To assess the impact of glycemic control in gestational on neonatal weight and metabolic complications of twin and singleton pregnancies.
MethodsAn observational, retrospective study to monitor 120 twin and 240 singleton pregnancies in women with GDM. Maternal glycemic parameters during pregnancy (oral glucose tolerance test results, treatment, insulinization rate, mean HbA1c in the third trimester), and neonatal complications and weight were recorded.
ResultsA higher infant birth weight ratio (IBWR 1.02±0.12 vs. 0.88±0.12, p<0.001) and a lower rate of newborns small for gestational age (severe SGA 2.5% vs. 8.3%, p=0.012) were seen after singleton pregnancies as compared to twin pregnancies. The rates of newborns large for gestational age (LGA 12.6% vs. 12.5%, p=0.989); macrosomic (6.7% vs. 7.5%, p=0.777); or small for gestational age (SGA 6.7% vs. 10.8%, p=0.175) were similar in both groups. Neonates from twin pregnancies had a higher risk of hypoglycemia (adjusted OR 4.71; 1.38–16.07, p=0.013) and polycythemia (adjusted OR 10.05; 1.82–55.42, p=0.008). A linear relationship was seen between third trimester HbA1c levels and IBWR in singleton (r=.199, p=0.003), but not in twin pregnancies (r=0.049, p=0.610).
ConclusionsRisk of severe SGA, hypoglycemia, and polycythemia was significantly higher in twin pregnancies of women with GDM. Neonatal weight outcomes and metabolic complications in twin pregnancies of women with GDM were not related to glycemic control. Moreover, in our study population, fasting glucose at diagnosis and mean HbA1c in the third trimester showed a linear relationship with higher birth weights in singleton, but not in twin pregnancies.
Evaluar el impacto del control glucémico de la diabetes mellitus gestacional (DMG) en el peso y las complicaciones de origen metabólico neonatales de embarazos gemelares y de feto único.
MétodosEstudio observacional retrospectivo que incluyó gestantes con DMG: 120 embarazos gemelares y 240 embarazos de feto único como controles. Registramos los parámetros de control glucémico durante el embarazo (resultados de la sobrecarga oral de glucosa diagnóstica, tratamiento, insulinización, HbA1c media del tercer trimestre), las complicaciones neonatales y el peso neonatal.
ResultadosLos neonatos de embarazos únicos tuvieron mayor índice ponderal fetal (IPF 1,02±0,12 vs. 0,88±0.12, p<0,001) y menor incidencia de pequeños para la edad gestacional grave (2,5% vs. 8,3%, p=0.012). La tasa de neonatos grandes para edad gestacional, macrosómicos y pequeños para la edad gestacional fue similar en ambos grupos. Los recién nacidos de embarazos gemelares tuvieron un mayor riesgo de hipoglucemia: OR ajustada 4,71 (1,38–16,07, p=0,013) y poliglobulia: OR ajustada 10,05 (1,82-55,42, p=0,008). El IPF se correlacionó con la glucosa basal en la sobrecarga oral de glucosa al diagnóstico (r=0,223, p=0,001) y la HbA1c media del tercer trimestre (r=0,199, p=0,003) en los embarazos únicos, pero no en los gemelares (r=0,003, p=0,748; r=0,049, p=0,610; respectivamente).
ConclusionesEl riesgo de pequeño para la edad gestacional grave, hipoglucemia y poliglobulia fue mayor en los embarazos gemelares con DMG. Los resultados de peso neonatal y las complicaciones de origen metabólico no se relacionan con el control metabólico materno en los embarazos gemelares.
In Spain, a diagnosis of gestational diabetes mellitus (GDM) is established in 8.8% of all pregnancies. Depending on the diagnostic criteria used, the prevalence may be even higher in other geographical settings.1,2
Multiple deliveries account for approximately 2% of all births in Spain,3,4 with an increasing trend in recent decades due to an older maternal age and the increasing use of fertility treatments. The incidence of twin births increased 76% between 1980 and 2009 in developed countries.5 Neonates from multiple pregnancies involving the use of assisted reproduction techniques account for between 14.7 and 29.0% of all infants born of multiple pregnancies, depending on the country.6 Gestational diabetes mellitus and multiple pregnancies achieved through fertility treatments share a number of risk factors (older maternal age, overweight and obesity), multiplying the probability that both conditions may coexist. In addition, twin pregnancies pose an increased risk of neonatal complications,7 as well as of gestational hypertension, compared with singleton pregnancies.8
Gestational diabetes mellitus in singleton pregnancies is associated with an increased risk of macrosomia, infants large for gestational age (LGA), and neonatal complications of metabolic origin, including hypocalcemia, hypoglycemia, polyglobulia and hyperbilirubinemia. However, there is controversy regarding the impact of GDM in multiple pregnancies. Some studies have found an increased risk of LGA,9 macrosomia4,10 and asymmetrical fetal growth,11 while others have observed no differences.12,13 On the other hand, there appears to be consistent evidence that GDM exerts a “protective effect”, reducing the risk of infants small for gestational age (SGA) and severe fetal growth restriction.4,11,14,15
A number of authors have proposed a differential impact of GDM upon twin pregnancies as compared to singleton pregnancies. Population-based studies have found a lesser increase in the risk of preterm delivery and macrosomia, and a decrease in the risk of low Apgar scores at 5min and of neonatal death in twin pregnancies, suggesting a milder impact of GDM in such situations.9,10 It has even been reported that blood glucose control during pregnancy does not influence neonatal weight in twin pregnancies with GDM,12 in contrast to what has been extensively demonstrated in singleton pregnancies with GDM.16
The purpose of this study was to assess the influence of glycemic control during pregnancy in accounting for differences between twin and singleton pregnancies with regard to neonatal weight (the fetal ponderal index [FPI], LGA, macrosomia, SGA and severe SGA) and neonatal complications of metabolic origin in pregnancies complicated by GDM.
Material and methodsA retrospective observational study was carried out in the Diabetes and Pregnancy Unit of Hospital Universitario La Paz (Madrid, Spain) from January 1999 to December 2012. A total of 360 women were included: 120 with twin pregnancies and 240 with singleton pregnancies, assisted during pregnancy and at delivery at our center.
