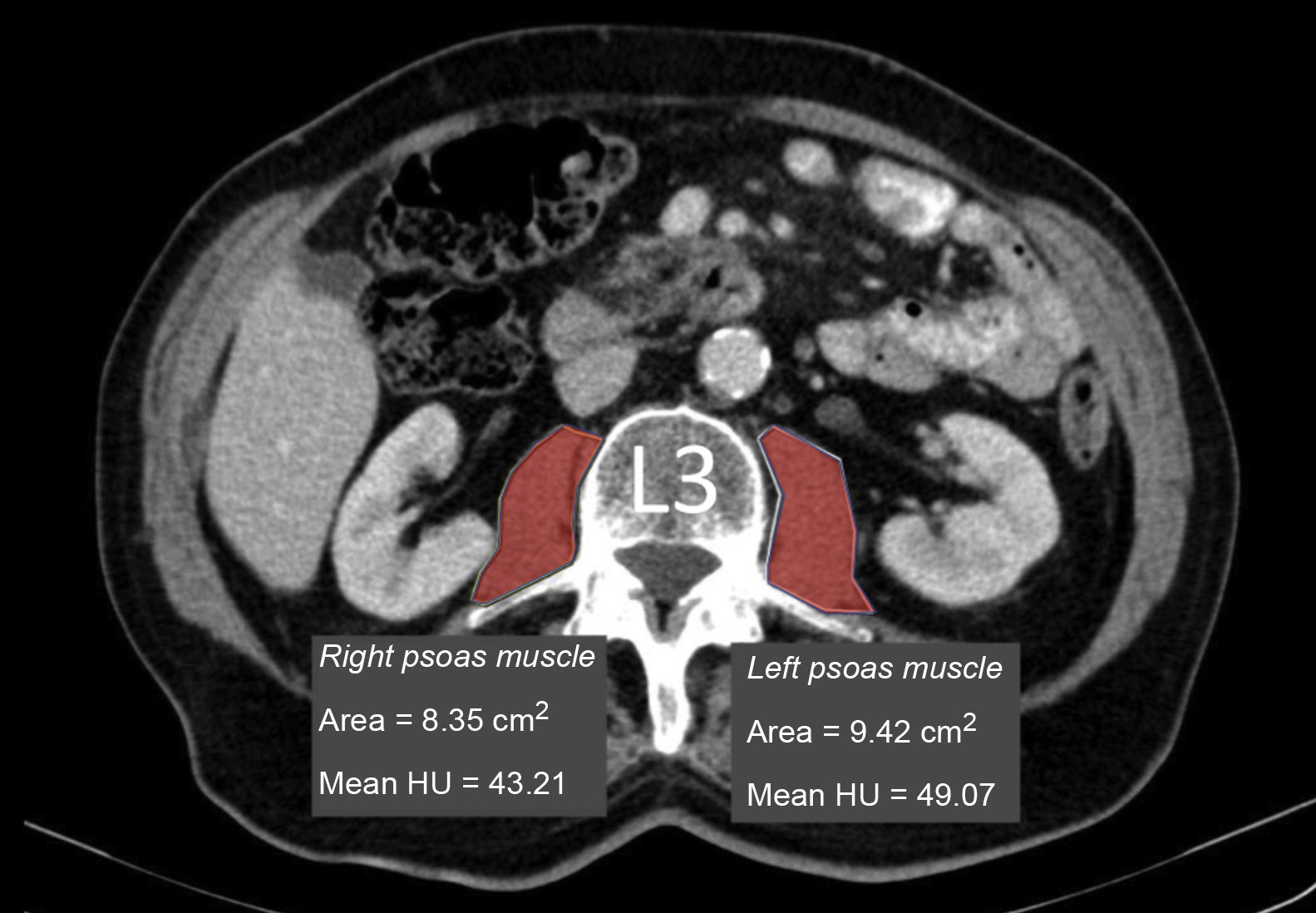
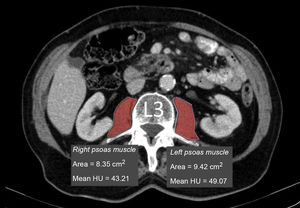
Measurement of muscle densityHounsfield Unit Average Calculation (HUAC) was recorded (Fig. 1) for the psoas muscles, as described by Joglekar et al.7 in 2015.