Controls were matched for maternal age and year of delivery from among 1436 singleton pregnancies complicated by GDM, in a proportion of 2:1. Newborn infants weighing <0.5kg and with gestational age <24 weeks were excluded. Patients with pregestational type 1 or type 2 diabetes were also excluded. The study was approved by the local Ethics Committee.
The diagnosis of GDM was established using a two-step approach: universal screening in gestational week 24–28, or in the first trimester in women with risk factors (maternal age >35 years, pregestational obesity, a personal history of gestational diabetes mellitus or macrosomia in the infant, a first degree relative with diabetes mellitus). In positive cases (first hour plasma glucose ≥140mg/dl in the 50g oral glucose tolerance test [OGTT]), OGTT was performed with 100g. The diagnosis of GDM was based on the criteria of the National Diabetes Data Group.17 Gestational age at diagnosis of GDM was also recorded.
Gestational age was determined in spontaneous pregnancies from the date of last menstruation corrected by first trimester ultrasound. In pregnancies achieved through assisted reproduction techniques, the date of oocyte recovery corrected by first trimester ultrasound was used.
The women were classified according to the pregestational body mass index (BMI): low weight (BMI <18.5kg/m2), normal weight (BMI 18.5–24.9), overweight (BMI 25–29.9), and obesity (BMI ≥30).18 The maternal weight gain rate was calculated as: maternal weight gain - neonatal weight/total weeks of gestation.19
All women received dietary advice from qualified nurses based on their pregestational BMI and routine physical activity (25–40kcal/kg/day).
Capillary glucose measurements were assessed every 2–3 weeks at medical visits. Insulin was added to dietary management if fasting glucose was ≥95mg/dl or ≥120mg/dl two hours postprandial. No oral medication is used in our center for glycemic control. Glycosylated hemoglobin (HbA1c) was determined monthly after the diagnosis of GDM, and poor metabolic control was defined as mean HbA1c values of the third trimester above the third quartile ([median 5.1%, 32mmol/mol] [interquartile range 4.9–5.4%, 30–36mmol/mol]).
Serum glucose was measured by the glucose oxidase method (Hitachi 717, Boehringer, Mannheim, Germany). Glycosylated hemoglobin was measured by high performance liquid chromatography on Diamat and Variant autoanalyzers (BioRad, Richmond, CA, USA), with interchangeable results calibrated for the normal range according to the Diabetes Control and Complications Trial.20–22 All measurements were made after overnight fasting.
Spanish neonatal weight tables for singleton and twin pregnancies23 were used to determine the FPI (actual birth weight/P50 for gestational age),24 LGA (neonatal weight >P90 or at least one twin with weight >P90), SGA (neonatal weight
The occurrence of neonatal complications of metabolic origin was recorded27: hypocalcemia (serum calcium <7mg/dl or ionic calcium <4mg/dl), hypoglycemia (<40mg/dl in the first 24h of life), polyglobulia (venous blood hematocrit >65%) and hyperbilirubinemia (according to gestational age and extrauterine life time).28
Other maternal-fetal variables were also documented, including the method of conception, maternal parity, hypertensive pregnancy disorders, gestational age at delivery, preterm delivery (before 37 weeks of gestation) and early preterm delivery (before 34 weeks of gestation), the delivery route, respiratory distress syndrome (defined as progressive dyspnea in the first 24h of life, requiring supplemental oxygen), obstetric trauma (shoulder dystocia, brachial paralysis and/or cephalic-pelvic disproportion), neonatal sepsis (positive culture accompanied by consistent clinical manifestations), serious congenital malformations (those causing neonatal death, significant disability, or requiring major surgery for correction), and neonatal death.
Pregnancy-induced hypertension was defined as the presence of systolic blood pressure ≥140mmHgor diastolic blood pressure ≥90mmHgafter 20 weeks of gestation, while preeclampsia was considered if accompanied by proteinuria ≥30mg/mmol creatinine.29
In twin pregnancies, chorionicity (monochorionic/dichorionic) was assessed by ultrasound and confirmed after delivery by obstetricians and by pathology reports, where available.
The primary objective was to compare the effect of glycemic control in GDM upon neonatal weight (the FPI, LGA, macrosomia, SGA and severe SGA) and fetal metabolic complications between twin and singleton pregnancies.
In the statistical analysis, a complicated twin pregnancy was considered as a unit with one or both twins affected. Comparisons between the study groups were made using the Student t-test for quantitative variables, and the chi-squared test or Fisher's exact test for categorical variables. Adjusted multiple logistic regression was used to assess the influence of possible confounding factors upon the association between multiple pregnancy and birth weight (adjusted for maternal BMI before pregnancy, maternal weight gain rate, premature delivery, serious fetal malformations, chorionicity, hypertension or preeclampsia, smoking habit and parity), and between multiple pregnancy and neonatal complications (maternal age, maternal BMI before pregnancy, the rate of maternal weight gain, gestational age at delivery, serious fetal malformations, chorionicity, hypertension or preeclampsia, smoking habit, delivery route and parity).
In analyzing the influence of glycemic control during pregnancy, the Pearson correlation coefficient was used to measure the relationship between FPI and fasting glucose in the OGTT at diagnosis, and mean HbA1c in the third trimester. A subanalysis was also performed of the incidence of fetal metabolic complications in patients with poor metabolic control (HbA1c of the third trimester above the third quartile [>5.4%, 36mmol/mol]). In addition, the neonatal weight variables were analyzed in the subgroup of patients requiring insulin. The SPSS version 20 statistical package (Chicago, IL, USA) was used for data analysis. Statistical significance was considered for p<0.050.
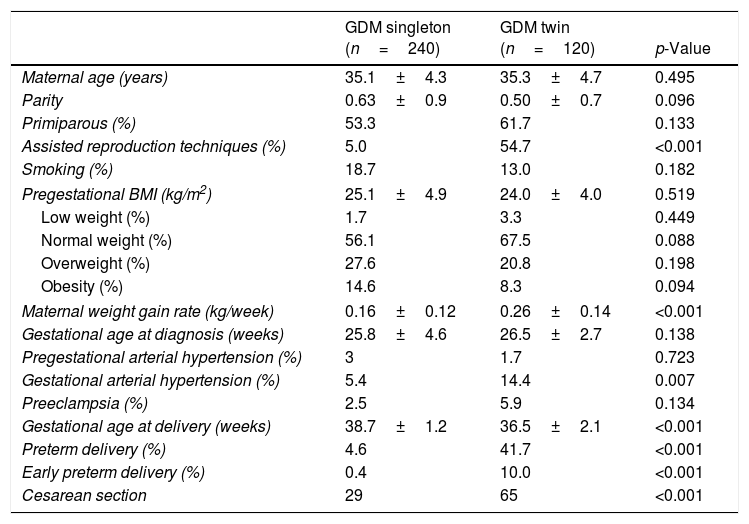
ResultsMaternal outcomesTable 1 shows the characteristics of the pregnant women with gestational diabetes. A higher pregnancy rate achieved by assisted reproduction techniques was recorded in the twin pregnancy group. A total of 87.9% of the twin pregnancies were dichorionic. The maternal weight gain rate was significantly higher in the twin pregnancy group: 0.26±0.14 vs. 0.16±0.12kg/week; p<0.001. The gestational hypertension rate was significantly greater in twin pregnancies (14.4% vs. 5.4%; p=0.007), though not so the preeclampsia rate (5.9% vs. 2.5%; p=0.134).
Characteristics of women with GDM and singleton and twin pregnancies.
| GDM singleton (n=240) | GDM twin (n=120) | p-Value | |
|---|---|---|---|
| Maternal age (years) | 35.1±4.3 | 35.3±4.7 | 0.495 |
| Parity | 0.63±0.9 | 0.50±0.7 | 0.096 |
| Primiparous (%) | 53.3 | 61.7 | 0.133 |
| Assisted reproduction techniques (%) | 5.0 | 54.7 | <0.001 |
| Smoking (%) | 18.7 | 13.0 | 0.182 |
| Pregestational BMI (kg/m2) | 25.1±4.9 | 24.0±4.0 | 0.519 |
| Low weight (%) | 1.7 | 3.3 | 0.449 |
| Normal weight (%) | 56.1 | 67.5 | 0.088 |
| Overweight (%) | 27.6 | 20.8 | 0.198 |
| Obesity (%) | 14.6 | 8.3 | 0.094 |
| Maternal weight gain rate (kg/week) | 0.16±0.12 | 0.26±0.14 | <0.001 |
| Gestational age at diagnosis (weeks) | 25.8±4.6 | 26.5±2.7 | 0.138 |
| Pregestational arterial hypertension (%) | 3 | 1.7 | 0.723 |
| Gestational arterial hypertension (%) | 5.4 | 14.4 | 0.007 |
| Preeclampsia (%) | 2.5 | 5.9 | 0.134 |
| Gestational age at delivery (weeks) | 38.7±1.2 | 36.5±2.1 | <0.001 |
| Preterm delivery (%) | 4.6 | 41.7 | <0.001 |
| Early preterm delivery (%) | 0.4 | 10.0 | <0.001 |
| Cesarean section | 29 | 65 | <0.001 |
Data presented as mean±standard deviation (SD) or as percentage; preterm delivery <37 weeks; early preterm delivery <34 weeks. Statistical significance was considered for p<0.050.
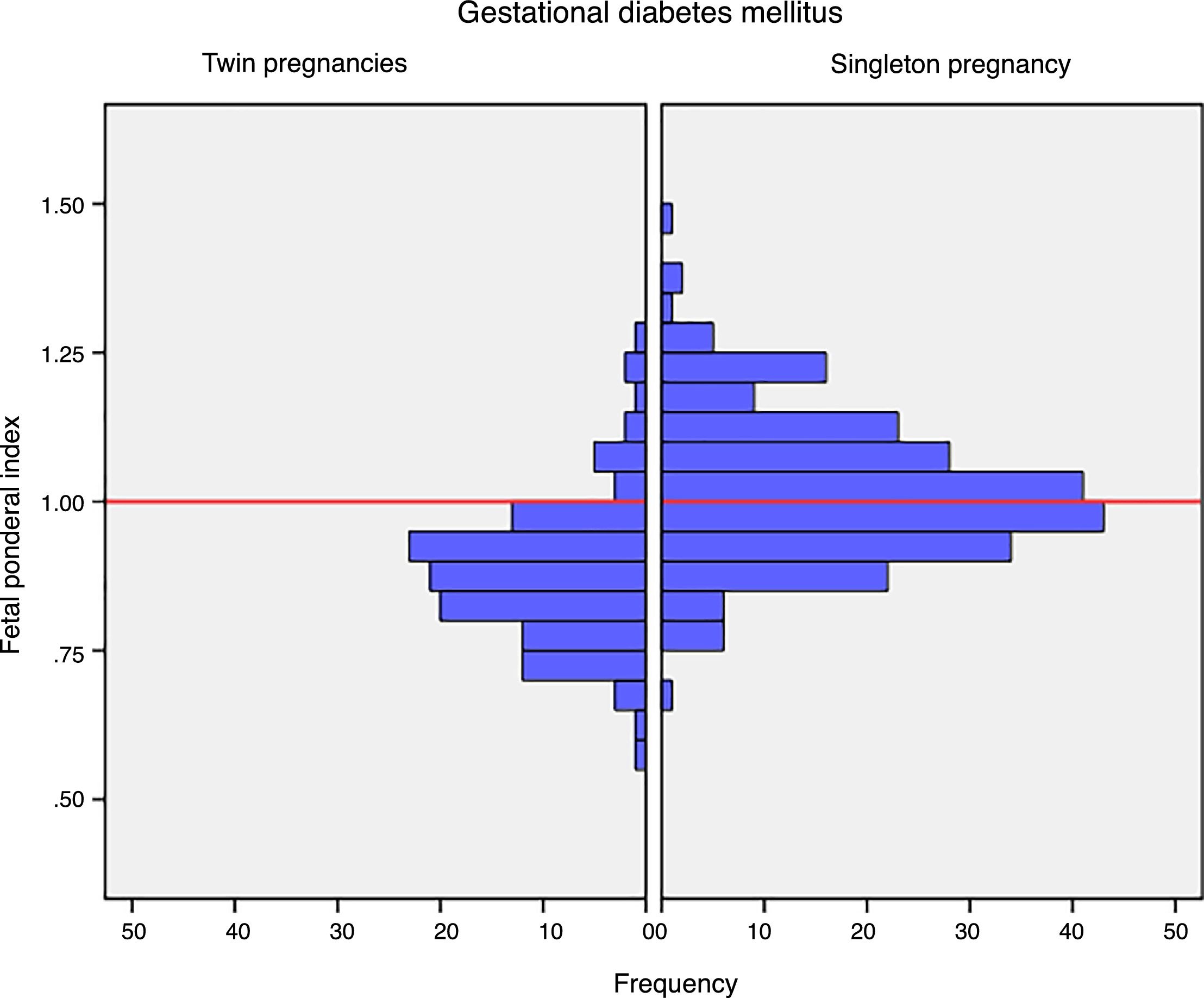
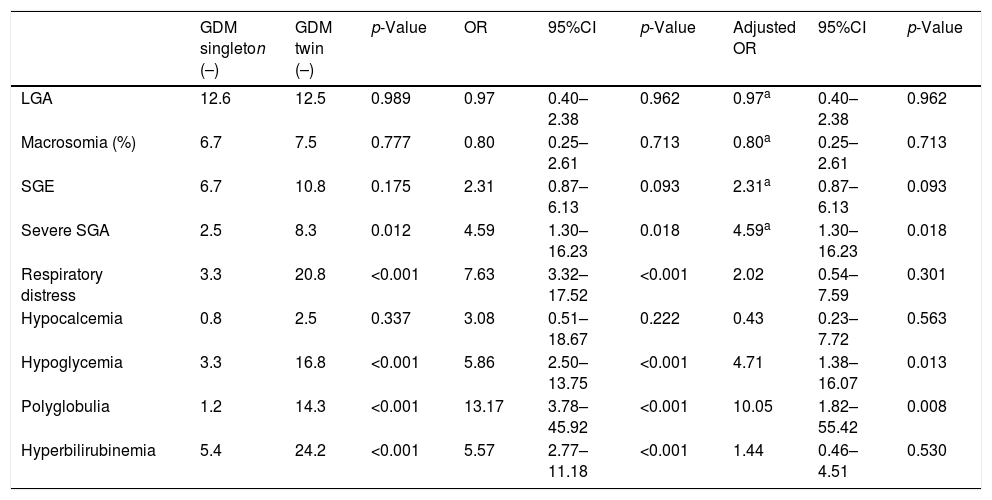
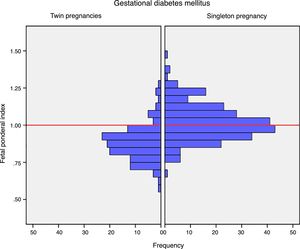
Mean birth weight was higher in singleton newborn infants with GDM (3206.4g±381.6g vs. 2382.3g±408.9g; p<0.001). The FPI was also higher in singleton pregnancies: 1.02±0.12 vs. 0.88±0.12 in twin pregnancies; p<0.001 (Fig. 1). The incidence of severe SGA was significantly lower in the singleton pregnancy group (2.5% vs. 8.3%; p=0.012). There were no differences in the incidence of LGA, macrosomia or SGA in the singleton pregnancy group versus the twin pregnancy group (Table 2).