The HUAC is a measure that assesses lean mass density and fat infiltration based on the Hounsfield units (HU) of the area (in cm2) of both psoas muscles at the level of the third lumbar vertebra (L3), using the CT performed at the time of diagnosis of the disease.
The following formula was used to calculate the HUAC value7Firstly, we obtained the Right Hounsfield Unit Calculation (RHUC)=(Hounsfield Unit right psoas x right psoas area) / (total psoas area).
Then we recorded the Left Hounsfield Unit Calculation (LHUC)=(Hounsfield Unit left psoas x left psoas area) / (total psoas area).
Lastly, we recorded HUAC=(RHUC+LHUC)/2.
The HUAC has been associated in particular with outcomes following hepatobiliary-pancreatic and gastrointestinal cancer surgery.7–9
Statistical analysisThe age of the patients was evaluated, expressed as the mean and standard deviation (SD). Baseline PSA, HUAC in the baseline CT scan, PSA at 6 months and testosterone at 6 months after the start of ADT were analysed, and reported as the median and interquartile range (IQR). The number of bone metastases was categorized as 0, 1–2, 3–9 and ≥10, the result being reported as total number and percentage. The presence of visceral metastases (M1c) was also reported as total number and percentage.
Overall survival was calculated using the Kaplan-Meier method.