Neonatal complications in singleton and twin pregnancies complicated by GDM.
| GDM singleton (–) | GDM twin (–) | p-Value | OR | 95%CI | p-Value | Adjusted OR | 95%CI | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| LGA | 12.6 | 12.5 | 0.989 | 0.97 | 0.40–2.38 | 0.962 | 0.97a | 0.40–2.38 | 0.962 |
| Macrosomia (%) | 6.7 | 7.5 | 0.777 | 0.80 | 0.25–2.61 | 0.713 | 0.80a | 0.25–2.61 | 0.713 |
| SGE | 6.7 | 10.8 | 0.175 | 2.31 | 0.87–6.13 | 0.093 | 2.31a | 0.87–6.13 | 0.093 |
| Severe SGA | 2.5 | 8.3 | 0.012 | 4.59 | 1.30–16.23 | 0.018 | 4.59a | 1.30–16.23 | 0.018 |
| Respiratory distress | 3.3 | 20.8 | <0.001 | 7.63 | 3.32–17.52 | <0.001 | 2.02 | 0.54–7.59 | 0.301 |
| Hypocalcemia | 0.8 | 2.5 | 0.337 | 3.08 | 0.51–18.67 | 0.222 | 0.43 | 0.23–7.72 | 0.563 |
| Hypoglycemia | 3.3 | 16.8 | <0.001 | 5.86 | 2.50–13.75 | <0.001 | 4.71 | 1.38–16.07 | 0.013 |
| Polyglobulia | 1.2 | 14.3 | <0.001 | 13.17 | 3.78–45.92 | <0.001 | 10.05 | 1.82–55.42 | 0.008 |
| Hyperbilirubinemia | 5.4 | 24.2 | <0.001 | 5.57 | 2.77–11.18 | <0.001 | 1.44 | 0.46–4.51 | 0.530 |
Odds ratio (OR) adjusted for maternal age, the maternal pregestational BMI, the maternal weight gain rate, gestational age at delivery, serious congenital malformations, chorionicity, high blood pressure, preeclampsia, smoking, the delivery route, and parity.
LGE: large for gestational age; SGA: small for gestational age.
On comparing the neonatal complications rate, the singleton pregnancies showed a lower incidence of respiratory distress syndrome (3.3% vs. 20.8%; p<0.001), as well as a lower incidence of hypoglycemia (3.3% vs. 16.8%; p<0.001), polyglobulia (1.2% vs. 14.3%; p<0.001) and hyperbilirubinemia (5.4% vs. 24.2%; p<0.001). There were no neonatal deaths in our study population.
After adjusting for potential confounders, the risk of hypoglycemia and polyglobulia remained higher in the twin pregnancy infants. Regarding the association between twin pregnancy and neonatal weight, the risk of severe SGA was 4.59 times higher in the twin pregnancy group compared to the singleton pregnancy group (CI: 1.3–16.23; p=0.018) (Table 2).
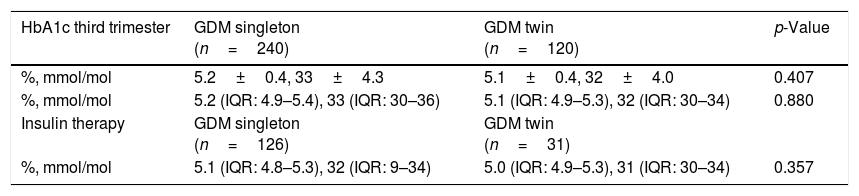
Glycemic controlWe analyzed a possible effect of glycemic control and the type of treatment received during pregnancy upon newborn weight. In our study population, the mean HbA1c concentration in the third trimester was 5.2±0.4% (33mmol/mol±4.2), with a median of 5.1% (32mmol/mol) (interquartile range 4.9–5.4%, 30–36mmol/mol). There were no differences in the HbA1c values between the study groups (Table 3).