The Cox regression test was used to determine whether HUAC (assessed as a continuous variable) was unilaterally associated with OS. A multivariate analysis was then performed using the backward stepwise selection method, including the factors found to be significant in the univariate study, as well as the classical prognostic factors.
Statistical significance was considered for p<0.05.
ResultsWe identified 70 patients with metastatic onset prostate cancer diagnosed at our centre during the study period. Of these subjects, only 59 could be analysed, because the remainder had had no baseline abdominal CT scan at the time of diagnosis.
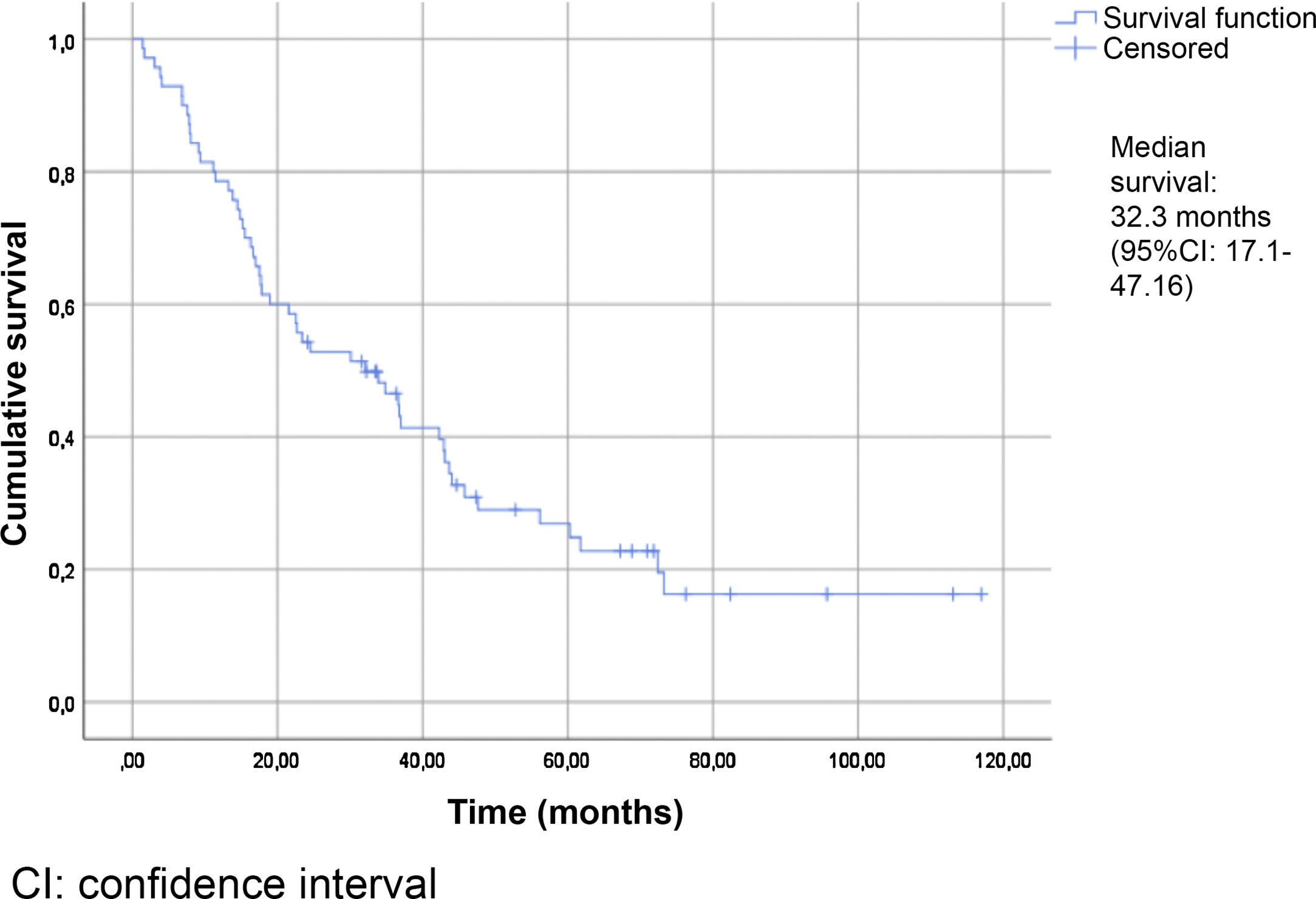
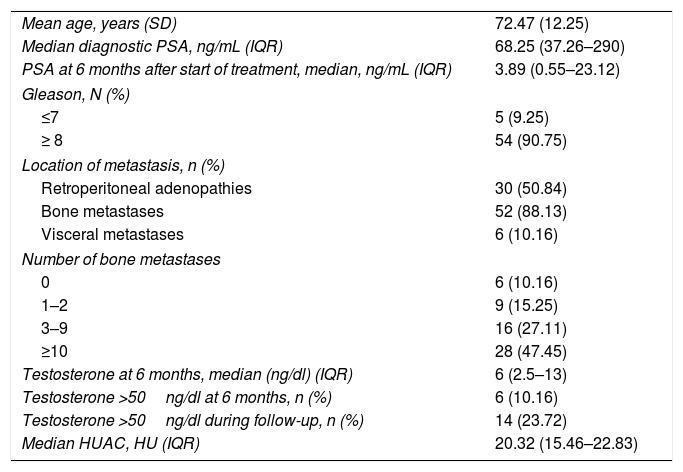
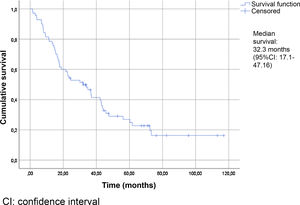
Table 2 summarizes the patient characteristics. The mean age of the patients was 72.47±12.25 years. The great majority (90.75%) presented a Gleason score ≥8. A total of 88.13% were diagnosed with bone metastases, and 74.56% presented more than three bone metastases. The presence of visceral metastases (M1c) was recorded in 10.16% of the patients. The median follow-up time was 30.5 months (IQR: 15–45.75). The median survival based on the Kaplan-Meier method was 32.3 months (95% confidence interval [95%CI] 17.1–47.16) (Fig. 2).
Characteristics of the study population.
| Mean age, years (SD) | 72.47 (12.25) |
| Median diagnostic PSA, ng/mL (IQR) | 68.25 (37.26–290) |
| PSA at 6 months after start of treatment, median, ng/mL (IQR) | 3.89 (0.55–23.12) |
| Gleason, N (%) | |
| ≤7 | 5 (9.25) |
| ≥ 8 | 54 (90.75) |
| Location of metastasis, n (%) | |
| Retroperitoneal adenopathies | 30 (50.84) |
| Bone metastases | 52 (88.13) |
| Visceral metastases | 6 (10.16) |
| Number of bone metastases | |
| 0 | 6 (10.16) |
| 1–2 | 9 (15.25) |
| 3–9 | 16 (27.11) |
| ≥10 | 28 (47.45) |
| Testosterone at 6 months, median (ng/dl) (IQR) | 6 (2.5–13) |
| Testosterone >50ng/dl at 6 months, n (%) | 6 (10.16) |
| Testosterone >50ng/dl during follow-up, n (%) | 14 (23.72) |
| Median HUAC, HU (IQR) | 20.32 (15.46–22.83) |
SD=standard deviation; PSA=prostate-specific antigen; IQR=interquartile range; HUAC=Hounsfield Unit Average Calculation; HU=Hounsfield units.
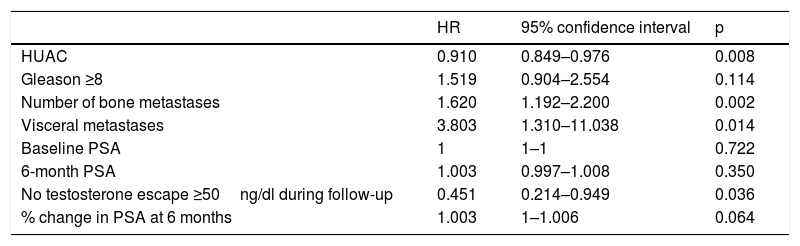
The variables significantly associated with a decrease in OS in the univariate analysis were the number of bone metastases (p=0.002), the presence of visceral metastases (p=0.014) and the absence of testosterone levels above castration levels (p=0.036). In contrast, the HUAC value of the psoas muscles (p=0.008) showed a protective effect and was associated with improved OS (Table 3).
Univariate analysis of the association of different variables with patient survival.
| HR | 95% confidence interval | p | |
|---|---|---|---|
| HUAC | 0.910 | 0.849–0.976 | 0.008 |
| Gleason ≥8 | 1.519 | 0.904–2.554 | 0.114 |
| Number of bone metastases | 1.620 | 1.192–2.200 | 0.002 |
| Visceral metastases | 3.803 | 1.310–11.038 | 0.014 |
| Baseline PSA | 1 | 1–1 | 0.722 |
| 6-month PSA | 1.003 | 0.997–1.008 | 0.350 |
| No testosterone escape ≥50ng/dl during follow-up | 0.451 | 0.214–0.949 | 0.036 |
| % change in PSA at 6 months | 1.003 | 1–1.006 | 0.064 |
HUAC=Hounsfield Unit Average Calculation; PSA=prostate-specific antigen; HR=hazard ratio.
All variables with a significant impact in the univariate analysis were entered in the multivariate study. Although not found to be significant in the univariate analysis, the Gleason score of the biopsy was also included because of its classically recognized prognostic impact. The change in PSA value at 6 months, expressed as a percentage, was also included, since it came close to statistical significance in the univariate analysis (p=0.064).
The multivariate study (Table 4) found a Gleason score ≥8 (hazard ratio [HR]=2.001, 95%CI=1.02–3.923; p=0.044), the presence of visceral metastases (M1c) at diagnosis (HR=7.404, 95%CI=2.233–24.549; p=0.001), and a greater number of bone metastases (HR=1.573, 95%CI=1.103–2.243; p=0.012) to be negatively correlated to patient survival.
Multivariate analysis of the association of different variables with patient survival.
| HR | 95%CI | p | |
|---|---|---|---|
| HUAC | 0.902 | 0.835–0.973 | 0.008 |
| Gleason ≥8 | 2.001 | 1.02–3.923 | 0.044 |
| Number of bone metastases | 1.573 | 1.103–2.243 | 0.012 |
| Visceral metastases | 7.404 | 2.233–24.549 | 0.001 |
| Baseline PSA | 1.000 | 0.999–1.000 | 0.357 |
| % change in PSA at 6 months | 0.998 | 0.994–1.003 | 0.467 |
| No testosterone escape ≥50ng/dl during follow-up | 0.636 | 0.261–1.548 | 0.318 |
HUAC=Hounsfield Unit Average Calculation; PSA=prostate-specific antigen; HR=hazard ratio; CI=confidence interval.