HbA1c levels in the third trimester in singleton and twin pregnancies complicated by GDM.
| HbA1c third trimester | GDM singleton (n=240) | GDM twin (n=120) | p-Value |
|---|---|---|---|
| %, mmol/mol | 5.2±0.4, 33±4.3 | 5.1±0.4, 32±4.0 | 0.407 |
| %, mmol/mol | 5.2 (IQR: 4.9–5.4), 33 (IQR: 30–36) | 5.1 (IQR: 4.9–5.3), 32 (IQR: 30–34) | 0.880 |
| Insulin therapy | GDM singleton (n=126) | GDM twin (n=31) | |
| %, mmol/mol | 5.1 (IQR: 4.8–5.3), 32 (IQR: 9–34) | 5.0 (IQR: 4.9–5.3), 31 (IQR: 30–34) | 0.357 |
Data presented as mean±standard deviation, or as median and interquartile range (IQR). Statistical significance considered for p<0.050.
The insulinization rate was higher in the singleton pregnancies as compared to twin pregnancies (52.5% vs. 26.1%; p<0.001). The mean insulin dose required to achieve adequate glycemic control was 0.29±0.14IU/kg in singleton pregnancies vs. 0.34±0.16 (p=0.028) in twin pregnancies.
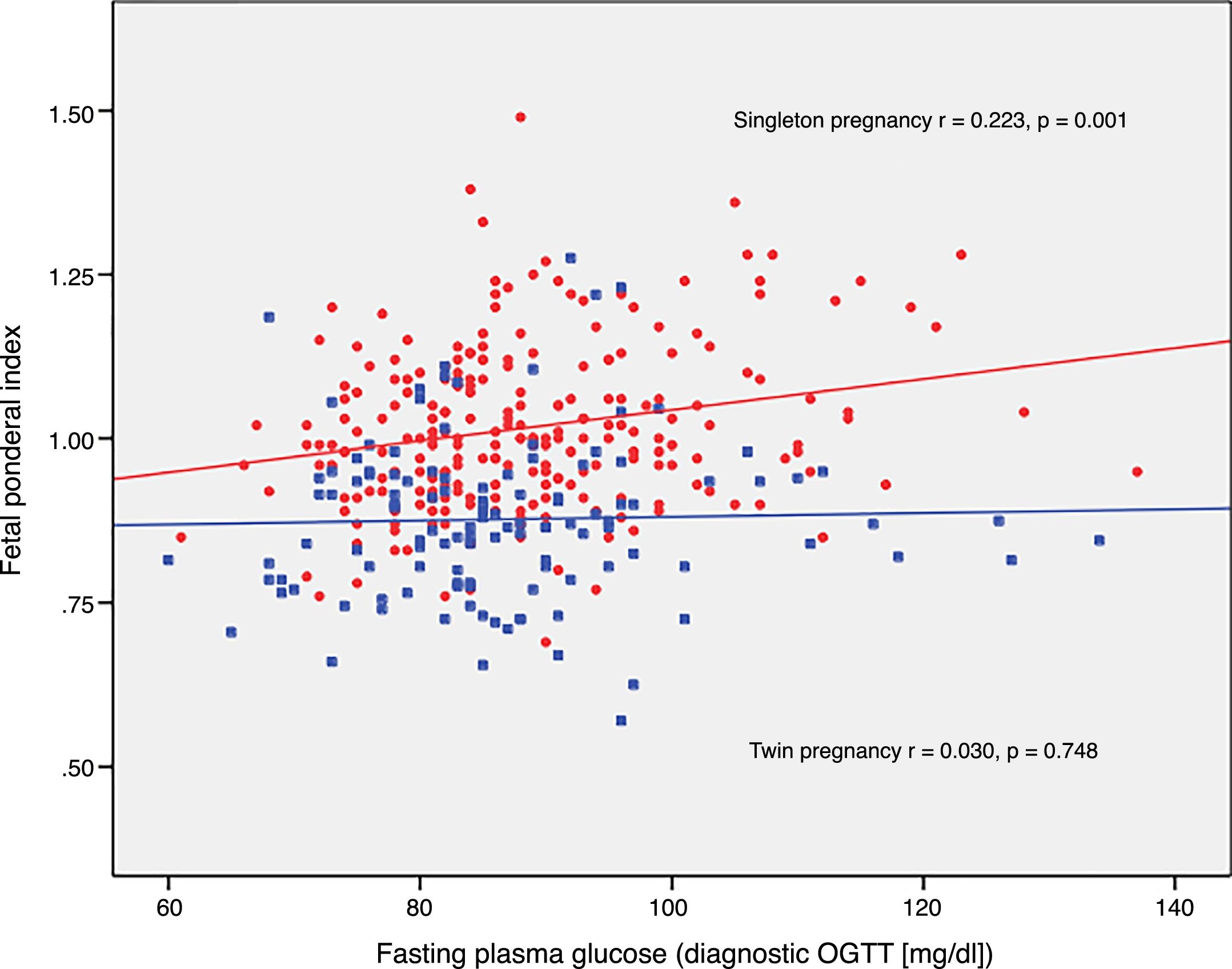
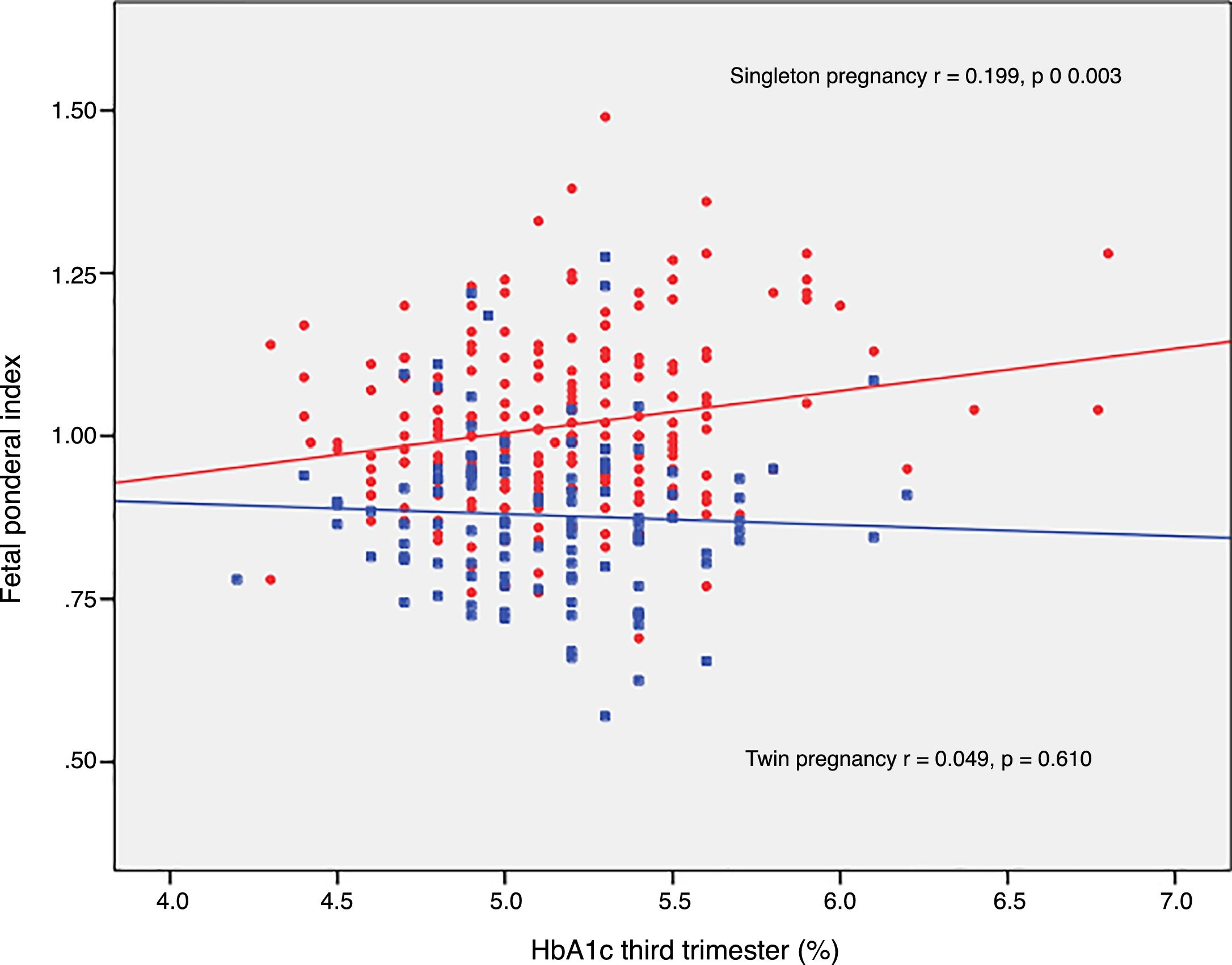
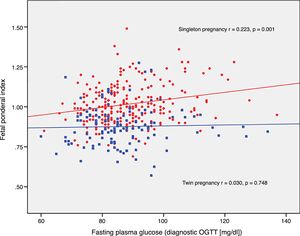
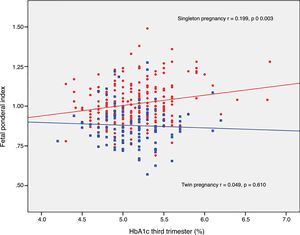
The FPI correlated to fasting glucose in the OGTT at diagnosis (r=0.223; p=0.001) and to mean HbA1c in the third trimester (r=0.199; p=0.003) in singleton pregnancies, though not in twin pregnancies (r=0.030, p=0.748; r=0.049, p=0.610, respectively) (Figs. 2 and 3).
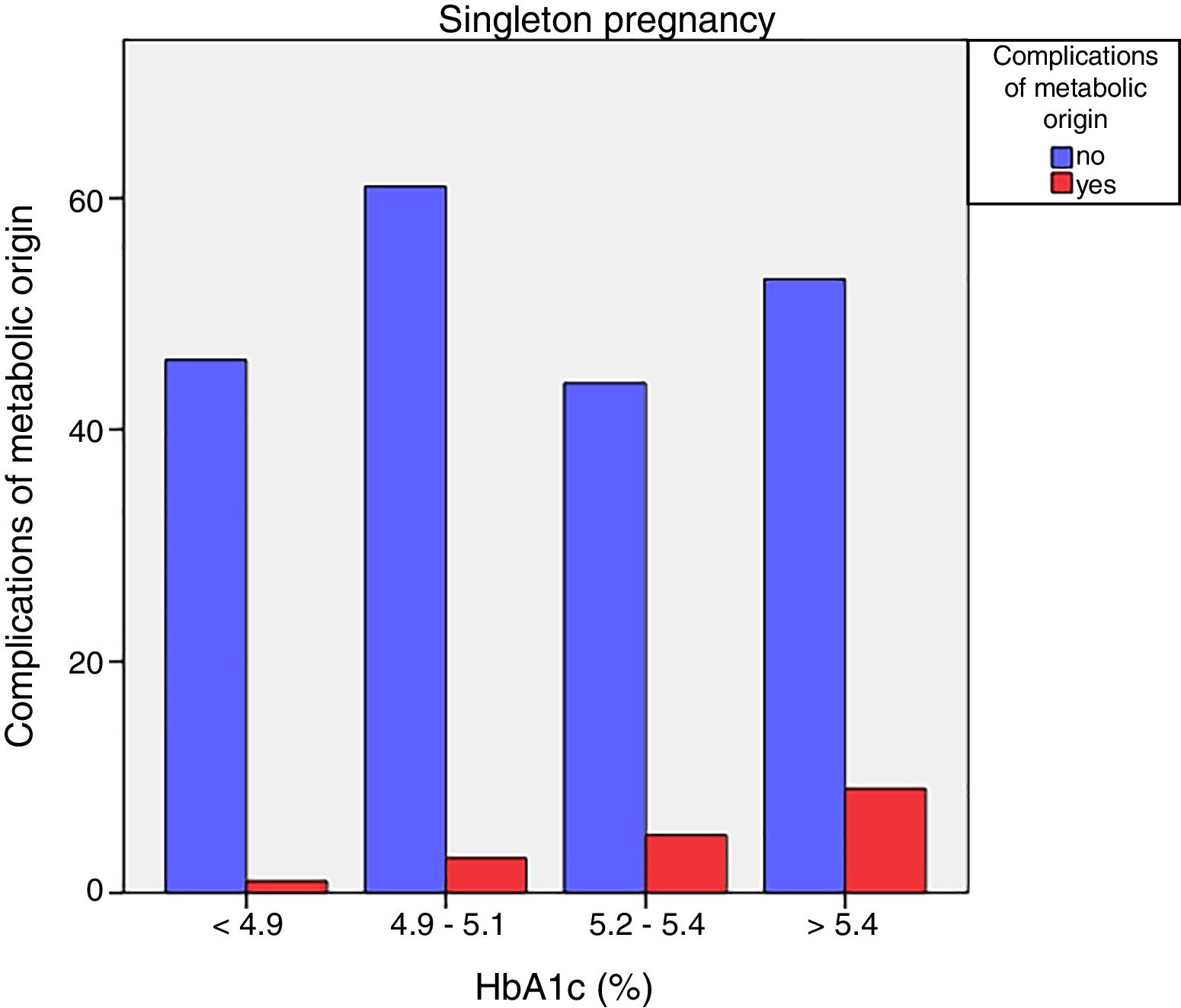
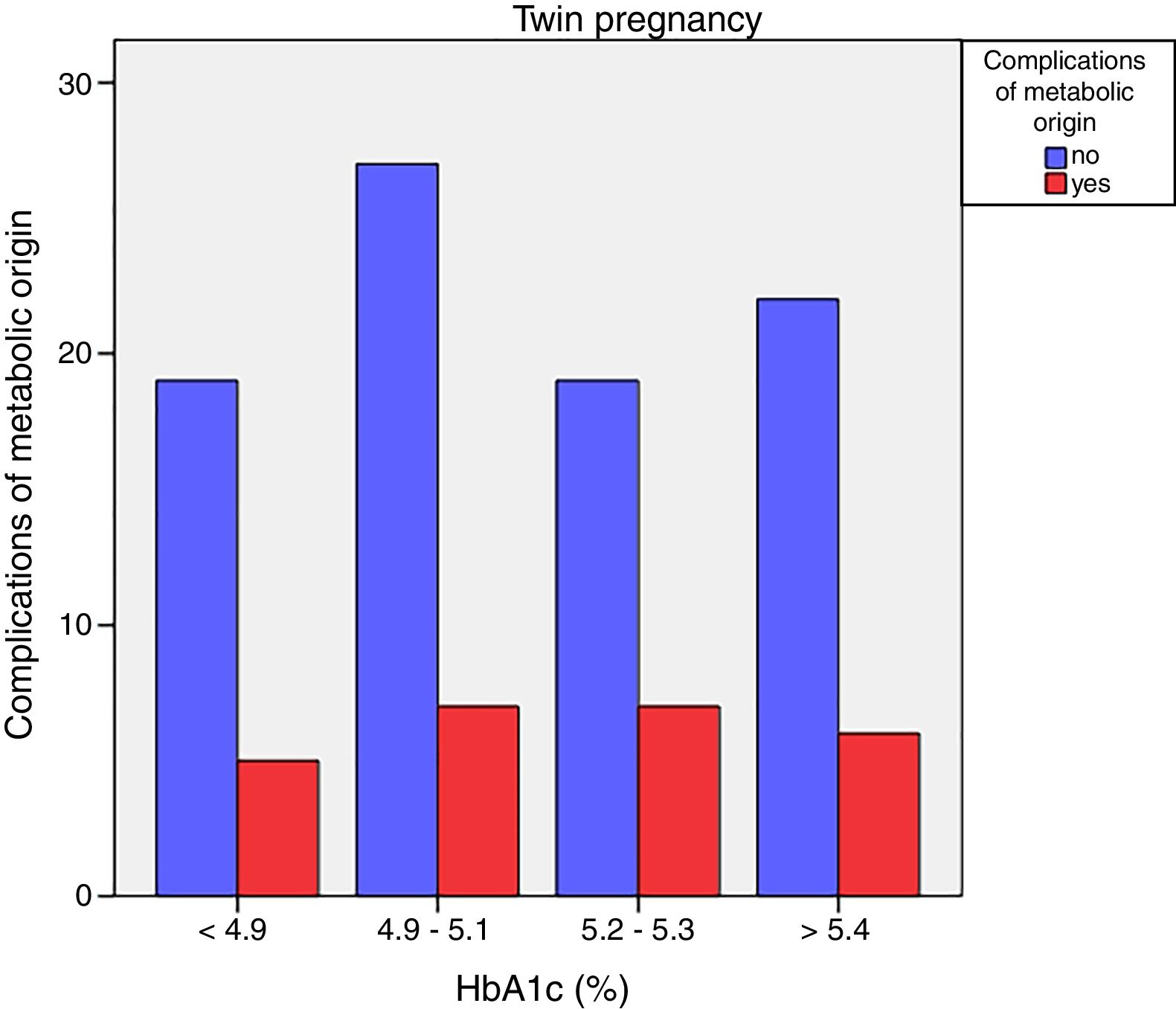
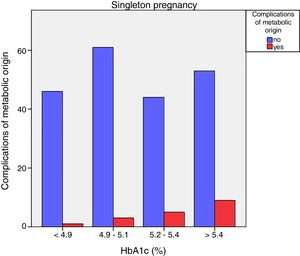
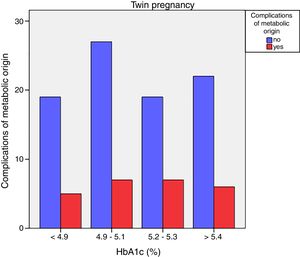
Neonatal metabolic complications (hypocalcemia, hypoglycemia, polyglobulia and hyperbilirubinemia) were more frequent in women with third trimester HbA1c values >5.4%: 14.5% vs. 5.6%; p=0.050 in singleton pregnancies, but not in twin pregnancies (22.6% vs. 21.4%; p=1.000) (Figs. 4 and 5).