By contrast, a higher muscle density of both psoas muscles, assessed by the HUAC, was significantly correlated to improved OS in patients with MPCa (HR=0.902, 95%CI=0.835–0.973; p=0.008). Neither treatment response assessed as a change in PSA value 6 months after starting ADT nor the presence of testosterone levels above castration levels had an impact upon survival (HR=0.998, 95%CI=0.994–1.003, p=0.467 and HR=0.636, 95%CI=0.261–1.548; p=0.318, respectively).
DiscussionThe treatment of metastatic onset prostate cancer has changed in recent years. Androgen deprivation therapy was initially considered to be the standard of care for these patients. The publication of new studies beginning in 2015, demonstrating increased OS with the administration of docetaxel, abiraterone, apalutamide and enzalutamide,10–14 has since modified the management paradigm for these patients. Our study was conducted in patients diagnosed with MPCa between 2009 and 2015, i.e., prior to the advent of these new therapies, so consequently they were only treated with ADT.
In 1988, Soloway et al.15 reported that a greater number of bone metastases is associated with poorer survival. In 2003, Glass et al.16 analysed the possible survival prognostic factors in patients with MPCa. The study variables included the location of the metastases (appendicular and/or visceral versus axial), the patient performance status (0 versus 1–3), baseline PSA at diagnosis (<65 versus ≥65ng/mL) and the Gleason score (<8 versus ≥8). The patients with a poorer prognosis were those with appendicular and/or visceral bone metastases, performance status ≥1, and PSA ≥65ng/mL (HR=2.8). Gravis et al.,2 in the patient population enrolled in the GETUG-15 trial,17 found that alkaline phosphatase, LDH, hemoglobin and pain intensity also behave as prognostic factors. In our series we found the Gleason score, the number of bone metastases, and the presence of visceral metastases to have an impact upon the OS of these patients, these data being consistent with those reported in the previously published series.
Following the introduction of the new treatments, a number of different poor outcome factors have been identified. The term high volume disease was defined in patients treated with docetaxel (the presence of visceral metastases or ≥4 bone metastases with ≥1 bone lesion beyond the vertebral bodies and pelvis).10 In patients treated with abiraterone, the concept of high risk was defined as the presence of two or more of the following factors: a Gleason score ≥8, at least three bone lesions, and the presence of visceral metastases.11
Perachino et al.1 described the prognostic importance of PSA as a response 6 months after starting treatment with ADT, and also of the testosterone levels in that same period. In our study, the testosterone levels at 6 months, the presence of testosterone >50ng/dl at some point during follow-up, and PSA at 6 months had no impact upon survival.
Sarcopenia has been shown to be a poor outcome indicator in a number of tumours such as melanoma, esophageal cancer,18 gastrointestinal or lung cancer19 and hepatocarcinoma.20 The negative impact of sarcopenia in urological tumours such as metastatic renal cancer21 and urothelial carcinoma (both in patients subjected to cystectomy22 and after nephroureterectomy23) has also been investigated. In most of these studies, sarcopenia was defined based on the muscle mass area using abdominal CT scans, but without assessing either loss of strength or physical performance.
Versteeg et al.24 found sarcopenia (calculating mass and density with CT and assessing muscle strength) to be associated with poorer survival in advanced tumour disease, including patients with prostate cancer. Stangl-Kremser et al.25 in turn found low muscle volume to be an independent poor prognosis factor for disease progression in patients with metastatic castration-resistant prostate cancer. In our population, the presence of higher muscle density acted as a protective factor. To our knowledge, the present study is the first to explore the association between muscle density using HUAC of the psoas muscles and OS in patients with metastatic onset prostate cancer.
Muscle mass measured from abdominal CT scans has been shown to be associated with total body muscle mass.26 In addition, it has been seen that even when only evaluating psoas muscle mass with CT, the data obtained are correlated to total muscle mass.27 As important as muscle volume is the radiation attenuation of the muscle, assessed in the CT scan, and its density. The lesser the muscle density, the poorer is the quality of the muscle.28
One of the problems in assessing sarcopenia is the different criteria used in the published series. In addition, most published studies are retrospective and only assess muscle mass without taking into account the recommendations of the EWGSOP, which state that muscle function should also be considered.6 In our study, we assessed HUAC because it integrates both muscle area (quantity) and the degree of fat infiltration (quality) into a single value. Joglekar et al.7 were the first to define HUAC and assess it as a predictor of complications in patients subjected to pancreatectomy, though they erroneously associated it indirectly with the presence of sarcopenia, since they did not assess muscle strength. In the case of a relationship, the HUAC value would be better associated with the presence of pre-sarcopenia. Many other studies have subsequently evaluated the association between HUAC and oncological and postoperative outcomes, erroneously associating muscle density with sarcopenia.8,9
Although clear evidence is lacking, it seems logical to assume that improving muscle mass will have a beneficial impact upon oncological patients,29 since it is a modifiable prognostic factor. The European Society for Clinical Nutrition and Metabolism (ESPEN) recommends maintaining or increasing physical activity in cancer patients in order to improve muscle mass and physical function, with a high level of evidence and a strong grade of recommendation. With regard to the type of exercise, resistance exercises are advised, together with aerobic exercises, though with a low level of evidence and a weak grade of recommendation.30 On the other hand, in a review of patients with prostate cancer, Peisch et al.31 found that regular vigorous physical exercise appeared to decrease the risk of progression.
The assessment of muscle density by recording HUAC from CT scans is an easy and reproducible technique that could be correlated to the presence of pre-sarcopenia, though further studies are needed to demonstrate this possible association. In addition, pre-sarcopenia could be defined as sarcopenia if the patient also presents a decrease in strength.
Our study has limitations. It involves a retrospective design and the sample size is limited, thus precluding the drawing of firm conclusions. Another limitation of the study is the fact that a normal muscle density cut-off value cannot be established using HUAC, because our entire population consisted of metastatic patients, and we assumed that they had a lower HUAC value as compared to the healthy population. Lastly, another limitation is the fact that 11 of the 70 patients with metastatic onset prostate cancer in the study period had had no baseline CT scan for assessing HUAC and thus could not be included in the study. Despite the above, the results obtained are in line with those published in similar articles, confirming their soundness. Prospective studies involving larger sample sizes are needed for more robust conclusions to be drawn regarding muscle density assessed with HUAC in the studied target population.
ConclusionsIn our series, the increase in HUAC values of the psoas muscles, reflecting the absence of sarcopenia in the diagnosis of MPCa, had a protective effect upon the OS of patients diagnosed with metastatic onset prostate cancer.
Sources of fundingThe present study received no specific financial support from public agencies, the commercial sector or from non-profit entities.
Conflicts of interestThe following authors report lecture fees: Jesus Muñoz-Rodriguez: Astellas Pharma, Bayer, Janssen. Arturo Dominguez: Astellas Pharma, Janssen. Victor Parejo: Janssen. Enrique Gallardo: Astellas Pharma, Bayer, Janssen, Ipsen, Sanofi, Pfizer. Teresa Bonfill: Astellas Pharma. Dario Garcia-Rojo: Janssen. Leticia De Verdonces: Astellas Pharma.
The following authors report advisory board fees: Jesus Muñoz-Rodriguez: Astellas Pharma, Bayer, Janssen; Enrique Gallardo: Astellas Pharma, Bayer, Janssen, Ipsen, Pfizer.
The other authors declare that they have no conflicts of interest.
The authors wish to thank Joan Carles Oliva for his contribution to the statistical analysis.
Please cite this article as: Muñoz-Rodríguez J, Domínguez A, Rosado MA, Centeno C, Parejo V, Costa-Trachsel I, et al. Efecto de la densidad muscular en pacientes con cáncer de próstata metastásico tratados con terapia de privación androgénica. Endocrinol Diabetes Nutr. 2021;68:92–98.