With regard to pregnancies requiring insulin, the mean FPI was significantly lower in twin pregnancies in this subgroup as well (1.03±0.13 vs. 0.90±0.15; p<0.001). No differences were found between singleton and twin pregnancies in the incidence of LGA (17.5% vs. 19.4%; p=0.805) or macrosomia (10.3% vs. 6.5%; p=0.737). The incidence of SGA (5.6% vs. 9.7%; p=0.415) or severe SGA (1.6% vs. 6.5%; p=0.099) was greater in the twin pregnancy group, though the difference was not statistically significant.
DiscussionThe results of our study support a divergent effect of gestational diabetes mellitus upon neonatal weight in singleton and twin pregnancies. In fact, the FPI showed a different distribution in the comparator groups. In singleton pregnancies the distribution was normal around percentile 50, but deviated toward percentile 25–50 in twin pregnancies. This change in neonatal weight distribution resulted in an increase in the incidence of SGA below percentile 5 in twin pregnancies (8.3% vs. 2.5%). In addition, fasting glucose at diagnosis and HbA1c showed a linear association with the FPI in singleton pregnancies but not in twin pregnancies.
In twin pregnancies the high prevalence of risk factors for growth restriction and low birth weight (an increased incidence of hypertensive disorders of pregnancy and premature delivery),7,8 probably counteracts the impact of GDM upon excessive fetal growth.
In a previous study, Tward et al. described excessive asymmetrical growth in twin pregnancies with GDM as measured by the abdominal circumference/head circumference ratio at last ultrasound evaluation before delivery, and this finding correlated to blood glucose at the diagnosis of GDM. The authors also found a higher proportion of newborn infants in the highest birth weight percentile group, and a lower proportion of fetuses in the lowest weight percentile.11 In fact, this fetal growth promoting effect has been demonstrated in a number of studies, which have documented that GDM in twin pregnancies causes a decrease in the incidence of SGA.4,11,14,15
Because of the low probability of untoward events such as macrosomia, shoulder dystocia or traumatic delivery in twin pregnancies, the decrease in the incidence of low birth weight infants may contribute to decreased perinatal morbidity in general.9,10
In our study population, neonatal weight in twin pregnancies with GDM was not related to fasting blood glucose at diagnosis or to glycemic control during pregnancy, this being consistent with the data previously reported by our group on comparing twin pregnancies with and without GDM.12 There were no differences in mean HbA1c values in the third trimester, despite the fact that women with twin pregnancies had a higher weight gain rate and a lower insulinization rate (52.5% vs. 26.1%).
With regard to neonatal complications of metabolic origin, we observed an increased risk of hypoglycemia and polyglobulia in twin pregnancies as compared to singleton pregnancies. The main risk factor for an increased incidence of neonatal complications is prematurity and the low birth weight inherent in prematurity.30 Preterm newborns from multiple pregnancies have been reported to suffer an additional increase in mortality compared to singletons of the same gestational age.31
Gestational diabetes mellitus as well as prematurity and multiple pregnancies increase the risk of complications of metabolic origin in the immediate perinatal period, since they are associated with a poorer adaptation to extrauterine life. In our cohort, gestational age at delivery and birth weight were significantly lower in the multiple pregnancy group, thus multiplying the likeliness of such complications.
A limitation to be taken into account in our study is its retrospective design, and we cannot rule out the impact of other factors such as placental insufficiency, perinatal acute hypoxia, delayed umbilical cord clamping, and feto-fetal transfusion syndrome upon the increased frequency of neonatal polycythemia.
Likewise, neonatal complications of metabolic origin were more common in women with higher HbA1c levels in the third trimester only in singleton pregnancies, as previously documented in the literature.12,16
In our cohort, twin pregnancies required a higher insulin dose than singleton pregnancies, 0.34±0.16 vs. 0.29±0.14IU/kg, to achieve the same glycemic control. This difference may be due to selection bias caused by a conservative management approach, in the absence of excessive fetal growth, treatment being intensified only in twin pregnancies with apparent hyperglycemia.
Population-based studies have proposed a differential impact of GDM upon singleton and twin pregnancies. An important limitation of these studies however is the fact that data on other known fetal growth modifiers such as chorionicity, maternal BMI before pregnancy, management protocols and glycemic control were not available.9,10 This is the first study to compare glycemic control during pregnancy in gestation complicated by GDM in singleton and twin pregnancies. There was no relationship between the weight variables or neonatal complications and maternal glycemic control or the type of treatment received (diet or diet alone plus insulin) in twin pregnancies in our study population.
The present study supports the hypothesis that there are other factors such as the incidence of premature delivery (both spontaneous and medically indicated) that produce a greater contribution to neonatal weight and increased perinatal morbidity than glycemic control of GDM in twin pregnancies.
A limitation of our study was the limited sample size involved, which could mask small differences. It should also be taken into consideration that in our cohort all patients were treated following a strict glycemic management protocol, and such intervention could minimize the impact seen upon excess fetal growth. Of note however was the increased risk of severe SGA in twin pregnancies, in which the influence of overtreatment during pregnancy cannot be ruled out.
Future studies are needed to determine whether women with twin pregnancies complicated by GDM and their offspring are at high long-term metabolic and cardiovascular risk, as has been documented in singleton pregnancies.
Specific management protocols for twin pregnancies complicated by GDM, with less strict control objectives, may be appropriate for these women, particularly in the absence of excessive fetal growth or asymmetrical growth.
ConclusionsThe incidence of severe SGA, hypoglycemia and polyglobulia was higher in twin pregnancies with GDM than in singleton pregnancies with GDM. In our study population, basal glucose at diagnosis and the HbA1c value in the third trimester showed a linear association with increased neonatal weight only in singleton pregnancies.
AuthorshipMaría A. Guillén, Lucrecia Herranz, Natalia Hillman and María A. Burgos collaborated in data collection. María A. Guillén analyzed the data and wrote the manuscript. Lucrecia Herranz and Beatriz Barquiel reviewed and edited the manuscript. All the authors participated in the interpretation and discussion of the submitted manuscript.
Conflicts of interestNone declared.
Please cite this article as: Guillén-Sacoto MA, Barquiel B, Hillman N, Burgos MÁ, Herranz L. Diabetes mellitus gestacional: control glucémico durante el embarazo y su relación con los resultados neonatales en embarazos gemelares y de feto único. Endocrinol Diabetes Nutr. 2018;65:319–327.